* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Insect olfactory memory in time and space
Metastability in the brain wikipedia , lookup
Neural coding wikipedia , lookup
Central pattern generator wikipedia , lookup
Nervous system network models wikipedia , lookup
Sensory cue wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Emotion and memory wikipedia , lookup
Synaptogenesis wikipedia , lookup
Childhood memory wikipedia , lookup
Collective memory wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Music-related memory wikipedia , lookup
Chemical synapse wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroanatomy wikipedia , lookup
Prenatal memory wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Sparse distributed memory wikipedia , lookup
State-dependent memory wikipedia , lookup
Memory consolidation wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Reconstructive memory wikipedia , lookup
Optogenetics wikipedia , lookup
Olfactory bulb wikipedia , lookup
Synaptic gating wikipedia , lookup
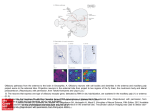
Insect olfactory memory in time and space Xu Liu1 and Ronald L Davis1,2 Recent studies using functional optical imaging have revealed that cellular memory traces form in different areas of the insect brain after olfactory classical conditioning. These traces are revealed as increased calcium signals or synaptic release from defined neurons, and include a short-lived trace that forms immediately after conditioning in antennal lobe projection neurons, an early trace in dopaminergic neurons, and a medium-term trace in dorsal paired medial neurons. New molecular genetic tools have revealed that for normal behavioral memory performance, synaptic transmission from the mushroom body neurons is required only during retrieval, whereas synaptic transmission from dopaminergic neurons is required at the time of acquisition and synaptic transmission from dorsal paired medial neurons is required during the consolidation period. Such experimental results are helping to identify the types of neurons that participate in olfactory learning and when their participation is required. Olfactory learning often occurs alongside crossmodal interactions of sensory information from other modalities. Recent studies have revealed complex interactions between the olfactory and the visual senses that can occur during olfactory learning, including the facilitation of learning about subthreshold olfactory stimuli due to training with concurrent visual stimuli. Addresses 1 Department of Molecular and Cellular Biology 2 Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX 77030 USA Corresponding author: Davis, Ronald L ([email protected]) Current Opinion in Neurobiology 2006, 16:679–685 This review comes from a themed issue on Neurobiology of behaviour Edited by John H Byrne and Wendy Suzuki genes involved in olfactory learning were identified using the fruit fly, Drosophila melanogaster. Several of the identified genes were found to encode components in the cAMP signaling pathway, such as the adenylyl cyclase encoded by the rutabaga (rut) gene; the cAMP phosphodiesterase encoded by the dunce (dnc) gene; the cAMPdependent protein kinase (PKA); the predicted product of the amnesiac (amn) gene, which has similarities to pituitary adenylyl cyclase activating peptide (PACAP); and the transcription factor cAMP-response element binding protein (CREB) [1,2]. At the level of neural circuits, a large body of evidence has accumulated that suggests that mushroom bodies (MBs) are primary sites for olfactory learning [2], and this evidence has biased investigators to focus their studies almost exclusively on the contribution of the MBs. These discoveries have begun to reveal some of the mechanisms underlying olfactory memory formation, and they have also posed new and challenging questions. For instance, is olfactory memory formed and stored exclusively in the MBs until retrieval? Where do cellular changes (cellular memory traces) occur within the olfactory nervous system in response to learning? What is the relationship of cellular memory traces that form in the brain to the various temporal phases (short-, medium-, and long-term) of behavioral memory? How do different sensory modalities interact to support learning? To answer these questions, powerful new tools were needed. Recently, researchers have developed new techniques to visualize the formation of olfactory memory traces in the brain and to dissect the contributions of various neurons to temporal phases of olfactory memory using molecular genetic strategies. We focus, here, on the progress made in these areas in the past few years. Available online 3rd November 2006 0959-4388/$ – see front matter # 2006 Elsevier Ltd. All rights reserved. DOI 10.1016/j.conb.2006.09.003 Introduction All animals modify their behavior through learning, which increases chances of survival. Insects rely heavily on olfaction for various behaviors, including foraging, mating and predator avoidance. The heavy reliance on olfactory information, in part, has made insects attractive model systems for dissecting olfactory memory formation. Significant progress was made in the last three decades of the 20th century in understanding the molecular biology and anatomy that underlies insect olfactory memory. Many www.sciencedirect.com Functional optical imaging reveals multiple, distributed cellular memory traces Electrophysiological recordings have been widely used to study the cellular or neuronal circuitry changes that occur with learning in mammalian model systems. However, such recordings are difficult to perform in insects because of the small size of their bodies, brains and neurons. Progress with functional optical imaging has helped to solve this problem. In functional optical imaging, specially engineered molecules (often fluorescent proteins) are used to report the activity of neurons in living animals during stimulation with sensory cues, such as an odor applied to an insect, before or after olfactory learning has occurred. Synthetic chemical reporters were first used for functional optical imaging in the honeybee, Apis mellifera. Faber et al. Current Opinion in Neurobiology 2006, 16:679–685 680 The neurobiology of behaviour [3] applied the calcium sensitive dye, calcium-green-2AM, through a small window cut in the bee’s head cuticle and using the window, were able to image activity in the antennal lobe. The investigators observed the activity in response to odor stimuli presented before and after associative conditioning with sucrose as a rewarding stimulus. They discovered that specific areas of the antennal lobe exhibit a calcium signal in response to odor presented to naı̈ve animals, and that this signal is increased for at least 30 min after conditioning. In addition, the patterns of activation changed after learning, as evaluated by the correlation in the pattern of activity elicited by a rewarded odor before and after learning compared with that elicited by an unrewarded one. Thus, olfactory conditioning of the honeybee leads to quantitative and qualitative changes in the calcium responses of undefined neurons that innervate the antennal lobes. These and other changes in the response properties of neurons described below could underlie behavioral memory. In Drosophila, functional optical imaging has employed protein-based optical reporters expressed in specific neurons with the GAL4–UAS expression system. In this binary system, one transgenic fly line carries the yeast transcription activator GAL4 controlled by a tissue specific promoter, and a second transgenic fly line carries a gene encoding a neuronal reporter downstream of the binding site for GAL4, the upstream activating sequences (UAS). Progeny from mating these two transgenic lines express the neuronal reporter only in cells defined by the tissue specific promoter [4]. Protein reporters that have been used to report calcium influx include G-CaMP [5], cameleon [6] and camgaroo [7], all of which have been built on a green fluorescent protein (GFP) framework by adding calcium binding motifs, such that the reporters emit stronger fluorescence when intracellular calcium levels are elevated. Wang et al. [5] expressed G-CaMP in the primary olfactory sensory neurons and projection neurons, and imaged antennal lobe calcium responses that occurred when different odorants were presented to the flies. They discovered that different odors generated responses in specific sets of glomeruli that were unique to the odor type, but conserved for each odor across flies. A similar but less comprehensive activity map has also been generated for the MBs using G-CaMP [8]. In addition to calcium-sensitive reporters, a pH-sensitive reporter for synaptic transmission (synapto-pHluorin) has also been developed and used [9]. As synaptic vesicles fuse with the plasma membrane to release neurotransmitter into the synaptic cleft, the pH inside the vesicles increases from acidic to neutral. Transgenically expressed synaptopHluorin, which resides on the lumen side of the synaptic vesicle, reports this pH change and, therefore, the fusion of synaptic vesicles that are releasing neurotransmitter. Synaptic activity maps of antennal lobe neurons responding to odors have been made using this reporter, similar to those generated using G-CaMP [9]. Current Opinion in Neurobiology 2006, 16:679–685 Recent studies using the protein-based reporters of neuronal activity have confirmed and extended the observation made in the honeybee that memory traces form in the antennal lobes. The growing impression from these studies is that the antennal lobe is a site for olfactory memory formation, in addition to the longer-held view that the function of the lobe is to encode the identity of odor type. Yu et al. [10] expressed synapto-pHluorin in specific types of neurons that innervated the antennal lobes, and imaged synaptic release in antennal lobe glomeruli in living flies through a small window in the dorsal head cuticle. Consistent with previous studies [5,9], they observed that different odors stimulated synaptic release in groups of glomeruli that were unique to each odor tested but conserved among flies. This was observed when synaptic release was monitored from olfactory receptor neurons, GABAergic interneurons, or the projection neurons, which have presynaptic release sites in the antennal lobe glomeruli and postsynaptic sites that receive information from the olfactory receptor neurons. Surprisingly, the activation pattern of sets of projection neurons exhibited a qualitative rather than a quantitative change in response to one cycle of conditioning with an odor paired with electric shock. For instance, the odor 3-octanol stimulated synaptic release from four sets of projection neurons (glomeruli) in naı̈ve flies, but from five sets of projection neurons after conditioning (Figure 1b, left). Thus, the synapses of some sets of projection neurons that were silent in the naı̈ve state became active after conditioning, and this occurred within 3 min of training. This synaptic memory trace persisted for only about 5 min after training, suggesting that the antennal lobes have a role in olfactory memory for only a few minutes after learning. Electrophysiology and behavioral data from locust [11] and moth [12] also support a role for the antennal lobes in short-term olfactory memory formation. In addition, the antennal lobe projection neurons might participate in long-term memory formation. Ashraf et al. [13] constructed a reporter transgene for synaptic protein synthesis by fusing a GFP coding cassette with the 30 untranslated region (30 UTR) of the Drosophila calcium– calmodulin protein kinase II (CaMKII) gene. The 30 UTR of the CaMKII gene contains sequences that target its mRNAs to the synaptic compartments of neurons for synaptic protein synthesis. When the reporter transgene was expressed in projection neurons, the investigators were able to monitor changes in synaptic protein synthesis due to neural activity of these neurons by following the expression level of GFP. Remarkably, they discovered that synaptic protein synthesis, as measured with this reporter, was increased in some sets of projection neurons 24 h after the flies received multiple pairings of the odor and the electric shock in a temporally spaced configuration (with a rest between each pairing; Figure 1b, right). Spaced olfactory conditioning produces www.sciencedirect.com Insect olfactory memory in time and space Liu and Davis 681 Figure 1 Neurons with defined olfactory memory traces. (a) Olfactory-learning related structures in Drosophila. MB cells (blue circles) extend dendrites into the calyx (C) region and send axons through peduncles (P) that branch into different lobes (a, a’, b, b’ and g). Projection neurons from the antennal lobes (AL, green) make synaptic connections with MB cells in the calyces. Dorsal paired medial neurons (DPM, red) and dopaminergic neurons (DA, yellow) innervate MB lobes. Memory traces in specific glomeruli of the AL, DPM and DA neurons have been detected with functional optical imaging. (b) Short-term and long-term memory traces in the ALs. An illustration of the AL with glomeruli labeled is shown for both short and long term traces. Left: for the short-term memory trace [10], orange indicates glomeruli responding to 3-octanol in both naı̈ve and trained flies; magenta indicates glomeruli showing detectable synaptic responses to the odor only after training. Right: for the long-term memory trace [13], orange indicates some of the glomeruli that were assayed for synaptic protein synthesis in response to spaced conditioning; magenta indicates glomeruli that exhibited increased synaptic protein synthesis after spaced conditioning. Note the overlap of the responding glomeruli between the short term (synaptic transmission) and the long term (synaptic protein synthesis) traces. (c) Time course of memory traces in different neurons. These memory traces were detected by increased calcium influx, synaptic transmission or synaptic protein synthesis using optical reporters for these processes. Solid lines indicate experimental results; dashed lines indicate hypothetical extensions. AL traces (green) are from [10] and [13]; DPM (red) trace from [17]; DA trace (yellow) from [19]. the formation of long-term, protein synthesis-dependent behavioral memory, whereas a single training trial or massed training with multiple trials (with no rests between pairings) fails to produce this form of memory. Moreover, the specific sets of projection neurons showing increased synaptic protein synthesis after spaced conditioning were, strikingly, overlapping with those previously described to have increased synaptic activity after single pairing (shortterm) training [10], which infers that the mechanisms that lead to the increased synaptic activity over the short-term might also lead to synaptic protein synthesis for long-term memory (Figure 1b). The regulation of synaptic protein synthesis for normal long-term memory is apparently under the control of the RNA-induced silencing complex (RISC) pathway, because genetic disruptions of protein components of the RISC pathway blocked the traininginduced changes in synaptic protein synthesis and longterm behavioral memory. Spaced conditioning induces a prolonged activation of PKA in the honeybee antennal lobes. A single training trial along with the release of caged cAMP in the antennal lobes by photostimulation also induces long-term memory [14]. This study provides www.sciencedirect.com further evidence that supports a role for the antennal lobes in long-term memory formation. Amnesiac (amn) is a Drosophila olfactory memory mutant with a strong effect on post-training performance that begins 15–30 min after conditioning [15]. Immunohistochemical studies revealed that the putative product of the gene is expressed in the dorsal paired medial (DPM) neurons, a pair of neurons in the brain sending two major processes into the neuropil areas that house the axons of MB cells [16]. The behavioral effect of the mutant has been ascribed to the DPM neurons, because expression of a wild type amn gene in these neurons is sufficient to rescue the behavioral phenotype. The gene is regarded as primarily influencing medium-term memory, because there is no memory defect immediately after acquisition, and synaptic transmission from these neurons is not required during acquisition, as revealed by synaptic blocking experiments (see below). Yu et al. [17] expressed G-CaMP or synapto-pHluorin in the DPM neurons and imaged the odor-response Current Opinion in Neurobiology 2006, 16:679–685 682 The neurobiology of behaviour properties of DPM neuronal process after olfactory learning. They found that pairing odor with shock increased subsequent odor-evoked calcium influx and synaptic release from the DPM neurons, but this increase was delayed, appearing first at 30 min after training and persisting for at least an hour (Figure 1c, middle). Remarkably, this delayed memory trace was restricted to the DPM processes that innervate the vertical lobes of mushroom bodies and not the horizontal lobes, and its formation required the activity of the amn gene in the DPM neurons themselves. These functional imaging studies have, therefore, identified a branch-specific, delayed memory trace that forms in DPM neurons with kinetics similar to those of behavioral medium-term memory. The synaptic activity of dopaminergic neurons is required for odor–electric shock learning, as shown in experiments using a conditional block of dopaminergic synaptic transmission at the time of training (see below) [18]. Riemensperger et al. [19] expressed the optical reporter cameleon in the dopaminergic neurons, and imaged the flies before and after olfactory learning. Surprisingly, they detected calcium responses in these neurons when odors were presented to the flies, even though the dopaminergic neurons are thought to be part of the unconditioned stimulus (US) pathway (electric shock) and not the conditioned stimulus (CS) pathway (odor). The odor responses of the neurons were also prolonged after training, indicating that conditioning alters the response of these neurons to odor [19] (Figure 1c, bottom). These data, therefore, surprisingly indicate that cellular memory traces also form in dopaminergic neurons after training. These recent studies suggest that cellular memory traces form in a distributed fashion in the olfactory nervous system after olfactory conditioning, but they do not challenge a central role for the MBs in olfactory learning. The neurons that form these memory traces (antennal lobe projection neurons, DPM neurons and dopaminergic neurons) are all thought to synapse onto the MB neurons, potentially suggesting that these cellular memory traces ultimately work through the MB neurons. Nevertheless, MBs must not be viewed as the only anatomical structure in which olfactory memories form. Future studies will undoubtedly reveal the mechanisms underlying these newly discovered memory traces and the general issue of whether they are dependent or independent of one another. Molecular genetic approaches to dissect behavioral memory in time and space Functional optical imaging experiments, described above, have provided evidence that olfactory memory traces can form at different times and in different places in the insect brain. Molecular genetic experiments have also allowed for time and space dissections of behavioral memory. Current Opinion in Neurobiology 2006, 16:679–685 A central question for any mutant with memory deficits is whether the loss of function of a specific gene alters the development of the nervous system so as to perturb memory formation or whether it alters the cellular mechanisms underlying memory formation. To address this important issue, systems that offer the experimenter control of transgene expression in both time and space have been developed. The traditional GAL4–UAS system relies on the inherent temporal expression pattern of a tissue-specific promoter to drive the GAL4 transgene. Two recently developed systems, named Gene-Switch and TARGET, provide extensions of the GAL4–UAS system for temporally controlling expression [20]. The TARGET system uses a transgene encoding a temperature-sensitive inhibitor of GAL4, named GAL80ts, in addition to the GAL4 and UAS components. At low temperatures (18 8C), GAL80ts represses GAL4 activity to inhibit expression of the UAS-transgene. At high temperatures (32 8C), the repression is released and GAL4 activates expression from the UAS component in the spatial pattern defined by the promoter driving GAL4. In Gene-Switch, a GAL4-progesterone receptor chimera replaced the GAL4 component, which functions as a transcriptional activator only in the presence of mifepristone (RU486). The tissue-specific promoter, therefore, confers the pattern of expression, and administration of RU486 through feeding provides temporal control. McGuire et al. [21] used the TARGET system to express a wild type rut transgene in rut null mutant flies. They discovered that expression of this transgene in the MBs only during adulthood was sufficient to rescue the olfactory learning phenotype. This demonstrates that the rut learning phenotype is due to the loss of rut activity in the adult brain after development is completed. The same conclusion was reached in experiments using GeneSwitch to provide temporal control [22]. Ferris et al. [23] disrupted olfactory learning by expressing pertussis toxin, an inhibitor of heterotrimeric G(o) proteins, in adult fly mushroom bodies using both TARGET and Gene-Switch systems to prove that G(o)-based signaling is required for learning. One useful division of memory is formed on the basis of its persistence, such as short-, medium- and long-term memory. But memory can also be divided operationally into acquisition, stabilization, consolidation and retrieval. A major challenge in the functional dissection of memory is to assign the specific phases and operations of memory to different brain regions, neurons, cellular processes and genes. New and old techniques and reagents have begun to make this possible in Drosophila. Structural brain mutants have been studied for decades for their effects on memory formation in an attempt to dissect the underlying anatomy. Pascual et al. [24] www.sciencedirect.com Insect olfactory memory in time and space Liu and Davis 683 identified a new structural brain mutant that causes the variable loss of the vertical and/or the horizontal lobes of the MBs in different animals. Although the mutant flies exhibit normal performance at all times after training when evaluated all together, if analyzed as a subgroup, those flies missing the vertical lobes exhibit no long-term but normal short-term memory. Animals missing the horizontal lobes have no phenotype. These structural studies, therefore, suggest that long-term memory is either formed within the vertical lobes of the MBs or distributed from these lobes. The mutant shibirets (shits) causes a rapid and reversible block in synaptic transmission at high temperatures (>29 8C), owing to the function of the gene product in replenishing the neurotransmitter vesicle population at synapses. This mutant gene was placed under UAS control (UAS-shits) in a transgene as a new tool to evaluate the importance of synaptic transmission from specific neurons. Two different groups expressed this transgene in the MBs at elevated temperatures at different time points during olfactory learning. They showed that synaptic transmission from these neurons is only required at the time of retrieval [25,26]. Blocking synaptic transmission from these neurons at the time of acquisition or during the subsequent consolidation phase was without effect on performance, which was tested at times up to 3 h after training. These studies, therefore, indicate that early memories are formed in the MB or upstream neurons, because blocking MB output had no effect on acquisition or stability for the first few hours. Keene et al. [27] performed similar experiments to block synaptic transmission from the DPM neurons between 0 and 2 h after odor–electric shock training, a time window during which consolidation is thought to occur. When certain odor combinations were used for training, this blockade impaired performance when tested at 3 h after training, suggesting that synaptic output onto the MB neurons during this period is mechanistically part of the consolidation process. Blocking synaptic transmission during the acquisition or retrieval phases is without effect on memory tested 3 hours after training. Similar results have been obtained using odor–sucrose reward training, indicating that the mechanisms provided by DPM synaptic transmission onto the following neurons are used for consolidating both aversive and appetitive unconditioned stimuli [28]. Finally, UAS-shits was used to demonstrate that synaptic release from dopaminergic neurons is required during acquisition for normal memory formation [18]. Olfactory memory formation across extended space: crossmodal interactions for olfactory learning Learning is generally a complex process that integrates different sensory cues. Olfactory memory, visual memory, taste memory and other forms of memories represent www.sciencedirect.com unimodal memories within the whole of ‘memory space’, and they can interact extensively with each other in this space. To dissect how olfactory memory interacts with other forms of memories within this extended space, crossmodal learning paradigms have been invented. Guo and Guo [29] combined the flight simulator with an odor delivery system to study these interactions. They used a noxious heat stimulus as a negative reinforcer to train flies suspended by a thin wire to fly towards different shapes, odors or their combinations in an artificial flight space. They first defined the discriminatory threshold of the visual cue (horizontal bars presented at different vertical positions relative to the horizon) required for learning an association between one visual cue and the heat reinforcer, and independently the odor concentration threshold necessary for learning to discriminate a punished and an unpunished odor. When presented with subthreshold visual and olfactory cues together, Drosophila display significant learning to either cue, although each cue by itself is inadequate to support learning. Moreover, when flies were pre-exposed to both visual and olfactory cues simultaneously without reinforcement, and then trained to associate only one of the cues with heat punishment, they were able to respond to the cue representing the other sensory modality during testing. This suggests that the association of visual and olfactory cues during preconditioning provided the cross-modal memory space to transfer subsequent reinforced associations across modalities. Crossmodal learning studies have also been performed in honeybees. To test if honeybees were capable of complex learning tasks, Giurfa et al. [30] passed honeybees through series of training chambers, each of which contained one of two odors. The bees were rewarded with sucrose if they entered the final chamber with the same odor as that experienced in the first chamber. In some trials, the honeybees were put through a similar series of chambers except that the two odor cues were replaced by two different colors. They preferred the final chamber with the same color as the first chamber. The opposite was observed if the honeybees were trained to choose the odor that is different from the one experienced in the first chamber. These experiments show that honeybees can transfer the concept of ‘sameness’ and ‘difference’ across olfactory and visual memories. Reinhard et al. [31] tested crossmodal interactions in honeybees with a setting closely mimicking the natural environment. They trained bees to forage at sugar water feeders associated with different odors and colors that were placed at different outdoor locations. Presenting a specific odor into the hive prompted the bees to locate the feeders previously associated with that odor, even though the odor was not present during testing. Thus, memory of a specific odor triggers complex navigational behavior in the honeybee that engages visual memory. Current Opinion in Neurobiology 2006, 16:679–685 684 The neurobiology of behaviour Conclusions Olfactory memory formation is a complicated process that is being dissected with many different techniques. The dissections of when and where memory traces are formed in the brain, of when and where gene products are required in the brain for normal learning, and of when and where synaptic transmission is required to support memory formation are among the most fundamental issues in the field of learning and memory. New techniques and tools are starting to help unravel these issues. Current and future research will enable not only the dissection of olfactory memory formation in both time and space but will also incorporate this information into a general multi-sensory learning program. Deeper insights are anticipated using combined molecular–genetic and systems neuroscience approaches to study olfactory learning. References and recommended reading Papers of particular interest, published within the annual period of review, have been highlighted as: of special interest of outstanding interest 1. McGuire SE, Deshazer M, Davis RL: Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol 2005, 76:328-347. 2. Davis RL: Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci 2005, 28:275-302. in primary olfactory networks. Proc Natl Acad Sci USA 2004, 101:10476-10481. 13. Ashraf SI, McLoon AL, Sclarsic SM, Kunes S: Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 2006, 124:191-205. Synaptic protein synthesis is crucial for long-term memory. The authors used a new reporter containing the 30 UTR of the CaMKII gene to monitor synaptic protein synthesis in projection neurons of the Drosophila antennal lobes after long-term olfactory associative learning. They found that the mRNA reporter was directed to synaptic regions and translated in specific sets of projection neurons after spaced training. The authors also found that this process is regulated by the RISC pathway. 14. Muller U: Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron 2000, 27:159-168. 15. Quinn WG, Sziber PP, Booker R: The Drosophila memory mutant amnesiac. Nature 1979, 277:212-214. 16. Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG: The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 2000, 103:805-813. 17. Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL: Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell 2005, 123:945-957. Using G-CaMP and synapto-pHluorin as reporters, the authors imaged the activity and synaptic release of DPM neuronal processes after olfactory conditioning. They detected a memory trace registered as increased calcium influx and increased synaptic release at 30 minutes after training but not before, and that persisted for at least 1 hour. This memory trace is restricted to the DPM neuronal processes that innervate the vertical lobes of MBs but not the horizontal lobes. Formation of the memory trace also requires normal amn gene function in the DPM neurons. These observations are consistent with behavioral data indicating a role for the DPM neurons in medium-term memory. 18. Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M: Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 2003, 23:10495-10502. 3. Faber T, Joerges J, Menzel R: Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci 1999, 2:74-78. 4. Duffy JB: GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis 2002, 34:1-15. 19. Riemensperger T, Voller T, Stock P, Buchner E, Fiala A: Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol 2005, 15:1953-1960. 5. Wang JW, Wong AM, Flores J, Vosshall LB, Axel R: Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 2003, 112:271-282. 20. McGuire SE, Roman G, Davis RL: Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet 2004, 20:384-391. 6. Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud J-M, Buchner E, Galizia CG: Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons. Curr Biol 2002, 12:1877-1884. 21. McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL: Spatiotemporal rescue of memory dysfunction in Drosophila. Science 2003, 302:1765-1768. 7. Yu D, Baird GS, Tsien RY, Davis RL: Detection of calcium transients in Drosophila mushroom body neurons with camgaroo reporters. J Neurosci 2003, 23:64-72. 8. Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, Svoboda K, Zhong Y: Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent proteinbased Ca2+ imaging. J Neurosci 2004, 24:6507-6514. 9. Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G: Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 2002, 36:463-474. 10. Yu D, Ponomarev A, Davis RL: Altered representation of the spatial code for odors after olfactory classical conditioning: memory trace formation by synaptic recruitment. Neuron 2004, 42:437-449. 11. Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G: Fast odor learning improves reliability of odor responses in the locust antennal lobe. Neuron 2005, 46:483-492. 12. Daly KC, Christensen TA, Lei H, Smith BH, Hildebrand JG: Learning modulates the ensemble representations for odors Current Opinion in Neurobiology 2006, 16:679–685 22. Mao Z, Roman G, Zong L, Davis RL: Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci USA 2004, 101:198-203. 23. Ferris J, Ge H, Liu L, Roman G: G(o) signaling is required for Drosophila associative learning. Nat Neurosci 2006, 9:1036-1040. 24. Pascual A, Preat T: Localization of long-term memory within the Drosophila mushroom body. Science 2001, 294:1115-1117. 25. McGuire SE, Le PT, Davis RL: The role of Drosophila mushroom body signaling in olfactory memory. Science 2001, 293:1330-1333. 26. Dubnau J, Grady L, Kitamoto T, Tully T: Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 2001, 411:476-480. 27. Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S: Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron 2004, 44:521-533. www.sciencedirect.com Insect olfactory memory in time and space Liu and Davis 685 28. Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S: Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol 2006, 16:1524-1530. 29. Guo J, Guo A: Crossmodal interactions between olfactory and visual learning in Drosophila. Science 2005, 309:307-310. The authors trained flies with both olfactory and visual cues in the flight simulator. They found subthreshold visual and olfactory cues elicited significant learning when presented simultaneously (crossmodal enhancement). They also found visual or olfactory memory is transferable www.sciencedirect.com across modalities if both stimuli are presented together before training (crossmodal transfer). 30. Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV: The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 2001, 410:930-933. 31. Reinhard J, Srinivasan MV, Guez D, Zhang SW: Floral scents induce recall of navigational and visual memories in honeybees. J Exp Biol 2004, 207:4371-4381. Current Opinion in Neurobiology 2006, 16:679–685