* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Systematic Regional Variations in the Loss of Cortical Cholinergic
Neurogenomics wikipedia , lookup
Brain Rules wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroscience and intelligence wikipedia , lookup
Haemodynamic response wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neuroanatomy wikipedia , lookup
Metastability in the brain wikipedia , lookup
Perception of infrasound wikipedia , lookup
Neuropsychology wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Time perception wikipedia , lookup
Neuroesthetics wikipedia , lookup
Microneurography wikipedia , lookup
Environmental enrichment wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Synaptic gating wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Circumventricular organs wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Human brain wikipedia , lookup
Cortical cooling wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuroeconomics wikipedia , lookup
Alzheimer's disease wikipedia , lookup
Aging brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroplasticity wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Systematic Regional Variations in the
Loss of Cortical Cholinergic Fibers in
Alzheimer's Disease
The loss of cortical cholinergic fibers in Alzheimer's disease was investigated using choline acetyltransferase immunohistochemistry and
acetylcholinesterase histochemistry. Within both the normal and Alzheimer's cerebral cortex, the two methods revealed an identical pattern of fiber staining. In the normal brain, cholinergic fiber density
was highest in limbic and paralimbic cortical zones, intermediate in
most sensory-motor and association zones, and lowest within the primary visual and visual association areas of the occipital lobe. In general, supragranular cortical layers contained a higher density of cholinergic fibers, and most of these were oriented vertically. In Alzheimer's disease, an overall 55% loss of cortical cholinergic fibers was
detected. There was, however, marked regional variations in the extent of this loss in different cortical areas. Cortical areas within the
temporal lobe, particularly the temporal association areas, displayed
a dramatic loss of cholinergic fibers. By contrast the anterior cingulate cortex, primary visual, primary somatosensory, and primary motor
cortex displayed a relative preservation of cholinergic fibers. As a
whole, greater loss of cholinergic fibers was detected in supragranular layers and in fibers oriented vertical to the cortical surface. These
results indicate that cholinomimetic therapies are likely to have different effects on cholinergic transmission in various cortical areas.
The precise mechanisms that lead to the regional variations in cortical
cholinergic denervation in Alzheimer's disease remain to be elucidated.
The human cerebral cortex contains a complex and extensive
network of cholinergic axons (Geula and Mesulam, 1990; Mesulam et al., 1992; Mesulam and Geula, 1994). Virtually all of
these axons originate from the cholinergic neurons of the
basal forebrain (Chl-Ch4), -which are rich in the enzymes
choline acetyltransferase (ChAT) and acetylcholinesterase
(AChE) (Mesulam et al., 1983, 1986; Mesulam and Geula,
1988a). Within the human cerebral cortex, AChE and ChAT
staining reveal a completely overlapping pattern of axons
(Mesulam and Geula, 1992).
A marked loss of cortical cholinergic innervation was the
first neurotransmitter abnormality detected in the brains of
patients suffering from Alzheimer's disease (AD). In 1976, Davies and Maloney (1976) and Bowen et al. (1976) reported a
dramatic loss in levels of biochemically determined ChAT activity in postmortem tissue from cerebral cortex of AD patients. Since that time, a severe loss of cortical ChAT activity
(up to 95%) has become established as one of the most consistent findings in AD (Perry et al., 1977; Bowen et al., 1979;
Davies, 1979; Rossor et al., 1982a; Wilcock et al., 1982; Bird et
al., 1983; Wood et al., 1983; DeKosky et al., 1985). Biochemical
studies have also found an up to 90% loss in the activity of
cortical AChE in AD (Davies and Maloney, 1976; Davies, 1979;
Reinikainen et al., 1988; Zubenko et al., 1989).
A small number of studies have investigated the fate of
individual cholinergic axons in AD cortex using AChE histochemistry (Henke and Lang, 1983; McGeer et al., 1986; Brashear et al., 1988; Geula and Mesulam, 1989) or ChAT immunohistochemistry (Ransmayr et al., 1989), and all of these
studies have investigated the cholinergic loss in only a few
Changiz Geula1 and M.-Marsel Mesulam2
•Laboratory for Neurodegenerative and Aging Research,
Department of Medicine, Harvard Medical School and
Section of Geriatric Medicine, New England Deaconess
Hospital, Boston, Massachusetts 02215; and behavioral and
Cognitive Neurology and Alzheimer Program, Department of
Neurology, Northwestern University Medical School,
Chicago, Illinois 60611
cortical areas. Consistent with biochemical findings, these histochemical studies have demonstrated a marked loss of cortical cholinergic innervation in AD.
To date, there is little consistent information on the regional variability of cholinergic loss in different cortical areas
of the AD brain. Biochemical studies of cortical cholinergic
denervation in AD include many inconsistencies with respect
to the extent of this loss in various cortical regions (Davies
and Maloney, 1976; Perry et al., 1977; Davies, 1979; Araujo et
al., 1988; Reinikainen et al., 1988). Our earlier observations
using AChE histochemistry of three cortical areas showed
considerable variation in the loss of cholinergic fibers iri different cortical areas (Geula and Mesulam, 1989). Here, we report marked regional variations in the loss of cortical cholinergic fibers using AChE histochemistry and ChAT immunohistochemistry combined with a survey of a large number of
cortical areas.
Materials and Methods
Tissue Preparation and Pathological Observations
The observations described in this report were made in 10 brains
from normal-aged individuals with no prior history of neurologic or
psychiatric disorders and 10 brains from patients with a history of
dementia of the Alzheimer type. The characteristics of the subjects
are summarized in Table 1. Each brain was cut into 1-2 cm hemispheric coronal slabs and examined for the presence of atrophy, ventricular enlargement, and other gross abnormalities. The slabs of tissue were placed in cold 4% paraformaldehyde in 0.1 M phosphate
buffer (pH 7.4) for 24-30 hr, then into graded concentrations of
sucrose (10-40% in 0.1 M phosphate buffer, at 4°C) for cryoprotection. The slabs were then sectioned at 40 |un on a freezing microtome into 0.1 M phosphate buffer and stored at 4°C until used. Representative sections from each tissue block were stained with hematoxylin-eosin and thioflavin-S for neuropathological observations
and with cresyl echt violet for delineation of cytoarchitectonic
boundaries. In most of the brains (six normal and six AD cases),
tissue was available from the whole extent of the hemisphere and
all cortical areas. In the rest, tissue was available from temporal, anterior parietal, and posterior frontal areas, and in some of these from
the occipital and anterior frontal cortex as well.
Only brains with no gross or microscopic abnormalities and no
or very few cortical plaques and tangles, consistent with normal aging (Khachaturian, 1985), were designated as normal (cases 1-10,
Table 1). The brains from the demented individuals (cases 10-20)
contained numerous cortical plaques and tangles in a density and
distribution consistent with the neuropathological diagnosis of AD
(Khachaturian, 1985). Brains that displayed other gross or microscopic neuropathological abnormalities were not used in this study.
Acetylcbolinesterase Histocbetnistry
Acetylcholinesterase activity within cortical axons and perikarya was
visualized in a representative series of sections from each brain with
the help of a new and highly sensitive histochemical method. The
principles of this method (incubation in a dilute Karnovsky-Roots
medium followed by metal ion-diaminobenzidine intensification)
have been described by Hanker et al. (1973) and Tago et al. (1986).
We have introduced a number of changes in this method as described
elsewhere (Geula and Mesulam, 1989; Mesulam and Geula, 1994).
Cerebral Cortex Mar/Apr 1996;6:l65-177; 1047-3211/96/$4.00
Tablet
Summary of cases
P
Case
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
•
Age
55Y
64Y
71 Y
72Y
74 Y
75Y
76 Y
84Y
86Y
91 Y
62Y
67Y
76 Y
77Y
79 Y
80Y
81 Y
82Y
86Y
87Y
Table 2
Loss of cortical cholinergic (AChE-positive) fibers in Alzheimer's disease
Postmortem
*
* ii 11 an
1
Cortical area
U A ^ A I J * *
interval
8hr
21 hr
14 hr
8 hr
24 hr
12 hr
17 hr
4 hr
8 hr
3 hr
18 hr
8 hr
19 hr
17 hr
6 hr
9 hr
20 hr
6 hr
20 hr
12 hr
horo
Cov
nere ocx
R
R
L
R
R
R
R
R
L
R
R
R
R
R
R
L
R
R
R
R
All
AJJ
F
F
M
F
M
M
M
F
M
M
F
+
F
F
F
F
F
F
F
F
F
+
+
+
+
+
+
+
+
+
Lause of death
Cardiac arrest
Myocardial infarction
Cardiac arrest
Cardiac arrest
Lymphoma
Cardiac arrest
Myocardial infarction
Myocardial infarction
Respiratory arrest
Cerebellar hemorrhage
Pneumonia
Pneumonia
Cardiac arrest
Pneumonia
Cardiac arrest
Cardiac arrest
Pneumonia
Myocardial infarction
Cardiac arrest
Pneumonia
Normal
Alzheimer's disease % Loss
1 ATPJK rfi^nlflvino oreator thsn 75% lice
Temporal visual association (20)'
Temporal visual association (21)
Auditory association (22)
Entorhinal cortex (28)
572
524
617
1218
± 115
±77
± 123
± 170
86
77
101
239
85
85
84
80
±51*
±43*
±59*
±81*
II. Areas displaying 45-75% loss
Granular temporal pole (38)
Granular insula
Primary auditory (41-42)
Superior parietal association (7)
Visual association (19)
Granular orbitofrontal (11-12)
Inferior parietal lobule (39-40)
Somatosensory association (5)
Subiculum
Dysgranular orbitofrontal (11-12)
Prefrontal association (9)
CA1 sector of hippocampus
Visual association (18)
Frontal operculum (44)
839 ± 182
984 ± 167
742 ± 141
427 ± 4 5
373 ± 91
734 ± 162
517 ± 54
495 ± 89
1325 ±277
1224 ± 289
464 ± 4 8
1862 ± 505
340 ± 103
570 ± 131
220 ± 82*
262 ± 9 9 *
228 ± 80*
135 ± 49*
124 ±23*
265 ± 122*
200±92*
194 ± 41*
540 ± 161*
500 ± 90*
198 ±39*
856 ± 5 0 *
173 ± 3 8 *
296±96t
497 ± 143
257±103t
Att
ricTroniai association tot
Frontal pole (10)
74
73
69
68
67
64
61
61
59
59
57
54
49
48
40
48
III. Areas displaying less than 45% loss
To inhibit butyrylcholinesterase (BChE, nonspecific cholinesterase),2 X 10-* M ethopropazine (MW 348.9) or 10"* M ISO-OMPA (MW
342.4, Sigma Chemical Company, St. Louis, MO) were used in the
incubation medium. The specific AChE inhibitor BW284C51 (MW
556.4, Sigma Chemical Company) was added (10"* M) to demonstrate
the specificity of the AChE staining.
Cboline AcetyUransferase Immunobistocbemistry
Representative series of sections from three normal and three AD
cases were processed for ChAT immunohistochemistry using a wellcharacterized polyclonal antibody (generously provided by Dr. L. B.
Hersh, University of Kentucky, Lexington, KY) raised in the rabbit
against human placenta] ChAT (German et al., 1985). The antibody
•was used at a dilution of 1:500 to 1:700 in an avidin-biotin-peroxidase (ABC) immunohistochemical procedure employing the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). The final
immunohistochemical reaction product -was intensified according to
the method described by Kitt et al. (1988).
Two types of control procedures were used. In one set of control
sections, an irrelevant IgG was substituted for the primary antibody.
In a second set, adsorption procedures were carried out by incubating the antibody in the presence of purified ChAT before being used
for immunohistochemistry. Sections from both control procedures
also underwent the intensification procedure.
Assessment of Cbolinergic Fiber Density
All sections processed for AChE nistochemistry and ChAT immunohistochemistry were subjected to a qualitative surveyforthe assessment of regional variations in fiber density in the normal brains and
in fiber loss in AD brains. In normal (N = 6) and AD (N = 6) brains
from •which whole hemispheric sections were available, an intersect
analysis was used (Geula and Mesulam, 1989) to obtain a quantitative
estimate of fiber density in 28 cytoarchitectonically and functionally
distinct cortical areas (Tables 2, 3). For this purpose, tissue sections
processed for AChE nistochemistry were viewed at 200X magnification through a square 10 X 10 grid ( 5 X 5 mm actual dimensions)
placed in the ocular of a Nikon Compound microscope (Fig. 1). At
the above magnification, the grid contained a square of tissue 250 X
250 |Am. The grid was adjusted such that one side of it was parallel
to the cortical surface. The number of fibers intersecting the 10 lines
parallel (for determination of vertically oriented fibers) and perpendicular (for determination of horizontally oriented fibers) to the cortical surface were counted and recorded in lower lamina in (lamina
HJc) and upper lamina V of each cortical area examined. In areas
with more primitive lamination, such as the hippocampus and the
cingulate cortex, counting was performed in a superficial and a deep
layer. To ensure that intersects from all stained fibers within the full
166 Alzheimer's Cholinergic Dcncrvation • Gcula and Mesulam
Premotor association (6)
Posterior cingulate (23)
Primary visual (17)
Primary somatosensory (3,1, 2)
Parolfactory area (25)
Anterior Cingulate (32)
Anterior Cingulate (24)
Primary motor (4)
527 ± 105
923 ± 118
304±94t
537 ± 174*
376 ± 96
597 ± 101
s
1420
975
1193
619
± 466
± 123
±218
± 108
231 ± 41"
390 ± 94*
953 ± 283"s
718 ± 102"s
884±123«
506 ± 351"
42
42
39
35
33
26
26
18
Each value represents combined vertical and horizontal fibers in layers Illc and V averaged across
subjects ± standard deviation. Repeated measures ANOVA indicated significant effects for variables
of cortical area (F = 7142, p < 0.0001) and disease state ( f = 66.86, p < 0.0001). Newman-Keuls
pairwise comparisons: *p < 0.001, t p < 0.025, and t p < 0.05, NS, not significantly different from
control (p > 0.051. The probability values referred to in the test indicate results of Newman-Keuls
pairwise comparisons.
•Numbers in parentheses refer to cortical areas according to Brodmann's classification.
40 |xm thickness of each section were counted, the plain of focus on
the microscope was systematically varied while counting. The best
stained areas within each cytoarchitectonic region were chosen for
this analysis. The counts obtained from the lines of the grid were
then added to obtain an estimate of fiber density (Tables 2, 3).
These counts were subjected to analysis of variance for repeated
measures with Newman-Keuls post hoc tests to determine significant differences.
Results
General Staining Characteristics
Immunohistochemistry with the ChAT antibody revealed-very
thin cholinergic fibers and varicosities throughout the cerebral cortex (Fig. 2). No staining was observed when nonspecific IgG was substituted for the antibody or when the antibody was adsorbed with purified ChAT. No specific ChAT
staining was observed in cortical neurons.
The AChE histochemical procedure also revealed a dense
plexus of fibers throughout the cerebral cortex. When compared with the ChAT-positive fibers, AChE-positive fibers appeared thicker and more distinctly stained (Fig. 2). The histochemical procedure used also revealed many AChE-positive
cortical neurons (see Figs. 4, 5). The great majority of these
neurons were pyramidal in shape and were distributed in laminae in and V of many cortical areas. We have previously described the staining characteristics and distribution of these
Table 3
Counts of AChE-positive cholinergic fibers as a function of lamination and fiber orientation
Alzheimer's disease
Normal
Layer III
Cortical regions
Vet
Layer III
Layer V
Vet
Hor.
Vet
Hor.
Layer V
Vet
Hor.
Hor.
Higher order association areas:
Prefrontal association (9)"
Frontal pole (10)
Frontal operculum (44)
Prefrontal association (10)
Temporal visual association (21)
Temporal visual association (20)
Inferior parietal lobule (39-40)
Superior parietal lobule (7)
147
145
162
163
155
169
154
126
± 18
±34
±36
±50
±17
±26
±25
± 18
106
117
136
127
135
142
126
93
± 11
± 47
±26
±32
±22
±33
±25
± 13
109
109
134
155
119
120
101
100
±11
±30
±35
±34
±35
±35
± 13
± 13
102
126
137
129
115
141
116
107
±18
+ 37
±43
±42
±25
±40
±57
±20
44
72
82
90
21
23
61
42
±31
±28
± 27
±31
±17
±15
±25
± 14
158
182
142
102
115
±32
±47
± 18
±31
±33
110 ± 17
146 ± 2 9
111 ± 21
81 ± 26
88±28
133
135
117
78
74
±34
±32
±37
±22
±9
126 ± 3 4
154 ± 4 4
125 ± 2 5
79 ± 3 2
95±33
214
181
169
111
±33
±35
±29
±28
185
145
148
97
164
143
138
77
±28
±38
±21
± 18
178
149
142
87
± 42
±25
±32
±26
67 ± 3 1
150 + 14
107 ± 2 8
58 ± 13
54 +
115 ±
33 i
47 +
18
21
22
10
53 + 15
126 ± 18
296
234
335
210
190
254
281
442
352
317
158
±65
± 18
± 107
±39
±55
i : 60
±36
± 107
±75
± 114
±37
246 ± 6 0
218 ± 5 4
323 ± 135
207 ± 3 2
198 ± 6 7
207 ± 5 0
286 ± 6 2
483 ± 152
282 i 36
243 ± 5 6
175 ± 58
277±36
215 ± 3 8
280 ± 8 4
156 ± 5 0
58 ± 2 1
78 ± 3 1
66 + 18
224 ± 2 9
145 ± 4 6
148 ± 3 6
77 ± 3 9
201 ± 26
184 ± 2 8
221 ±30
180 + 31
235 ± 84
127 ± 49
58 + 24
67 ± 2 9
56 + 16
194 ± 3 0
131 ± 5 3
126 ± 2 2
53 ± 2 1
51 ± 4
65 ±24
35 ± 10
56 it 25
61 ±23
62 + 22
18 ± 16
23 ±16
45 ±28
28±9
56 + 10
64 ± 3 1
88 ± 3 1
86 it 38
21 ± 13
20 it 13
47 ±22
34 it 17
65 + 29
62 ± 1 8
16 ± 6
20 it 10
54+12
31 ± 1 3
66
23
45
44
24
78
23
44
35
31
68 it 18
21 ± 12
49 ± 1 0
44 it 11
32 ± 9
Unimodal association areas
Premotor association (6)
Auditory association (22)
Somatosensory association (5)
Visual association (18)
Visual association (19)
•
92±36
34 ± 23
56 ± 2 0
51 ± 1 4
37 ± 9
+ 28
±12
± 10
=t 13
±6
± 17
it 18
it 9
it 8
±7
Primary sensory and motor areas
Primary auditory (41-42)
Primary motor (4)
Primary somatosensory (3,1,2)
Primary visual (17)
±37
±23
±28
±27
87 it 24
59 ±11
55
111
102
67
±23
± 16
+ 29
±16
Limbic and paralimbic areas
Anterior cingulate (24)
Anterior cingulate (32)
Parolfactory area (25)
Posterior cingulate (23)
Granular temporal pole (33)
Granular insula
Entorhinal (28)
CA1 Sector of hippocampus
Subiculum
Dysgranular orbitofrontal (11-12)
Granular orbitofrontal (11-12)
366 ± 6 0
279 ± 31
384 ±104
286 ± 4 0
251 ± 2 9
300 ± 5 3
331 ± 6 6
471 ± 144
367 ± 98
374 ± 7 7
231 ± 5 4
284±48
236 ± 60
357 ±140
219 ±43
199 ±47
222 ±31
320 ±33
465 ± 131
324 ± 101
297 ±72
169 ±27
220 i t 56
133 ± 4 4
51 ± 2 4
66 ±26
62 ± 3 1
230 ± 35
140 ± 4 0
111 ± 27
67 ± 4 2
186 ± 4 5
140 ± 3 1
217 ± 70
121 ±38
53 it 21
52 ± 18
55 ± 2 0
207 ± 19
125 ± 2 8
115 + 24
68 ± 3 2
Each value represents the sum of counts for fibers with vertical or horizontal orientation in layer Illc or V averaged across subjects + standard deviation. Repeated measures AN0VA indicated significant effects
for variables of cortical area (F = 71.69, p < 0.0001), disease state ( f = 67.20, p < 0.0001), cortical layer (F = 114.33, p < 0.0001), and fiber orientation IF = 85.52, p < 0.0001). The probability values referred
to in the text indicate results of Newman-Keuls pairwise comparisons.
' Numbers in parentheses refer to cortical areas according to Brodmann's classification.
neurons in both the normal and AD cortex (Mesulam and
Geula, 1988b; Mesulam and Geula, 1991; Heckers et al., 1992)
and therefore will not dwell on them in this report. The AChE
activity in both fibers and neurons was reliably and completely inhibited by 1O~4 M of the specific AChE inhibitor
BW284C51 but was unaffected by an equal concentration of
the specific BChE inhibitor Iso-OMPA.
Within the brains that were processed for ChAT immunohistochemistry and AChE histochemistry, the two procedures revealed an identical regional and laminar pattern and
density of cortical fibers. In both the normal and AD brains,
areas that showed a high density of ChAT-positive fibers also
displayed a high density of AChE-positive fibers, and areas
with a low density of ChAT-positive fibers also displayed a
low density of AChE-positive fibers (Tig. 2). This matched pattern of ChAT and AChE stained fibers was observed in all
cortical areas examined. For this reason, and because the very
thin and varicose ChAT-positive fibers did not lend themselves
to counting, we carried out our quantitative assessments of
fiber density using AChE stained material.
Cortical Cbottnergic Fibers in the Normal Brain
A prominent plexus of cholinergic fibers was present in all
cortical areas of the normal brains. The density of these fibers,
however, displayed considerable regional variations (Table 2).
Among the areas examined, the hippocampal formation displayed the highest density of fibers (p < O.OO1). Paralimbic
cortical areas such as the cingulate, entorhinal, orbitofrontal
cortex, and the temporal pole and insula displayed the next
highest density of cholinergic fibers (p < 0.025). The primary
auditory, somatosensory, and motor cortex displayed an intermediate density of fibers and tended to contain slightly more
fibers than most association cortical areas, while the primary
visual and visual association areas displayed the lowest density of cholinergic fibers. The later differences, however, were
not statistically significant (p > 0.05).
In all cortical areas, fibers travelled vertically, horizontally,
and tangentially. Overall, a greater number of fibers were oriented vertically as compared with horizontally oriented fibers.
This pattern of fiber orientation was a regularly encountered
characteristic of extragranular (superficial) layers. Our counts
in layer in showed this difference to be significant in 50% of
the areas examined <p < 0.05) and to be a consistent trend
in the rest (Table 3). Within the deep layers, horizontally oriented fibers were encountered almost as frequently as vertical
fibers (p > 0.05). The overall density of fibers was greater in
superficial layers as compared with the deep cortical laminae.
Quantitative comparison of fiber density in layer El versus V
revealed consistently higher fiber counts in layer HI, but this
difference did not reach significance (p > 0.05).
Cerebral Cortex Mar/Apr 1996, V 6 N 2 167
Figure 1. Rber tracing of entorhinal cortex depicting the method used for quantification
of the density of AChE-positive fibers. The fibers were viewed at 200x magnification
through a 10 x 10 grid placed in the ocular of the microscope. One side of the grid
was oriented parallel to the cortical surface (top side in this figure). The number of
fibers intersecting each line horizontal or vertical to the cortical surface were counted
and combined to provide a quantitative index of fiber density.
Loss of Cortical Cbolinergic Fibers in
Alzheimer's Disease
All cortical areas examined in AD brains displayed some degree of cholinergic fiber loss •when compared with matching
areas in normal brains (Table 2). Our quantitative estimate
revealed on average a 55% loss of cortical cholinergic fibers
in AD. Comparison of individual cortical areas, however, revealed marked and systematic variations in the extent of this
loss. These variations were apparent on gross examination of
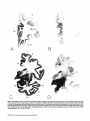
ChAT and AChE stained whole hemispheric sections (Fig. 3).
In general, cortical areas within the ventral aspects of the
hemisphere displayed a greater loss of fibers when compared
with cortical regions within the dorsal portions of the hemisphere. Cholinergic fibers displayed a major depletion in the
temporal neocortical areas, most of which were virtually empty of fibers (Fig. 4). By contrast, the cholinergic fibers in the
premotor cortex, the cingulate gyrus (Fig. 5), the sensorimotor cortex, and in some of the frontal association areas
seemed to be relatively well preserved.
The areas with the greatest loss (> 80% reduction) of cholinergic innervation were all in the temporal lobe and included areas 20, 21, 22, and 28 of Brodmann (Table 2, Fig. 4). The
frontal, parietal, and occipital association areas and paralimbic
areas such as the insula, temporal pole, and orbitofrontal cortex showed an intermediate magnitude of loss (40-75%). The
anterior cingulate gyrus, primary motor, primary somatosensory, and primary visual cortex displayed less than 40% loss
of cholinergic fibers (Table 2, Fig. 5). In the primary visual,
anterior cingulate (Brodmann areas 24, 25, and 32) and the
primary motor cortex of AD brains, the loss of cholinergic
fibers was not statistically significant (p > 0.05). Within the
hippocampal formation, fiber density appeared to be reduced
in all sectors (Fig. 3), although the very high density of fibers
in the CA2-CA4 sectors and the dentate gyrus precluded an
accurate count, especially in the normal brain. We did find a
54% loss of cholinergic fibers within the CA1 sector of Ammon's horn and a 59% loss in the subiculum of AD brains.
No consistent pattern was observed in the loss of cholinergic axons in functionally similar cortical areas. Some trends,
168 Alzheimer's Cholinergic Denervation • Geula and Mesulam
however, were observed. Primary motor and sensory areas,
except the primary auditory cortex, displayed among the lowest percentages of loss in AD (18-39%). Consistent with its
anatomical location within the temporal lobe, the primary auditory cortex displayed a relatively high degree (69%) of cholinergic fiber loss. In general, paralimbic cortical areas displayed a more marked loss of cholinergic fibers than most
neocortical areas. This trend was particularly apparent within
the entorhinal cortex, temporal pole, and insula, which displayed relatively high percentages (73-80%) of loss. The cingulate paralimbic areas, on the other hand, displayed among
the lowest loss of fibers (26-42%). All association cortical areas except those within the temporal lobe displayed an intermediate reduction in the density of cholinergicfibers.The
association cortical areas within the temporal lobe (Brodmann areas 20, 21, and 22) displayed the highest percentage
of cholinergic fiber loss (84-85%) amongst all areas examined.
The superficial cortical laminae tended to display a consistently greater magnitude of cholinergic fiber loss than deep
laminae. In most cortical areas, there was also a greater loss
of vertically oriented fibers as compared with fibers with a
horizontal orientation. These differences, however, were not
statistically significant (p > 0.05), except in a few cortical
areas such as the visual association cortex (area 18,p < 0.05).
Discussion
The histochemical methods used in this experiment revealed
a dense plexus of AChE- and ChAT-positive fibers throughout
the cerebral cortex within the normal brain, and a dramatically reduced density of these fibers in AD. Consistent with
our earlier observations (Mesulam and Geula, 1992), which
were based on tissue stained separately as well as concurrently for ChAT and AChE, we found a completely overlapping pattern and apparent density of cortical AChE- and ChATpositive fibers in the normal brain. We further observed an
overlap between these fibers in the AD cortex. Thus, within
both the normal and AD cerebral cortex, an AChE-positive
pattern of fiber staining is a good marker for cholinergic axons (See Mesulam et al., 1984; Geula and Mesulam, 1989, for
review).
Within the normal brain, we found variations in the regional pattern of cortical cholinergic fiber distribution as well
as in fiber lamination and orientation. The core limbic regions
displayed the highest density of cholinergic fibers, followed
closely by paralimbic areas. The majority of primary sensory
and association areas displayed an intermediate fiber density.
The primary visual cortex and the visual association areas
wkhin the occipital lobe displayed the lowest density of cholinergic fibers. In the majority of areas, fiber density tended
to be higher in the superficial layers within which vertically
oriented fibers predominated. These observations are consistent with our recent investigations based on AChE histochemistry and ChAT immunohistochemistry in the human brain
(Mesulam and Geula, 1988a, 1992; Geula and Mesulam, 1989,
1990). Several studies of biochemically determined ChAT and
AChE activities have shown a similar distribution pattern of
cholinergic markers in the human cerebral cortex (Perry et
al., 1977; Davies, 1979; Rossor et al., 1982a; Reinikainen et al.,
1988;Javoy-Agid et al., 1989).
Cortical Cbolinergic Fibers in Alzheimer's Disease
In the AD cerebral cortex, we found very substantial but regionally variable loss of cortical cholinergic fibers. The severity of this depletion was greatest (80-85%) in the temporal
lobe, including its paralimbic and association components.
The visual association areas within the temporal lobe (areas
20, 21, and 22) were virtually emptied of cholinergic fibers.
^
figure 2. Photomicrographs of ChAHA C, and £) and AChE-positive (B, 0, and fl fibers in the CA1 sector of the hippocampus [A and B), anterior cingulate cortex (area 24, C and
0), and granular insula (F and A from an AD case. Note the virtually identical pattern and density of ChAT- and AChE-positive fibers in each area. The two enzymes revealed the
same pattern of fiber staining in all cortical areas within both normal and AD brains. Magnification: A and B, 176.8x; C-F, 110.5x.
Cerebral Cortex Mar/Apr 1996, V 6 N 2 169
32
IF
11-12
B
A
8
24
22
28
21
c
20
D
Figure 3. Whole hemisphere sections of anterior {A and B), middle (C and 23), and posterior ( f and fl aspects of the brain in normal {A, C, and £) and AD (fi, D, and fl cases stained
for AChE. The numbers refer to cortical regions according to the classification of Brodmann (see tables for functional affiliations of various areas). Note the relative preservation
of cortical AChE staining (mostly of fiber type) within the dorsal and medial aspects of the hemisphere and the marked loss of staining within the ventral cortical areas in the AD
brain. AChE-positive fibers within the anterior cingulate cortex {areas 32 and 24) and the motor cortex {area 4) are well preserved, while the neocortical zones within the temporal
lobe {areas 20, 21, and 22) are virtually empty of fibers. The AChE-positive bands seen within some cortical areas {arrowheads) are collections of AChE-positive cortical pyramidal
neurons. Magnification: A and B, 1.26x; C-f, 0.84x.
170 Alzheimer's Cholinergic Denervatlon • Geula and Mesulam
39-40
Figure 3. Continued.
By contrast, cholinergic fibers within the anterior cingulate
areas (areas 24, 25, and 32), the primary visual, primary somatosensory, and primary motor cortex were relatively well
preserved (18-39% loss). The remaining cortical areas displayed an intermediate degree of loss (42-74%).
Among the functionally similar cortical areas, the greatest
loss of cholinergic fibers was consistently associated with cortical areas located within the ventral aspects of the hemisphere, while cortical areas located within the dorsal portions
of the hemisphere showed relative preservation of fibers. For
example, among the primary sensorimotor areas, the primary
auditory cortex, which is located within the temporal lobe,
displayed a relatively large percentage of loss (69%) while the
other primary areas, which are located dorsally, showed some
of the lowest percentages of fiber loss (18-39%). A similar
pattern of loss was observed amongst the paralimbic and association areas of cortex. Thus, the major determinant of the
severity of cholinergic fiber loss in AD cortex appears to be
anatomical location rather than functional affiliation, with cortical areas situated within the temporal lobe displaying the
most dramatic fiber loss and cortical areas situated within the
dorsal and particularly the dorsomedial aspects of the hemisphere displaying the least pronounced loss.
Our observations on the regional variability of cortical cholinergic innervation in AD are in agreement with previous
histochemical studies (Henke and Lang, 1983; Mesulam and
Geula, 1988a; Geula and Mesulam, 1989). hi an earlier study
of three cortical areas (Geula and Mesulam, 1989), we found
a greater loss of AChE-positive fibers in the cingulate cortex
than that described in the present report. This is most likely
due to slightly different methods of counting and a more severe overall pathology of the cholinergic system in the AD
sample used in the former study. It should be pointed out,
however, that the regional pattern of AD-related fiber loss dis-
played by the three areas investigated in our earlier study is
identical to that reported here. Biochemical studies have also
shown the greatest decrement in cholinergic enzymes in AD
to occur in cortical structures within the temporal lobe (Rossor et al., 1980, 1982a; Wilcock et al., 1982; Reinikainen et al.,
1988) while cingulate cortex (areas 24, 25, and 32) and cortical areas within the occipital lobe, particularly the primary
visual cortex (area 17) have been shown to display the least
depletion of cholinergic enzymes (Davies, 1979; Rossor et al.,
1980, 1982a; Henke and Lang, 1983). Biochemical studies,
however, include many inconsistencies with regards to the
extent of cholinergic loss in various cortical regions (Davies
and Maloney, 1976; Perry et al., 1977; Davies, 1979; Araujo et
al., 1988; Reinikainen et al., 1988). One likely reason for these
inconsistencies is the difficulty in precise dissection of various cortical areas. The failure by some investigators to observe regional variations in the depletion of AChE activity (Davies and Maloney, 1976; Davies, 1979; Reinikainen et al., 1988),
may also result from the inability of biochemical measurements to distinguish the AChE activity within cholinergic fibers from that present within AChE-positive (but ChAT-negative) cortical neurons. Observations based on histochemical
methods are relatively immune from these limitations.
Although the loss of cortical cholinergic markers is found
in all cortical laminae, a greater loss has been demonstrated
within the superficial laminae (II-III) by both histochemical
(Geula and Mesulam, 1989) and biochemical (Henke and
Lang, 1983; Perry et al., 1984; DeKosky et al., 1985) studies.
Our results indicate a similar trend and further demonstrate
a tendency for greater loss of vertically oriented fibers as compared widi fibers with a horizontal orientation.
The regional variations in the loss of cortical cholinergic
fibers in AD could reflect one of two mechanisms. (1) The
effect of AD upon cholinergic innervation could vary from
Cerebral Cortex Mar/Apr 1996, V 6 N 2 171
Figure 4. Examples of cortical areas with the most severe depletion of cholinergic fibers in AD. The entorhinal cortex {A and 6) and the auditory association cortex (C and 0) of
AD (6 and 0) brains showed a marked loss of their AChE-positive fibers when compared to the same areas in the normal brain [A and 0. Magnification, 262x.
172 Alzheimer's Cholinergic Denervation • Geula and Mesulam
Rgure 5. Examples of cortical areas with relatively well-preserved cholinergic fibers in AD. The anterior cingulate cortex {area 24, A and 6) and the primary visual cortex [C and
0) of AD brains (6 and 0) showed only a minor and nonsignrficant loss of their cholinergic fibers.when compared with the same areas of the normal brains (4 and 0. Magnification:
A and B, 262x; C and D. 174.7x.
Cerebral Cortex Mar/Apr 1996, V 6 N 2 173
one cortical area to another, or (2) all areas could loose an
equal number (or percentage) of their cholinergic fibers but
the residual density could vary because of regional differences in premorbid density. Our observations tend to argue
against the latter possibility since many cortical areas, which
in the normal brain contain comparable densities of cholinergic fibers, showed different degrees of fiber loss in AD. For
example, the primary visual cortex and the superior parietal
association cortex normally display low densities of cholinergic fibers in the cerebral cortex. In AD, however, the superior parietal association cortex displayed a much more pronounced loss of cholinergic fibers than primary visual cortex
(68% vs 39%). Furthermore, the anterior cingulate area, which
normally contains quite high densities of cholinergic fibers,
displayed a modest depletion (26-33%) when compared to
the severe depletion (59-80%) seen in other paralimbic areas
(e.g., temporal pole, insula and entorhinal cortex) with a comparable baseline density of cholinergic fibers.
Relationship to Loss of Cholinergic Neurons of the
Basal Forebrain and to Cortical Pathology
A large body of evidence has indicated a consistent loss of
basal forebrain cholinergic neurons in AD brains, ranging in
magnitude from 30% to 95% (Perry et al., 1982; Whitehouse
et al., 1982; Arendt et al., 1983; Wilcock et al., 1983; Mann et
al., 1984; Rinne et al., 1987; Kobayashi et al., 1991). Biochemical investigations have also reported a significant loss of
ChAT activity (30-90%) in the nucleus basalis of Meynert
(nbM, Ch4) of AD patients (Perry et al., 1982; Rossor et al.,
1982b; Bird et al., 1983; Henke and Lang, 1983; Etienne et al.,
1986; Koshimura et al., 1987; Rinne et al., 1987).
The magnitude of cholinergic neuronal loss within the basal forebrain of AD patients seems to display regional variations. For example, studies that have compared the sectors of
Ch4 (nbM) (Arendt et al., 1985; Etienne et al., 1986; Doucette
and Ball, 1987; Mesulam and Geula, 1988a; Wilcock et al.,
1988; Mufson et al., 1989; Vogels et al., 1990; Iraizoz et al.,
1991; Lehericy et al., 1993) have demonstrated that posterior
(Ch4p) and anterolateral (Ch4al) sectors of this cell group
show the greatest and most consistent loss, followed by the
intermediate (Ch4i) and anteromedial (Ch4am) sectors. This
finding is consistent with the greater loss of cholinergic innervation within at least some of the cortical areas that are
innervated predominantly by the Ch4p and Ch4al neurons,
namely, the temporal cortex and the amygdala (Emre et al.,
1993). The few studies that have compared Ch4am to Ch4al
(Arendt et al., 1985; Wilcock et al., 1988; Mufson et al., 1989;
Lehericy et al., 1993), report that the former subsector was
less affected than the latter. This is consistent with the greater
preservation of the cholinergic innervation of the cingulate
gyrus, which is derived predominantly from Ch4am when
compared to that of the amygdala, which is derived predominantly from Ch4al.
It should be noted that correspondence between cholinergic loss within specific Chl-Ch4 sectors and the cortical
areas to which they project is not a universal finding. Although a significant correlation has been reported between
the depletion of cortical ChAT activity and reduction of nucleus basalis neurons (or basal forebrain ChAT activity) by
some investigators (Etienne et al., 1986; Koshimura et al.,
1987), insignificant or inconsistent correlation has been found
by others (Perry et al., 1982; Wilcock et al., 1983,1988; Rinne
et al., 1987). Furthermore, a number of investigations report
that the magnitude of cortical ChAT depletion is much larger
than the magnitude of cholinergic neuronal loss in the basal
forebrain (Perry et al. 1982; Wilcock et al., 1983,1988; Etienne
et al., 1986; Rinne et al., 1987). If substantiated, this could
favor the suggestion that the cholinergic pathology originates
174 Alzheimer's Cholinergic Denervation • Geula and Mesulam
in the axonal projections within cortex rather than in the
Chl-Ch4.
ChAT activity in many cortical areas of AD brains has been
found to show a significant negative correlation with the density of plaques (Perry et al., 1978,1981a; Mountjoy et al., 1984;
Zubenko et al., 1989). The size of this correlation, however, is
quite variable, ranging from —0.34 to —0.82. Moreover, some
studies show no significant relationship between the loss of
cholinergic en2ymes and plaque density (Wilcock et al., 1982;
Brashear et al., 1988; DeKosky et al., 1992). Some investigators
have also found a small but significant negative correlation
(-0.38 to -0.58) between residual cortical ChAT levels and
density of tangles (Wilcock et al., 1982; Mountjoy et al., 1984),
while others have found no such correlation (Zubenko et al.,
1989; Ransmayr et al., 1992). Almost all of these studies have
investigated the correlation of plaques and tangles with the
residual density of cholinergic input rather than its loss. This
is an important distinction because equivalent residual densities could result from greatly different magnitude of loss,
depending on premorbid density.
Relationship between Cholinergic Loss and Cognitive
Deficits in Alzheimer's Disease
The relationship of central cholinergic systems to learning
and memory has been the subject of extensive research. Lesions of the Ch4 cholinergic cells deplete the cortex of its
cholinergic innervation and impair learning and memory in a
number of animal species (Flicker et al., 1983; Aigner et al.,
1987; Irle and Markowitsch, 1987; Miyamoto et al., 1987; Peternel et al., 1988; Ridley et al., 1992). The cholinergic basis
of this deficit was demonstrated through the reversibility of
memory loss by cholinergic agonists (Dokla and Thai, 1988;
Tilson et al., 1988; Ueki and Miyashi, 1989). In humans, cholinergic antagonists such as scopolamine have been shown to
interfere with learning in young volunteers and to cause a
memory deficit reminiscent of that which arises in the course
of normal aging (Drachman and Leavitt, 1974).
In view of these relationships, considerable interest has
been generated by the possibility that the cognitive deficits
in AD are caused by the cholinergic denervation of the cerebral cortex. A significant negative correlation has been reported between cortical ChAT activity and the degree of dementia, as determined by neuropsychological tests (Perry et
al., 1978, 1981a; Wilcock et al., 1982; Ruberg et al., 1990;
DeKosky et al., 1992), whereas cortical levels of other neurochemicals, such as NE do not appear to show a significant
relationship with the degree of dementia (Palmer et al.,
1987b). The extent of Ch4 neuronal loss has also been shown
to be correlated with the degree of dementia (Lehericy et al.,
1993).
The possible involvement of the cholinergic system in the
processes of learning and memory, the relationship between
cholinergic loss and cognitive deficits in AD, and the observation that the loss of cortical cholinergic innervation in this
disorder is more severe and occurs earlier than the loss of
other cortically projecting neurotransmitter systems (Mann et
al., 1980; Perry et al., 1981a,b; Bowen et al., 1983; Cross et al.,
1984; D'Amato et al., 1987; Palmer et al., 1987a; Zweig et al.,
1988; Aletrino et al., 1992) have provided the basis for cholinergic replacement therapy in AD. Although many trials of
AChE inhibitors, cholinergic precursor substances, and receptor agonists have been undertaken (Etienne et al., 1981; Mohs
et al., 1985;Growdon et al., 1986;Mouradian et al., 1988; Weinstein et al., 1991), only a few based on AChE inhibition have
shown minor and mostly transient improvements (Gustafson
et al., 1987; Stern et al., 1987; Francis and Bowen, 1989).
The pattern of residual cholinergic innervation in AD (Table 2) suggests that cholinergic therapies based on the inhi-
bition of AChE are likely to have regionally variable consequences. For example, the inhibition of AChE is unlikely to
have much effect in temporal neocortical areas that are virtually empty of cholinergic fibers, specially late in the course
of the disease. Even late in the disease, however, entorhinal
cortex, hippocampus, and amygdala still have a substantial
residual density of cholinergic fibers whose activity could be
enhanced by the administration of AChE inhibitors or ACh
precursors.
Notes
We thank Leah Christie, Kristin Bouve, Daniel Saroff, and Tamar Hashimi for expert secretarial and technical assistance. We are grateful to
Dr. Louis B. Hersh (Department of Biochemistry, University of Kentucky Medical School, Lexington, KY) for the generous gift of ChAT
antibody, and to Drs. Deborah Mash (University of Miami Medical
School Brain Endowment Bank), Bruce Price (Department of Neurology, Beth Israel Hospital, Boston, MA), and Anna Sotrel (Department of Pathology, Beth Israel Hospital, Boston, MA) for providing
brain tissue. This work was supported by grants from the National
Institute on Aging (AG10282 and AG08812), Massachusetts Alzheimer's Disease Research Center (AG05134), and a Javitz Neuroscience
Investigator Award (NS20285).
Address correspondence to Changiz Geula, Ph.D., Laboratory for
Neurodegenerative and Aging Research, New England Deaconess
Hospital, 99 Brookline Avenue, Boston, MA 02215.
References
Aigner TG, Mitchell SJ, Aggleton JP, DeLong MR, Struble RG, Price DL,
Wenk GL, Mishkin M (1987) Effects of scopolamine and physostigmine on recognition memory in monkeys with ibotenic-acid
lesions of the nucleus basalis of Meynert. Psychopharmacology
(Berl) 92:292-300.
Aletrino MA, Vogels OJM, Van Domberg PH, Ten Donkelaar HJ (1992)
Cell loss in the nucleus raphe dorsalis in Alzheimer's disease. Neurobiol Aging 461-468.
Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R (1988)
Differential alteration of various cholinergic markers in cortical
and subcortical regions of human brain in Alzheimer's disease. J
Neurochem 50:1914-1923.
Arendt T, Bigl V, Arendt A, Tennstedt A (1983) Loss of neurons in
the nucleus basalis of Meynert in Alzheimer's disease, paralysis
agitans and korsakoffs disease. Acta Neuropathol (Berl) 61:101108.
Arendt T, Bigl V, Tennstedt A, Arendt A (1985) Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque
formation in cortical target areas in Alzheimer's disease. Neuroscience 14:1-14.
Bird TD, Stranaham S, Sumi SM, Raskind M (1983) Alzheimer's disease: choline acetyltransferase activity in brain tissue from clinical
and pathological subgroups. Ann Neurol 14:284-293.
Bowen DM, Smith CB, White P, Davison AN (1976) Neurotransmitterrelated enzymes and indices of hypoxia in senile dementia and
other abiotrophies. Brain 99:459-496.
Bowen DM, Spillane JA, Curzon G, Meier-Ruge W, White P, Goodhardt
MJ, Iwangoff P, Davison AN (1979) Accelerated ageing or selective neuronal loss as important cause of dementia? Lancet 1:1114.
Bowen DM, Allen SJ, Benton JS, Goodhardt MJ, Haan EA, Palmer AM,
Sims NR, Smith CT, Spillane JA, Esiri MM, Neary D, Snowdon JS,
Wilcock GK, Davison AN (1983) Biochemical assessment of serotonergic and cholinergic dysfunction and cerebral atrophy in
Alzheimer's disease.J Neurochem 41:266-272.
BrashearHR,GodecMS,CarlsenJ (1988) The distribution of neuritic
plaques and acetylcholinesterase staining in the amygdala in Alzheimer's disease. Neurology 38:1694-1699.
Cross AJ, Crow TJ, Ferrier IN, Johnson JA Bloom SR, Corsellis JAN
(1984) Serotonin receptor changes in dementia of the Alzheimer
t y p e j Neurochem 43:1574-1581.
D'Amato RJ, Zweig RM, Whitehouse PJ, Wenk GL, Singer HS, Mayeux
R, Price DL, Snyder SH (1987) Aminergic systems in Alzheimer's
disease and Parkinson's disease. Ann Neurol 22:229-236.
Davies P (1979) Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res 171:319-327.
Davies P, Maloney AJF (1976) Selective loss of central cholinergic
neurons in Alzheimer's disease. Lancet 2:1403.
DeKosky ST, Scheff SW, Markesbery WR (1985) Laminar organization
of cholinergic circuits in human frontal cortex in Alzheimer's disease and aging. Neurology 35:1425-1431.
DeKosky ST, Harbaugh RE, Schmitt FA, Bakay RAE, Chui HC, Knopman
DS, Reeder TM, Shetter AG, Senter HJ, Markesbery WR (1992)
Cortical biopsy in Alzheimer's disease: diagnostic accuracy and
neurochemical, neuropathological, and cognitive correlates. Ann
Neurol 32:625-632.
Dokla CJ, Thai LJ (1988) Effect of cholinesterase inhibitors on Morris
water task behavior following lesions of the nucleus basalis magnocellularis. Behav Neurosci 102:861-871.
Doucette R, Ball MJ (1987) Left-right symmetry of neuronal cell
counts in the nucleus basalis of Meynert of control and of Alzheimer-diseased brains. Brain Res 422:357-360.
Drachman DA, Leavitt J (1974) Human memory and the cholinergic
system: a relationship to aging? Arch Neurol 30:113-121.
Emre M, Heckers S, Mash DC, Geula C, Mesulam MM (1993) Cholinergic innervation of the amygdaloid complex in the human brain
and its alterations in old age and Alzheimer's disease. J Comp
Neurol 336:117-134.
Etienne P, Dastoor D, Gauthier S, Ludwick R, Collier B (1981) Alzheimer's disease: lack of effect of lecithin treatment for 3 months.
Neurology 31:1552-1554.
Etienne P, Robitaille Y, Wood P, Gauthier S, Nair NPV, Quirion R (1986)
Nucleus basalis neuronal loss, neuritic plaques, and choline acetyltransferase activity in advanced Alzheimer's disease. Neuroscience 19:1279-1291.
Flicker C, Dean RL, Watkins DL, Fisher SK, Bartus RT (1983) Behavioral and neurochemical effects following neurotoxic lesions of a
major cholinergic input to the cerebral cortex in the rat. Pharmacol Biochem Behav 18:973-981.
Francis PT, Bowen DM (1989) Tacrine, a drug with therapeutic potential for dementia: post-mortem biochemical evidence. Can J
Neurol Sci 16:504-510.
German DC, Bruce G, Hersh LB (1985) Immunohistochemical staining of cholinergic neurons in the human brain using a potyclonal
antibody to human choline acetyltransferase. Neurosci Lett 61:
1-5.
Geula C, Mesulam M-M (1989) Cortical cholinergic fibers in aging
and Alzheimer's disease: a morphometric study. Neuroscience 33:
469-481.
Geula C, Mesulam M-M (1990) Human brain cortical cholinergic innervation. Soc Neurosci Abstr 16:1057.
Growdon JH.CorkinS, Huff FJ, Rosen TJ (1986) Piracetam combined
with lecithin in the treatment of Alzheimer's disease. Neurobiol
Aging 7:269-276.
Gustafson L, Edvinsson L, Dahlgren N, Hagberg B, Risberg J, Rosen I,
Ferno H (1987) Intravenous physostigmine treatment of Alzheimer's disease evaluated by psychometric testing, regional cerebral
blood flow (rCBF) measurement, and EEG. Psychopharmacology
(Berl) 93:31-35.
Hanker, JS, Thornburg IP, Yates PE, Moore HG (1973) The demonstration of cholinesterases by the formation of osmium blacks at
the sites of Hatchett's brown. Histochemie 37:223-242.
Heckers S, Geula C, Mesulam M-M (1992) Acetylcholinesterase-rich
pyramidal neurons in Alzheimer's disease. Neurobiol Aging 13:
455-460.
Henke H, Lang W (1983) Cholinergic enzymes in neocortex, hippocampus and basal forebrain of non-neurological and senile dementia of Alzheimer-type patients. Brain Res 267:281-291.
Iraizoz I, de Lacalle S, Gonzalo LM (1991) Cell loss and nuclear hypertrophy in topographical subdivisions of the nucleus basalis of
Meynert in Alzheimer's disease. Neuroscience 41:33-40.
Irle E, Markowitsch HJ (1987) Basal forebrain-lesioned monkeys are
severely impaired in tasks of association and recognition memory.
Ann Neurol 22:735-743.
Javoy-Agid F, Scatton B, Ruberg M, L'Heureux R, Cervera P, Raisman R,
Maloteaux J-M, Beck H, Agid Y (1989) Distribution of monoaminergic, cholinergic, and GABAergic markers in the human cerebral cortex. Neuroscience 29:251-259.
Khachaturian Z (1985) Diagnosis of Alzheimer's disease. Arch Neurol 42:1097-1105.
Kitt CA Levey AI, Friedman DP, Walker LC, Koliatsos VE, Raskin LS,
Cerebral Cortex Mar/Apr 1996, V 6 N 2 175
Price DL G988) Immunocytochemical visualization of cholinergic fibers in monkey neocortex: enhanced visualization using silver nitrate. Soc Neurosci Abstr 14:631Kobayashi K, Miyazu K, Fukutani Y, Nakamura I, Yamaguchi N (1991)
Morphometric study on the Ch4 of the nucleus basalis of Meynert
in Alzheimer's disease. Mol Chem Neuropathol 15:193-205.
Kbshimura K, Kato T, Yohyama I, Nakamura S, Kameyama M (1987)
Correlation of Choline acetyltransferase activity between the nucleus basalis of Meynert and the cerebral cortex. Neurosci Res 4:
330-336.
Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh IB, Bakchine S, Piette
F, Duyckaerts C, Hauw J-J, Javoy-Agid F, Agid Y (1993) Heterogeneity and selectivity of the degeneration of cholinergic neurons
in the basal forebrain of patients with Alzheimer's disease. J Comp
Neurol 330:15-31.
Mann DMA, Lincoln J, Yates PO, Stamp JE, Toper S (1980) Changes
in the monoamine containing neurons of the human CNS in senile
dementia. BrJ Psychiatry 136:533-341.
Mann DMA, Yates PO, Marcyniuk B (1984) Changes in nerve cells of
the nucleus basalis of Meynert in Alzheimer's disease and their
relationship to ageing and to accumulation of lipofuscin pigment.
Mech Ageing Dev 25:189-204.
McGeer EG, McGeer PL, Kamo H, Tago H, Harrop R (1986) Cortical
metabolism, acetylcholinesterase staining and pathological
changes in Alzheimer's disease. Can J Neurol Sci 13:511-516.
Mesulam M-M, Geula C (1988a) Nucleus basalis (Ch4) and cortical
cholinergic innervation in the human brain: observations based
on the distribution of acetylcholinesterase and choline acetyltransferase.J Comp Neurol 275:216-240.
Mesulam M-M, Geula C (1988b) Acetylcholinesterase-rich pyramidal
neurons in the human neocortex and hippocampus: absence at
birth, development during the life span, and dissolution in Alzheimer's disease. Ann Neurol 24:765-773Mesulam M-M, Geula C (1991) Acetylcholinesterase-rich neurons of
the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol 306:193-220.
Mesulam M-M, Geula C (1992) Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons
in human cerebral cortex. Brain Res 577:112-120.
Mesulam M-M, Geula C (1994) Chemoarchitectonics of axonal and
perikaryal acetylcholinesterase along information processing systems of the human cerebral cortex. Brain Res Bull 33:137-153.
Mesulam M-M, Mufson EJ, Levey AI, Wainer BH (1983) Cholinergic
innervation of cortex by the basal forebrain: cytochemistry and
cortical connections in the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the
rhesus monkey.J Comp Neurol 214:170-197.
Mesulam M-M, Rosen AD, Mufson EJ (1984) Regional variations in
cortical cholinergic innervation: chemoarchitectonics of acetylcholinesterase-containing fibers in the macaque brain. Brain Res
311:245-258.
Mesulam M-M, Mufson EJ, Wainer BH (1986) Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline
acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Res
367:301-308.
Mesulam M-M, Hersh LB, Mash DC, Geula C (1992) Differential cholinergic innervation within functional subdivisions of the human
cerebral cortex: a choline acetyltransferase study. J Comp Neurol
318:316-328.
Miyamoto M, Kato J, Narumi S, Nagaoka A (1987) Characteristics of
memory impairment following lesioning of the basal forebrain
and medial septal nucleus in rats. Brain Res 419:19-31.
Mohs RC, Davis BM, Johns CA, Mathe AA, Greenwald BS, Horvath TB,
Davis KL (1985) Oral physostigmine treatment of patients with
Alzheimer's disease. Am J Psychiatry 142:28-33.
Mountjoy CQ, Rossor MN, Iverson LL, Roth M (1984) Correlation of
cortical cholinergic and GABA deficits with quantitative neuropathological findings in senile dementia. Brain 107:507-518.
Mouradian MM, Mohr E, Williams JA, Chase TN (1988) No response
to high-dose muscarinic agonist therapy in Alzheimer's disease.
Neurology 38:606-608.
Mufson EJ, Bothwell M, Kordower JH (1989) Loss of nerve growth
factor receptor-containing neurons in Alzheimer's disease: a quan-
176 Alzheimer's Cholinergic Denervation • Geula and Mesulam
titative analysis across subregions of the basal forebrain. Exp Neurol 105:221-232.
Palmer AM, Francis PT, Benton JS, Sims NR, Mann DMA, Neary D,
Snowden JS, Bowen DM (1987a) Presynaptic serotonergic dysfunction in patients with Alzheimer's disease. J Neurochem 48:815Palmer AM, Francis PT, Bowen DM, Benton JS, Neary D, Mann DMA,
Snowden JS (1987b) Catecholaminergic neurons assessed antemortem in Alzheimer's disease. Brain Res 414:365-375.
Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE (1977) Neurotransmitter enzyme abnormalities in senile dementia. J Neurol
Sci 34:247-265.
Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry
RH (1978) Correlation of cholinergic abnormalities with senile
plaques and mental test scores in senile dementia. Br Med J 2:
1457-1459Perry EK, Blessed G, Tomlinson BE, Perry RH, Crow TJ, Cross AJ, Dockray GJ, Dimaline R, Arragui A (1981a) Neurochemical activities
in human temporal lobe related to aging and Alzheimer-type
changes. Neurobiol Aging 2:251-256.
Perry EK, Tomlinson BE, Blessed G, Perry RH, Cross AJ, Crow TJ
(1981b) Neuropathological and biochemical observations on the
noradrenergic system in Alzheimer's disease. J Neurol Sci 51:279287.
Perry RH, Candy JM, Perry EK, Irving D, Blessed G, Fairbairn AF, Tomlinson BE (1982) Extensive loss of choline acetyltransferase activity is not reflected by neuronal loss in the nucleus of Meynert
in Alzheimer's disease. Neurosci Lett 33:311-315.
Perry EK, Atack JR, Perry RH, Hardy JA, Dodd PR, Edwardson JA, Blessed G, Tomlinson BE, Fairbairn AF (1984) Intralaminar neurochemical distributions in human midtemporal cortex: comparison between Alzheimer's disease and the normal.J Neurochem 42:14021410.
Peternel A, Hughey D, Wenk G, Olton D (1988) Basal forebrain and
memory: neurotoxic lesions impair serial reversals of a spatial discrimination. Psychobiology 16:54-58.
Ransmayr G, Cervera P, Hirsch E, Ruberg M, Hersh LB, Duyckaerts
C, Hauw J-J, DeUumeau C, Agid Y (1989) Choline acetyltransferase-like immunoreactivity in the hippocampal formation of control subjects and patients with Alzheimer's disease. Neuroscience
32:701-714.
Ransmayr G, Cervera P, Hirsch EC, Berger W, Fischer W, Agid Y (1992)
Alzheimer's disease: is the decrease of the cholinergic innervation
of the hippocampus related to intrinsic hippocampal pathology?
Neuroscience 47:843-851.
Reinikainen KJ, Riekkinen PJ, Paljarvi L, Soininen H, Helkala EL, Jolkkonen J, Laakso M (1988) Cholinergic deficit in Alzheimer's disease: a study based on CSF and autopsy data. Neurochem Res 13:
135-146.
Ridley RM, Gribble S, Clark B, Baker HF, Fine A (1992) Restoration
of learning ability in fornix-transected monkeys after fetal basal
forebrain but not fetal hippocampal tissue transplantation. Neuroscience 48:779-792.
Rinne JO, Paljarvi L, Rinne UK (1987) Neuronal size and density in
the nucleus basalis of Meynert in Alzheimer's disease. J Neurol
Sci 79:67-76.
Rossor M, Fahrenkrug J, Emson P, Mountjoy C, Iversen L, Roth M
(1980) Reduced cortical choline acetyltransferase activity in senile dementia of Alzheimer type is not accompanied by changes
in vasoactive intestinal polypeptide. Brain Res 201:249-253Rossor MN, Garrett NJ, Johnson AL, Mountjoy CQ, Roth M, Iversen LL
(1982a) A post-mortem study of the cholinergic and GABA systems in senile dementia. Brain 105:313-330.
Rossor MN, Svendsen C, Hunt SP, Mountjoy CQ, Roth M, Iverson LL
(1982b) The substantia innominata in Alzheimer's disease: an histochemical and biochemical study of cholinergic marker enzymes.
Neurosci Lett 28:217-222.
Ruberg M, Mayo W, Brice A, Duyckaerts C, Hauw J-J, Simon H, LeMoal
M, Agid Y (1990) Choline acetyltransferase activity and
[3H]vesamicol binding in the temporal cortex of patients with
Alzheimer's disease and rats with basal forebrain lesions. Neuroscience 35:327-333Stern Y, Sano M, Mayeux R (1987) Effects of oral physostigmine in
Alzheimer's disease. Ann Neurol 22:306-310.
Tago H, Kimura H, Maeda T (1986) Visualization of detailed acetyl-
cholinesterase fiber and neuron staining in rat brain by a sensitive
histochemical procedure. J Histochem Cytochem 34:1431-1438.
Tilson HA, McLamb RL, Shaw S, Rogers BC, Peiaditakis P, Cook L
(1988) Radial-arm maze deficits produced by colchicine administered into the area of the nucleus basalis are ameliorated by
cholinergic agents. Brain Res 438:83-94.
Ueki A, Miyoshi K (1989) Effects of cholinergic drugs on learning
impairment in ventral globus pallidus-lesioned rats. J Neurol Sci
90:1-21.
Vogels OJM, Broere CAJ, Ter Laak HJ, Ten Donkelaar HJ, Nieuwenhuys
R, Schulte BPM (1990) Cell loss and shrinkage in the nucleus
basalis Meynert complex in Alzheimer's disease. Neurobiol Aging
11:3-13.
Weinstein HC, Teunisse S, van Gool WA (1991) Tetrahydroaminoacridine arid lecithin in the treatment of Alzheimer's disease. Effect
on cognition, functioning in daily life, behavioral disturbances and
burden experienced by the carers.J Neurol 238:34-38.
Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, DeLong MR
(1982) Alzheimer's disease and senile dementia: loss of neurons
in the basal forebrain. Science 215:1297-1239.
WUcock GK, Esiri MM, Bowen DM, Smith CCT (1982) Alzheimer's
disease. Correlation of cortical choline acetyltransferase activity
with the severity of dementia and histological abnormalities. J
Neurol Sci 57:407-417.
WUcock GK, Esiri MM, Bowen DM, Smith CCT (1983) The nucleus
basalis in Alzheimer's disease: cell counts and cortical biochemistry. Neuropathol. Appl Neurobiol 9:175-179.
Wilcock GK, Esiri MM, Bowen DM, Hughes AO (1988) The differential involvement of subcortical nuclei in senile dementia of Alzheimer's type.J Neurol Neurosurg Psychiatry 51:842-849.
Wood PL, Etienne R Lai S, Nair NPV, Finlayson MH, Gauthier S, Palo J,
Haltia M, Paetau A, Bird ED (1983) A post-mortem comparison
of the cortical cholinergic system in Alzheimer's disease and
Pick's disease. J Neurol Sci 62:211-217.
Zubenko GS, Moossy J, Martinez AJ, Rao GR, Kopp U, Hanin I (1989)
A brain regional analysis of morphometric and cholinergic abnormalities in Alzheimer's disease. Arch Neurol 46:634-638.
Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ,
Folstein ME, Price DL (1988) The neuropathology of aminergic
nuclei in Alzheimer's disease. Ann Neurol 24:233-242.
Cerebral Cortex Mar/Apr 1996, V 6 N 2 177