* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Inferring a dual-stream model of mentalizing from associative white
Affective neuroscience wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Blood–brain barrier wikipedia , lookup
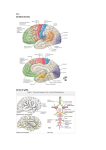
Temporoparietal junction wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Haemodynamic response wikipedia , lookup
Cortical cooling wikipedia , lookup
Nervous system network models wikipedia , lookup
Biology of depression wikipedia , lookup
Selfish brain theory wikipedia , lookup
Time perception wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroscience and intelligence wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Lateralization of brain function wikipedia , lookup
Human multitasking wikipedia , lookup
Persistent vegetative state wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neurolinguistics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neurotechnology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Dual consciousness wikipedia , lookup
Human brain wikipedia , lookup
Neurogenomics wikipedia , lookup
Brain Rules wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Metastability in the brain wikipedia , lookup
Brain morphometry wikipedia , lookup
Emotional lateralization wikipedia , lookup
Neurophilosophy wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
History of neuroimaging wikipedia , lookup
Aging brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
doi:10.1093/brain/awt370 Brain 2014: 137; 944–959 | 944 BRAIN A JOURNAL OF NEUROLOGY Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection Guillaume Herbet,1,2,3 Gilles Lafargue,4,5 François Bonnetblanc,6,7,8 Sylvie Moritz-Gasser,1,9 Nicolas Menjot de Champfleur10 and Hugues Duffau1,2 1 2 3 4 5 6 7 8 9 10 Department of Neurosurgery, Gui de Chauliac hospital, F-34295 Montpellier, France Institute for Neuroscience of Montpellier, INSERM 1051, Hôpital Saint Eloi, F-34091 Montpellier, France University of Montpellier 1, F-34967 Montpellier, France Functional Neuroscience and Pathologies Laboratory, EA-4559, Lille Nord de France University, F-59120 Loos, France Department of Psychology, Lille Nord de France University (Lille3), F-59653 Villeneuve d’Ascq, France Cognition, Action and Sensorimotor Plasticity Lab, INSERM U-1093, UFR STAPS, F-27877 Dijon, France University of Montpellier 2, LIRMM, DEMAR team, CNRS, INRIA, F-34095 Montpellier, France University Institute of France, F-75005 Paris, France Department of Neurology, Gui de Chauliac hospital, F-34295 Montpellier, France Department of Neuroradiology, Gui de Chauliac hospital, F-34295 Montpellier, France Correspondence to: Prof Hugues Duffau Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Centre, 80 avenue Augustin Fliche, 34295 Montpellier, France E-mail: [email protected] In the field of cognitive neuroscience, it is increasingly accepted that mentalizing is subserved by a complex frontotemporoparietal cortical network. Some researchers consider that this network can be divided into two distinct but interacting subsystems (the mirror system and the mentalizing system per se), which respectively process low-level, perceptive-based aspects and highlevel, inference-based aspects of this sociocognitive function. However, evidence for this type of functional dissociation in a given neuropsychological population is currently lacking and the structural connectivities of the two mentalizing subnetworks have not been established. Here, we studied mentalizing in a large sample of patients (n = 93; 46 females; age range: 18–65 years) who had been resected for diffuse low-grade glioma—a rare tumour that migrates preferentially along associative white matter pathways. This neurological disorder constitutes an ideal pathophysiological model in which to study the functional anatomy of associative pathways. We mapped the location of each patient’s resection cavity and residual lesion infiltration onto the Montreal Neurological Institute template brain and then performed multilevel lesion analyses (including conventional voxelbased lesion-symptom mapping and subtraction lesion analyses). Importantly, we estimated each associative pathway’s degree of disconnection (i.e. the degree of lesion infiltration) and built specific hypotheses concerning the connective anatomy of the mentalizing subnetworks. As expected, we found that impairments in mentalizing were mainly related to the disruption of right frontoparietal connectivity. More specifically, low-level and high-level mentalizing accuracy were correlated with the degree of disconnection in the arcuate fasciculus and the cingulum, respectively. To the best of our knowledge, our findings constitute the first experimental data on the structural connectivity of the mentalizing network and suggest the existence of a dual-stream hodological system. Our results may lead to a better understanding of disorders that affect social cognition, especially in neuropathological conditions characterized by atypical/aberrant structural connectivity, such as autism spectrum disorders. Received August 3, 2013. Revised November 2, 2013. Accepted November 11, 2013 ß The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: [email protected] A dual-stream model of mentalizing Brain 2014: 137; 944–959 | 945 Keywords: mentalizing system; mirror system; structural connectivity; arcuate fasciculus; cingulum Abbreviations: RME = Reading the Mind in the Eyes; VLSM = voxel-based lesion-symptom mapping Introduction It is now widely acknowledged that mentalizing (a key function in understanding and successfully performing complex social interactions) is subserved by a brain-wide neural network (Amodio and Frith, 2006; Carrington and Bailey, 2009; van Overwalle, 2009; Mar, 2011). Although the involvement of some brain areas is still subject to debate, it is generally accepted that this highly distributed neurocognitive network is formed by the temporoparietal junction, the precuneus and the medial and inferior-lateral areas of the prefrontal cortex. Group-based analyses in neuropsychological populations have repeatedly provided additional evidence for this type of frontotemporoparietal system, with some degree of right-hemisphere dominance (Winner et al., 1998; Happé et al., 2001; ShamayTsoory et al., 2005). Mentalizing is not necessarily an ‘all-in-one‘ cerebral process, but may emerge from the coherent orchestrated activity of at least two distinct neural subnetworks (Coricelli, 2005; Frith and Frith, 2006; Keysers and Gazzola, 2007; Uddin et al., 2007; Lieberman, 2007; Bohl and van den Bos, 2012; Spunt and Lieberman, 2012). The frontoparietal mirror system may subserve the low-level embodied processes (i.e. sensorimotor-based intersubjective resonance) that are involved (for example) in emotional empathy and the decoding of proximal (motor) intentions (Rizzolati and Craighero, 2004; Shamay-Tsoory et al., 2009; Rizzolatti and Sinigaglia, 2010). The mirror system may thus provide a neural route for the rapid, intuitive, pre-reflective understanding of another person’s internal affective or intentional states. Conversely, the mentalizing system per se may be concerned with sustaining the processes that underlie higher-level reflective inferences, such as the attribution of complex distal intentions or motives. Although a recent qualitative meta-analysis of functional MRI data suggested that these two mentalizing subsystems are relatively independent from a functional standpoint (van Overwalle and Baetens, 2009), data from a number of activation or effective connectivity functional MRI studies (Zaki et al., 2009; Lombardo et al., 2010a; Schippers et al., 2010; Spunt and Lieberman, 2012) do not support this view. The two subsystems may cooperate during the ascription of psychological states. Voxel-based lesion-symptom mapping (VLSM) (Bates et al., 2003) is an increasingly popular method for studying correlations between behavioural data and anatomic (lesion) data and thus drawing conclusions on the underlying neurocognitive organization. Although this inferential statistical method is free of the inherent weaknesses of group-based analyses of patients with defined lesions (because there is no need for a priori knowledge of a given brain process’s anatomical substrate), VLSM itself has several limitations. When white matter association pathways are damaged (and almost all VLSM studies show that the lesion peaks are located on these fascicles’ trajectories), behavioural impairments could also be due to disconnection mechanisms; this makes it difficult to draw reliable neuropsychological inferences in terms of brain location (Rorden and Karnath, 2004; Catani and Ffytche, 2005; Rudrauf et al., 2008a; van Overwalle, 2009; Duffau, 2011; Catani et al., 2012). Moreover, it is increasingly thought that high-level cognitive functions are subserved by complex, distributed neural networks (Mesulam, 1998; Bressler and Menon, 2010), and that cognitive disorders may result from the general dysfunction of a neurocognitive network induced by a loss of structural connectivity (He et al., 2007a). In the present study, we collected mentalizing data from a large sample of patients (n = 93) having undergone surgical resection of diffuse low-grade glioma, a rare lesion of the CNS that migrates preferentially along the associative white matter fibres. All patients had been operated on under local anaesthesia with a cortical and subcortical mapping through direct electrical stimulation. Electrical stimulation causes a transient, ‘virtual lesion’ and thus enables identification of the structures that are functionally essential at each stage of the resection (Duffau et al., 2002; Duffau, 2005). In fact, most of the associative white matter connectivity required for basic cognitive processes is never surgically removed, despite lesion invasion (Ius et al., 2011). Consequently, analysis of patients with diffuse low-grade glioma constitutes a unique opportunity to gain a better understanding of the functional counterpart of surgical excisions (at the cortical level) and the functional impact of lesion invasion on white matter bundles (at the subcortical level). In this latter case, the progressive disconnection of white matter pathway connectivity is likely to induce functional disruption of the underlying neurocognitive networks. Although our study addressed basic hypotheses on the involvement of specific cortical areas in each mentalizing subsystem (e.g. the involvement of the posterior inferior frontal gyrus and the dorsomedial prefrontal cortex in low-level and high-level mentalizing processes, respectively), we focused on predicting the structural connectivity of these networks. The mirror system a minima is composed of the pars opercularis of the inferior frontal gyrus, the ventral part of the premotor cortex and the inferior parietal lobule (Rizzolatti and Craighero, 2004). This cortical frontoparietal network is probably interconnected through the arcuate fasciculus and the lateral superior longitudinal fasciculus (Iacoboni and Dapretto, 2006), as both of the latter have cortical terminations in the anterior part of the mirror system. The arcuate fasciculus is known to connect the pars opercularis to posterior temporal and parietal regions, whereas the lateral superior longitudinal fasciculus connects the ventral premotor cortex and the pars opercularis to the supramarginal gyrus (Catani and Ffytche, 2005; Makris et al., 2005; Martino et al., 2013). Thus, if low-level aspects of mentalizing are indeed processed by the mirror network, we should be able to find correlations between decreases in mentalizing accuracy on one hand and the degree of damage to these fasciculi on the other (i.e. the volume of lesion infiltration). Conversely, the mentalizing system per se broadly overlaps with the brain’s default mode network (Schilbach et al., 2008, 2012; Spreng et al., 2009) that is reportedly involved in self-referential 946 | Brain 2014: 137; 944–959 processes, metacognition and reflexive awareness (Raichle et al., 2001; Greicius et al., 2003; Cavanna and Trimble, 2006). The cingulum is likely to be the structural ‘skeleton’ of the default network (van den Heuvel et al., 2008; Greicius et al., 2009) and interconnects the midline structures of the brain. In particular, the cingulum connects the medial prefrontal cortex with the medial posterior parietal cortex (including the precuneus and posterior cingulate cortices). Hence, one can legitimately hypothesize that this associative white matter fasciculus may play an important role in inference-based mentalizing. If so, a decrease in mentalizing accuracy should be correlated with the degree of damage to this tract. As detailed below, our results confirmed this hypothesis and provide a parsimonious anatomical basis for an integrated, interactive, dual-stream model of mentalizing. Materials and methods Participants A total of 93 native French speakers having undergone surgical resection for a diffuse low-grade glioma (as confirmed by postoperative neuropathological analyses) were recruited from Montpellier University Hospital’s Department of Neurosurgery over 42 years (see Supplementary Table 1 for a comprehensive overview of the sociodemographic and clinical data). All patients were operated on by the same experienced neurosurgeon (H.D.) under local anaesthesia (i.e. ‘awake’ surgery) with a cortical and subcortical brain mapping achieved by direct electrical stimulation. This validated surgical technique (Ojemann and Mateer, 1979; Duffau et al., 2002) spares the cortical and subcortical structures that remain eloquent for certain basic cerebral processes (e.g. visuospatial and language processes). It should be noted, however, that none of the patients were mapped for mentalizing processes during neurosurgery. The exclusion criteria were as follows: previous radiotherapy (which can impair cognition); any neurological impairment (i.e. hemianopia or contralateral superior motor disorders) or cognitive impairment (i.e. spatial neglect) that would prevent objective behavioural testing; a history of neurologic or psychiatric disorders; low or abnormal premorbid IQ (590, as assessed with the French version of the National Adult Reading Test) (Mackinnon and Mulligan, 2005). Ten patients were excluded by the latter criteria. The study population consisted of 45 females and 48 males, with a mean SD age of 38.35 10.31 years (range: 18–65 years), a mean educational level (years in full-time education) of 14 3.22 (range: 9–22 years) and a mean premorbid IQ of 107.52 6.91 (range: 90–123). The behavioural assessment was always performed by the same trained clinician neuropsychologist (G.H.) during the chronic phase (i.e. at least 3 months after surgery). A group of 60 healthy subjects was also enrolled in this study, to provide control data for low-level (n = 42) and high-level (n = 18) mentalizing tasks. All participants gave their written, informed consent to participation in the study. Additionally, patients agreed to the retrospective extraction of clinical and neuropsychological data from their medical records. Behavioural tasks We used two different behavioural tasks [the ‘Reading the Mind in the Eyes‘ (RME) task and the ‘comic strip‘ task] to assess primarily low- G. Herbet et al. level, perceptive-based aspects and high-level, inference-based aspects of mentalizing, respectively. Ninety and 85 patients were evaluated in the RME and comic strip tasks, respectively. These tasks have been extensively characterized and often used in lesion and imaging studies. The RME perception-based identification task (Baron-Cohen et al., 2001) consists of the presentation of 36 photographs of the eye region of human faces. Participants are asked to state which of four mental states best describes what the character ‘is feeling or thinking’ (BaronCohen et al., 1999). In our study, each photograph was presented separately on a PowerPointÕ slide. The four possible answers were shown at the bottom of the slide. Inference-based mentalizing was probed using the Brunet’s modified version of the comic strip task, which is known to engage the classical cortical mentalizing network (including the dorsomedial prefrontal cortex) (Sarfati et al., 1997; Brunet et al., 2000, 2003; Atique et al., 2011). In this behavioural paradigm, comic strip scenarios with three images are presented. The participant is then asked to select the most logical ending from among three possible answers (two distracters and the correct response). There are two experimental conditions, which require different types of causal inference: (i) the ‘attribution of intentions’ condition (corresponding to mentalizing); and (ii) the ‘physical causality‘ (control) condition. The comic strip scenarios were presented on a laptop computer (Windows 7; quad-core 2.8 i7; 16 Gb RAM) in a MATLABÕ environment (2008b, version 7.7, The Mathworks Inc.). Cogent 2000 toolbox (http://www.vislab.ucl.ac.uk) was used to generate the script. Comic strips were displayed at the top of the screen for 5 s. Next, the three possible answers (numbered from one to three, from left to right) were displayed at the bottom of the screen. Participants were asked to respond as quickly and accurately as possible by pressing keys 1 to 3 of the numeric pad with their right index finger. The answers were automatically recorded by the software. The attribution of intention and physical causality conditions each consisted of 28 scenarios. The order of the experimental conditions was counterbalanced. In each experimental condition, the scenarios were presented in a pseudo-random order. Before the experimental phase, a training session with 12 items (including attribution of intention and physical causality items) was performed. It should be noted that each of the two tasks preferentially recruits one mentalizing subsystem or the other. Given that the RME task requires explicit judgement (i.e. conscious processes), it necessarily engages the inference-based mentalizing network. Nevertheless, activation functional MRI studies have shown that the posterior part of the inferior frontal gyrus (including the pars opercularis) and the ventral premotor cortex, i.e. the anterior part of the mirror system, are involved in this task (Baron-Cohen et al., 1999; Russell et al., 2000; Baron-Cohen, 2006; Adams et al., 2010; Castelli et al., 2010; Moor et al., 2012). This involvement was formally demonstrated by lesion studies showing that RME task performance was specifically impaired by pars opercularis damage but not by medial prefrontal damage (Shamay-Tsoory et al., 2009; Herbet et al., 2013). Although the comic strip task is known to predominantly activate the mentalizing network (Brunet et al., 2000, 2003; Vollm et al., 2006; Atique et al., 2011) and be particularly affected by medial prefrontal damage (Herbet et al., 2013), a magnetoencephalography study has demonstrated the early activation of posterior brain regions belonging the mirror network (Vistoli et al., 2011) during this task. Furthermore, surgical resection of the right pars opercularis is associated with slight slowing of the inferential process associated with intentional attribution (Herbet et al., 2013). Based on these findings, we assumed that both tasks can simultaneously engage the mirror and mentalizing systems to some extent, but that the intactness of each subsystem is more important for success in one behavioural task or the other. A dual-stream model of mentalizing Neuroanatomical data acquisition In all patients, structural MRI data sets were acquired at the time of the behavioural assessment in the same medical centre as part of their standard care. The images had been acquired using conventional axial fluid-attenuated inversion recovery (FLAIR) or high-resolution 3D T1weighted sequences on a 1.5 T Siemens Avento or a 3 T Siemens Skyrya scanner (Siemens Medical Systems). Lesion mapping Resection cavities and residual lesion infiltrations were reconstructed in standardized MNI space by one of the investigators (G.H.). First, each reconstruction was compared with the initial non-normalized scans and the corresponding, detailed surgical report produced by the neurosurgeon (H.D.). Next, the work was carefully inspected by an independent, board-certified neuroradiologist (N.M.C.) who was blinded to the behavioural data. It should be noted that six patients were excluded at this point because structural abnormalities [abnormal ventricle size (n = 1) and brain hygroma (n = 5)] prevented accurate normalization. To spatially normalize individual data sets, we used SPM8 (implemented in a MATLABÕ environment) with cost function masking (Brett et al., 2001). This method has been extensively characterized and is proven to be the best option for normalization when the lesion is large (Andersen et al., 2010). During the registration process, cost function masking avoids bias caused by abnormal lesion-induced radiologic signals. Briefly, the lesion is contoured by hand and transformed into a binarized image. This image is used as a mask for the normalization process. The lesion is then drawn on the normalized scan to yield a volume of interest. We used high-resolution whole-brain 3D T1 images (resolution: 1 1 1 mm) to map the resection cavities. However, we preferred to use FLAIR images (23 axial slices, with a resolution of 0.898 0.898 6 mm) for the lesion infiltration maps because this sequence is known to yield the best contrast between normal brain tissue and infiltrated brain tissue. Voxel-based lesion-symptom analyses We used whole-brain VLSM analyses (Bates et al., 2003) to explore the putative relationships between mentalizing data on one hand and the spatial locations of the surgical resections or residual lesion infiltrations on the other. To this end, we used NPN software (version: December 2012) provided in MRIcron package (www.mricro.com/ mricron). We excluded voxels that were resected or infiltrated in fewer than three patients. Although this threshold may appear to be low, it has often been used in VLSM studies and, in view of the inhomogeneous spatial distribution of the surgical resections, was the best option here (Fig. 1). Diffuse low-grade glioma preferentially infiltrates the insular, frontal and anterior temporal cortices (Duffau and Capelle, 2004; Parisot et al., 2012). Given that right posterior temporal and parietal areas are of interest in mentalizing (Saxe and Kanwisher, 2003) and to not discount these regions of interest, it made sense to take account of voxels damaged at least in three patients. However, it should be noted that all the VLSM analyses were repeated with a minimum exclusion threshold of six and 10 patients for resection cavity maps (therefore excluding right posterior areas) and five patients for residual infiltration maps (to increase statistical power in more anterior areas). The overall results did not differ significantly as a function of the exclusion threshold, and so the latter analyses are not presented here. Brain 2014: 137; 944–959 | 947 The parametric t-test statistic was chosen to compute the Z-score statistical maps; a pairwise comparison is used to test for a statistically significant difference between behavioural scores for patients with or without damage in a given voxel (a resected or infiltrated voxel, in our case). We chose not to correct the type I error for multiple comparisons [with a Bonferroni correction or a false discovery rate (FDR) control method, for example]. In fact, this conservative approach can lead to a high false negative rate, especially for brain structures with low lesion coverage (Rudrauf et al., 2008b). Hence, we decided to threshold the resulting statistical maps at Z 4 3.09 (corresponding to P = 0.001 uncorrected), with an extent threshold of k = 70 contiguous voxels. This decision was justified by the fact that (i) we had strong prior assumptions (at least at the cortical level); and (ii) patients with diffuse lowgrade glioma show high levels of functional plasticity. Consequently, it was the best trade-off between sensibility and specificity (Rudrauf et al., 2008b). However, applying a FDR correction to the statistical maps with a threshold of q = 0.05 did not alter the main results; there were fewer significant voxels but the spatial location of significant peaks did not change. The VLSM analyses were performed on each behavioural accuracy score, expressed initially as the percentage of correct answers. However, we corrected the raw data before subsequent analyses. We specifically sought to remove the variance associated with certain sociodemographic and clinical variables of no interest—variance that otherwise may significantly distort the output results. To this end, we performed separate multiple regression analyses on the row score from the low-level task and the three row scores from the high-level task (the attribution of intention and physical causality conditions and the differential error rate when comparing the two conditions). Standardized residuals from each model (referred to as standardized residual–RME, standardized residual–attribution of intention, standardized residual–physical causality and standardized residual–differential error rate) were used as inputs in subsequent lesion-behaviour analyses. This is an acceptable approach when the design is substantially complicated by the entry of several covariates (Kimberg et al., 2007). Indeed, this procedure has been used by some authors to partial out unwanted (i.e. non-interest) factors in VLSM analyses (Schwartz et al., 2011; Gläscher et al., 2012). These residual scores were also used to study the correlation between the degree of disconnection of the associative pathways on one hand and mentalizing accuracy scores on the other (see below). For the residual lesion infiltration maps, VLSM analyses were performed with and without the lesion infiltration size as a covariate. This enabled us to check whether the degree of lesion infiltration in white matter associative pathways could account for worse mentalizing performance. Subtraction plots Resection cavity maps and residual lesion infiltration maps from patients with or without poor mentalizing accuracy were contrasted by means of MRIcron’s ‘subtraction plot’ function. This kind of imaging analysis is especially useful for detecting brain structures that are more frequently damaged in a set of patients with a given functional alteration (when compared with a set of control patients without this functional alteration) (Karnath et al., 2002). However, use of a subtraction plot is only appropriate when the two groups contain approximately the same number of individuals (Rorden and Karnath, 2004). Firstly, lesion maps from the two subgroups (impaired and unimpaired patients, in the present study) are overlaid. Secondly, these images are subtracted (the impaired image minus the unimpaired image) to create 948 | Brain 2014: 137; 944–959 G. Herbet et al. Figure 1 Lesion overlap maps for all patients. (A) Resection cavity overlaps (n = 93). The pars triangularis of the right frontal gyrus was the region with the greatest overlap (n = 26). (B) Residual lesion infiltration overlaps (n = 70). As expected, the residual infiltrations were located on the trajectory of the associative white matter fasciculi. The greatest degree of overlap occurred in the white matter fibres of the right inferior occipito-frontal fasciculus (n = 21). Histograms represent lesion density plots (i.e. the number of overlapping voxels). a new image that shows the specifically damaged cortical or subcortical regions. A colour bar is used to indicate the percentage overlap between the lesions. Infiltration estimation To estimate the degree to which white matter associative fasciculi had been infiltrated, each individual residual lesion map was first overlaid with the diffusion tensor imaging-based white matter fasciculus atlas from Catani and Thiebaut de Schotten (2008). Next, MRIcron’s ‘batch descriptive’ function was used to automatically compute the number of voxels that overlapped with each associative fasciculus (the inferior fronto-occipital, uncinate, inferior longitudinal, arcuate and lateral superior longitudinal fasciculi and the cingulum). The correlation between infiltration volumes (expressed as the number of infiltrated voxels) and mentalizing scores (raw scores and standardized residuals) were analysed using a non-parametric Spearman test. We adopted a constraining statistical context by including only patients showing infiltrated brain tissues. These analyses were supplemented by a group analysis approach based on the patient subdivisions established for subtraction lesion analyses. Using non-parametric Mann-Whitney U-test, we compared impaired patients and unimpaired patients in terms of the infiltration volumes in each fasciculus, the Z-score in the RME task and the differential error rate in the comic strip tasks. Results Sociodemographic and clinical data The patients’ sociodemographic and clinical data are summarized in Supplementary Table 1. The average volume of resected brain tissue was 72.25 61.49 cm3 (range 4.1–289.9 cm3); corresponding to 94.29 6.04% of the initial lesion size (range 70–100%). As shown in Fig. 1A, the spatial distribution of the surgical resections was inhomogeneous. This fits well with anteroposterior gradient typically observed for diffuse low-grade gliomas, where lesions infiltrating posterior areas are less frequent (Duffau and Capelle, 2004; Parisot et al., 2012). It is noteworthy that there were more patients with right-side lesions than patients with leftside lesions. This difference was expected because we excluded patients with lesions in the left middle/posterior temporal and A dual-stream model of mentalizing parietal regions, to avoid possible bias because of receptive language disorders. As expected, the remaining lesion infiltrations were mainly located on the anteroposterior trajectories of the associative white matter bundles (Fig. 1B). Our lesion mapping therefore replicated the probabilistic atlas of functional resectability published by Ius et al. (2011), which showed that most of the associative white-matter connectivity cannot be removed for functional reasons. Behavioural data analysis Raw mentalizing scores for each task are given in Supplementary Table 2. In the low-level mentalizing task, the mean standard deviation (SD) score in the patient group (n = 90) was 61.67 11% of correct responses (36.11–83.33). In the highlevel mentalizing task, the mean score in the patient group (n = 84) was 84.69 13.34% (39.90–100) of correct responses in the attribution of intention condition and 93.75% [ 7.29 (42.90–100)] of correct responses in the physical causality (control) condition. Low-level mentalizing scores were found to be moderately correlated with the attribution of intention scores (r84 = 0.254, P = 0.022), but not at all correlated with physical causality scores (r84 = 0.081, P = 0.470). All the accuracy scores were fed into separate multiple regression models with age, premorbid IQ, resection volume and time since surgery as potential predictors. For the low-level task, the overall model was not significant (R2 = 0.082, P = 0.11) but age (unlike all the other variables; P 4 0.16) was found to be a significant predictor (b = 0.24, P = 0.03). For the high-level task, the model was significant for all dependent variables (attribution of intention: R2 = 0.23, P = 0.0003; physical causality: R2 = 0.225, P = 0.0004; differential error rate: R2 = 0.11, P = 0.04). Age and premorbid IQ made significant contributions to these models contrary to clinical variables (the complete results of these analyses are reported in Supplementary Table 3). Standardized residuals from these multiple regressions were used instead of raw behavioural scores as inputs for subsequent VLSM analyses. This is enabled us to remove the unwanted variance associated with the covariates of non-interest mentioned above. The patient subgroups (unimpaired and impaired) for group and subtraction plot analyses (see below) were established by applying a clinical criterion for normality (4 1 SD versus 4 1 SD) based on control data from healthy participants. In clinical practice, patients performing below this cut-off are usually considered to be poor performers. Moreover, this cut-off was particularly convenient for dividing the patients into two equally sized subgroups. For the RME low-level mentalizing task, 42 healthy participants were divided into three age classes (Group 1: 18–34; Group 2: 35–49; Group 3: 50–65) and their behavioural scores were used to transform the patients’ raw scores into age-adjusted Z-scores. In contrast to the comic strip task, the RME task is known to be affected by age in French populations (Duval et al., 2011). For the comic strip task, 18 additional control participants provided normative data that enabled the patient’s performance levels to be transformed in Z-scores. The subtraction plot analysis was performed Brain 2014: 137; 944–959 | 949 on the differential error rate data only to identify patients with a specific decrease in inference-based mentalizing accuracy. For correlation analyses between mentalizing accuracy and the degree of lesion infiltration into associative white-matter fasciculi (see below), age-adjusted Z-scores (for the low-level RME task) and raw scores (for the high-level comic strip task) as well as standardized residuals from multiple regression models (see above) were used. Note that raw scores were directly selected for the comic strip task because scores were not adjusted for age, as earlier justified. Consequently, transformation into Z-scores for this task only served to segregate patients for group and subtraction plot analyses. Resections of the supplementary motor area and the dorsal premotor cortex are associated with poor inference-based mentalizing accuracy We performed VLSM analyses on standardized residuals (first for the resection cavity maps and then for the residual lesion infiltration maps; Fig. 1A and B). As explained in the ‘Materials and methods’ section, we chose to apply a liberal threshold for statistical significance (Z 4 3.09; P 5 0.001 uncorrected). For the sake of completeness, the summary table also reports the clusters that survived a correction for multiple comparisons (using a FDR control procedure with q = 0.05). For the low-level mentalizing task, our analyses of resection cavity maps (90 maps, with 589 001 voxels tested) were inconclusive and none of the voxels exceeded the critical value. The same was true for our analyses of residual lesion infiltration maps (68 maps, 33 126 voxels tested), regardless of whether or not the lesion infiltration volume was included as a covariate. For the high-level mentalizing task, VLSM analyses of the attribution of intention data and the resection cavity maps (84 maps, 572 302 voxels tested) revealed a cluster of significant voxels in the right angular gyrus (cluster size = 565 voxels, Zmax = 3.76) and the right supplementary motor area (cluster size = 211 voxels, Zmax = 4.11). However, the posterior parietal regions were not specific to intention mentalizing, as analyses performed on the physical causality data also highlighted a cluster of significant voxels in the same location (cluster size = 573 voxels, Zmax = 3.3). Accordingly, VLSM of the differential error rate data did not identity significant voxels in this region. In contrast, the latter analysis revealed a large cluster of significant voxels in the right supplementary motor area, which extended to the dorsal premotor cortex (cluster size = 2728 voxels, Zmax = 5.32) and— albeit to a lesser extent—the junction between the pars opercularis of the right inferior frontal gyrus, the pars triangularis and the posterior part of the right middle frontal gyrus (cluster size = 176 voxels, Zmax = 5.32). These results are reported in Table 1 and illustrated in Fig. 2. At the subcortical level, VLSM analysis of the residual lesion infiltration maps revealed a cluster of significant voxels in the white matter underlying the right occipito-temporoparietal junction when considering both attribution of intention data (cluster 950 | Brain 2014: 137; 944–959 G. Herbet et al. Table 1 Overall VLSM results Peak locations (all right-sided) BA Z P uncorrected Cluster size (at P 5 0.001) Cluster size (at P 5 0.05 FDR-corrected) VLSM results for resection cavity maps: standardized residual–attribution of intention SMA 6 4.1 0.000021 211 0 AG 39 3.76 0.000084 565 0 VLSM results for resection cavity maps: standardized residual–physical causality AG 39 3.3 0.00048 573 0 VLSM results for the resection cavity maps: standardized residual–differential error rate SMA 6 5.32 0.00000005 2728 2024 mid. FG 44 3.98 0.000034 176 0 VLSM results for residual lesion infiltration: standardized residual–attribution of intention WM TPOj 19 3.81 0.000069 210 177 VLSM results for residual lesion infiltration: standardized residual–physical causality WM TPOj 0 4.05 0.000026 257 863 WM AG 0 3.46 0.00027 88 VLSM results for residual lesion infiltration: standardized residual–differential error rate VLSM results in relation to RI maps + residual infiltration volumes as a covariate MNI coordinates x y z 9 49 12 60 70 43 49 60 43 21 48 8 28 64 41 31 59 22 30 25 58 48 22 46 - - - Analyses operated on standardized residual–attribution of intention, standardized residual–physical causality, and standardized residual–differential error ratewere inconclusive, and revealed systematically a volume effect (values in bold: P 5 0.00001 uncorrected; P 5 0.05 FDR-corrected). SMA = supplementary motor area; AG = angular gyrus; mid; FG = middle frontal gyrus; WM = white matter; TPOj = temporo-parieto-occipital junction; BA = Brodmann area; RL = residual lesion. Figure 2 Damage to the supplementary motor area and dorsal premotor cortex is associated with impaired accuracy in inference-based mentalizing. The statistical map is thresholded at P 5 0.001 uncorrected and rendered in three dimensions. Only significant voxels are shown. This lesion-symptom analysis was performed on the difference in error rate between the mentalizing condition (intention attribution) and the control condition (physical causality), after removing unwanted variance associated with nuisance variables (age, premorbid IQ, lesion volume and time since surgery). size = 210, Z = 3.81) and physical causality data (cluster size = 257, Z: 4.05). In the physical causality analysis, an additional cluster was observed in the white matter underlying the right angular gyrus (cluster size = 88, Z = 3.46). However, analysis of the differential error rate data did not identify any significant lesionbehaviour relationships, demonstrating that infiltration of these white matter fibres was not specifically associated with impaired mentalizing accuracy. Interestingly, the clusters identified for attribution of intention and physical causality disappeared when the analyses were repeated with the volume of residual infiltration as a covariate (Table 1). Brain regions most frequently damaged in patients with low mentalizing accuracy To visualize the brain areas the most frequently damaged in patients with mentalizing impairments, we generated maps by overlapping individual residual lesion infiltration maps of patients with poor accuracy and maps from patients with normal accuracy. In view of the difference in the numbers of patients with left-side lesions and those with right-side lesions, subtraction analyses were only performed on the latter group of patients. A dual-stream model of mentalizing Brain 2014: 137; 944–959 | 951 Figure 3 The most frequently damaged brain regions in patients with impaired (i) mentalizing accuracy. (A) Low-level mentalizing task, all right-lesion patients. (B) Low-level mentalizing task, only frontal right-lesion patients. (C) High-level mentalizing task, all right-lesion patients. The colour bar indicates increasing frequency. Only brain regions that overlapped in at least 10% of patients are shown. The lefthand plots depict accuracy scores for the two patient subgroups. ***P 5 10 6. DER = differential error rate; ui = unimpaired. For the low-level mentalizing task, the pars opercularis of the right inferior frontal gyrus (224 overlapping voxels in more than 25% of the patients, Fig. 3A) was more likely to be damaged in patients with impaired accuracy (n = 36, 53.7%) than in patients with unimpaired accuracy (n = 31, 46.3%). This was also true when considering only patients with a resection in the right frontal lobe (n = 20 patients with impaired accuracy versus n = 23 with unimpaired accuracy; 148 overlapping voxels in the right pars opercularis in 4 45% of the patients; Fig. 3B). For the high-level mentalizing task, the pars orbitalis of the right inferior frontal gyrus (42 voxels), the posterior part of the right middle frontal gyrus (142 voxels) and the supplementary motor area (52 voxels) were more likely to have been resected in patients with unimpaired performance (525%, Fig. 3C). Damage to the right arcuate fasciculus is specifically associated with impaired low-level mentalizing accuracy We estimated the degree of disconnection (i.e. the volume of infiltration) for each associative white matter fascicule and for each patient. These volumes were then related to the mentalizing data. We considered both age-adjusted Z-scores and standardized residuals from multiple regressions to test the robustness of the 952 | Brain 2014: 137; 944–959 G. Herbet et al. results after controlling for nuisance variables. Only patients with residual infiltrations were considered. We also restricted these analyses to right-sided lesion patients; there were not enough patients with left-side lesions to draw reliable and definitive conclusions on the possible involvement of left white matter pathways in mentalizing. As expected, we found significant negative correlations between low-level mentalizing performance and infiltration volumes in the right arcuate fasciculus (Z-scores: r47 = 0.38, P = 0.009; residuals: r47 = 0.35, P = 0.016) and the lateral superior longitudinal fasciculus (Z-scores: r47 = 0.35, P = 0.017; residuals: r47 = 0.35, P = 0.048). Performance was also significantly and negatively correlated with the total volume of infiltration (r47 = 0.29, P = 0.047; residuals: r47 = 0.69, P = 0.047). None of the other correlations were statistically significant (Table 2). In support of these results, impaired patients were significantly more infiltrated in the right arcuate fasciculus than unimpaired patients (Z = 1.95, P = 0.05, Fig. 4A), confirming the qualitative result from the subtraction lesion analyses (Fig. 4B). Damage to the right cingulum is specifically associated with impaired high-level mentalizing accuracy For the high-level mentalizing task, the infiltration volumes in the cingulum were negatively correlated with the differential error rate Table 2 Correlation analyses between infiltration volumes in right white matter associative pathways and mentalizing accuracy scores Age IQ Low-level perceptive-based mentalizing Z-scores 0.16 0.12 SR 0.016 0.02 High-level inference-based mentalizing AI -0.39** 0.19 SR AI 0.02 0.06 PhC -0.39** 0.25 SR PhC 0.06 0.12 DER 0.23 0.15 SR DER 0.02 0.002 RIV -0.29* -0.29* 0.14 0.17 0.003 0.004 0.11 0.12 IFOF ILF UF 0.18 0.20 0.2 0.21 0.025 0.002 0.032 0.012 0.062 0.03 0.06 0.04 0.04 0.05 0.036 0.03 0.02 0.03 0.07 0.005 0.02 0.07 0.13 0.08 AF -0.38** -0.35* 0.22 0.16 0.11 0.08 0.18 0.15 lSLF Cing. -0.35* -0.29* 0.19 0.19 0.13 0.03 0.16 0.086 0.07 0.027 -0.28* -0.27 0.03 0.042 -0.33* -0.31* Analyses were performed with a non-parametric correlation test. Numeric values in the table correspond to the Spearman’s rank correlation coefficient. RCV = resection cavity volume; RIV=residual infiltration volume; IFOF=inferior fronto-occipital fascicle, ILF=inferior longitudinal fascicle; UF=uncinate fascicle; AF=arcuate fascicle; lSLF=lateral longitudinal fascicle; Cing.=cingulum. *P 5 0.05; **P 5 0.01; P 5 0.1. Figure 4 Damage to the right arcuate fasciculus is specifically associated with decreased low-level mentalizing accuracy. (A) Group analyses: the residual lesion volumes in each associative pathway were compared in patients with impaired (i) and without impaired (ui) mentalizing performance. Only the arcuate fasciculus showed a group effect (Z = 1.95, P = 0.05). All other comparisons were nonsignificant (P 4 0.1, see also Table 2). (B) Subtraction plots: patients with impaired performance were more likely to have infiltration in the arcuate fasciculus. The bar indicates increasing frequency. Only brain regions that overlapped in at least 10% of patients are shown. The left arcuate fasciculus is plotted as a visual point of reference. IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; UF = uncinate fasciculus; AF = arcuate fasciculus; lSLF = lateral superior longitudinal fasciculus. A dual-stream model of mentalizing Brain 2014: 137; 944–959 | 953 Figure 5 Damage to the right cingulum is specifically associated with decreased high-level mentalizing accuracy. (A) Patients with an impaired differential error rate were more likely to have infiltration in the right cingulum than patients with a normal differential error rate (P = 0.03, see also Table 2). Subtraction plot: patients with impaired performance were more likely to have infiltration in the cingulum and in white matters fibres constituting the temporo-parieto-occipital junction. The bar indicates increasing frequency. The left cingulum is plotted as a visual point of reference. IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; UF = uncinate fasciculus; AF = arcuate fasciculus; lSLF = lateral superior longitudinal fasciculus. (r47 = 0.33, P = 0.024), even after controlling for nuisance variables (standardized residual–differential error rate: r47 = 0.33, P = 0.036). None of the other correlations were statistically significant. These analyses were also performed for the attribution of intention and physical causality data sets (Table 2). These findings were in accordance with group analyses showing that lesion infiltrations in the cingulum were significantly larger in impaired patients (n = 21) than in unimpaired patients (n = 26) (differential error rate, Z = 2.075, P = 0.038). None of the other correlations achieved statistical significance (Fig. 5A). Subtraction lesion analyses confirmed the above results (Fig. 5B). Impaired patients were likely to have infiltration in the cingulum and in the posterior part of the lateral frontoparietal network (including the white matter deep in the temporoparietal and temporo-parieto-occipital junctions). These findings are in line with data (Fig. 5A) showing that impaired and unimpaired patients also differed (albeit not significantly) in terms of infiltration volumes in the arcuate fasciculus and the lateral superior longitudinal fasciculus (but not in terms of the ventral associative fasciculi, including the inferior fronto-occipital fasciculus and inferior lateral fasciculus. Discussion Diffuse low-grade glioma migrates preferentially along white matter associative pathways. Accordingly, we used this neurological disorder as a physiopathological model of the functional anatomy of the associative fasciculi. To date, methods for relating cognitive or neurological impairments to the disconnection of long-range white matter fibres have been qualitative (Bartolomeo, 2012) and involved establishing whether a given lesion is located within associative fasciculi (Bartolomeo et al., 2007; Thiebaut de Schotten et al., 2008; Karnath et al., 2011). Quantitative methods are now being developed, in an attempt to gauge the respective roles of cortical and subcortical damage in the occurrence of cognitive disorders (Rudrauf et al., 2008a; Philippi et al., 2009). Here, we adopted the same type of approach by estimating the degree of disconnection of each associative fascicule to make specific predictions about the connectional anatomy of mentalizing. However, the main strength of the present study was our ability to identify critical structures for mentalizing processes at both the cortical and subcortical levels (by studying cortical resections and infiltrated deep connectivity, respectively) (Fig. 1). To the best of our knowledge, the present study is the first to provide data on the structural connectivity of mentalizing subnetworks. Our results suggest that this complex sociocognitive function is performed by a dual-stream hodological system. Disconnection of the right arcuate fasciculus/lateral superior longitudinal fasciculus and impaired perceptive-based mentalizing accuracy Dissection studies and diffusion tensor imaging-based studies have shown that the anterior part of the mirror neuron system (including the posterior inferior frontal gyrus and the ventral premotor cortex) is connected to posterior temporoparietal areas by the arcuate fasciculus and the lateral/superficial superior longitudinal fasciculus (Catani and Ffytche, 2005; Makris et al., 2005; Martino et al., 2013). As previously stated by some authors (Iacoboni and Dapretto, 2006), this perisylvian network might therefore constitute the main pathway for the frontoparietal mirror network (given its cortical terminations in putative mirror areas). Consequently, we hypothesized that damage to this tract 954 | Brain 2014: 137; 944–959 could account for impairments in low-level mentalizing performance. Our present results argue in favour of this hypothesis. Accuracy scores were specifically correlated with the degree of infiltration of the arcuate fasciculus and the left superior longitudinal fasciculus (Table 2), and patients with impaired versus unimpaired mentalizing performance differed significantly in terms of infiltration volumes in the arcuate fasciculus (Fig. 4A and B). This finding was also consistent with the fact that the junction between the right pars opercularis and the right ventral premotor cortex was the most frequently damaged brain region in impaired patients (Fig. 3A and B). Our data fit well with the results of two recent studies in which the right arcuate fasciculus/lateral superior longitudinal fasciculus was suggested to have an important role in social cognition. In a patient population, voxel-based lesion-symptom mapping showed that damage to these pathways was associated with poor social and emotional intelligence (Barbey et al., 2012). In healthy subjects, interindividual variability in emotional empathy (a brain process known to recruit the mirror system; Shamay-Tsoory et al., 2009) was positively correlated with the fractional anisotropy in certain parts of these associative fasciculi (and especially in the right hemisphere), suggesting that structural hyperconnectivity of the arcuate fasciculus/lateral superior longitudinal fasciculus is associated with greater empathic abilities (Parkinson and Wheatley, 2012). Disconnection of the right cingulum and impaired inference-based mentalizing accuracy In activation functional MRI studies, the medial prefrontal cortex (including the more rostral part of the anterior cingulate cortex) and the precuneus have always been systematically linked to inference-based mentalizing (Amodio and Frtih, 2006; Carrington and Bailey, 2009; van Overwalle and Baetens, 2009). Furthermore, connectivity studies have also showed strong functional interactions between the medial prefrontal cortex and the precuneus during sociocognitive tasks in general and inferencebased mentalizing in particular (Atique et al., 2011). Interestingly, these two cortical nodes also belong to the socalled default mode network, which is thought to be involved in self-referential cognition and overlaps greatly with the social cognition network (Schilbach et al., 2008, 2012; Spreng et al., 2009; Mars et al., 2012). Given that the cingulum provides strong connections between the rostral medial prefrontal cortex/anterior cingulate and the medial posterior parietal cortex (including the posterior cingulate cortex and ventral precuneus), the cingulum is likely to shape the structural connectivity of the default network, as recently suggested by two multimodal imaging studies (van den Heuvel et al., 2008; Greicius et al., 2009). Based on these findings, we hypothesized that disconnection of the cingulum was related to behavioural changes in inference-based mentalizing. Our present data provide direct support for this hypothesis. Impaired mentalizing accuracy was specifically correlated with the extent to which the right cingulum was infiltrated (Table 2). This result was corroborated by group analyses, which showed G. Herbet et al. that infiltration volumes in the right cingulum were significantly larger in patients showing specific impairments in inference-based mentalizing; this was not the case for the other fasciculi, and especially the ventral associative pathways (Fig. 5A and B). Understanding other’s intentions is impaired by damage to the supplementary motor area/dorsal premotor cortex Both subtraction lesion analyses and VLSM analyses highlighted the large number of significantly involved voxels in the right supplementary motor area (extending to the dorsal premotor regions) (Fig. 2). These brain regions are known to harbour neurons with mirror properties (Mukamel et al., 2010) and have a crucial role in understanding intentions and actions (Iacoboni et al. 2005; Spunt and Lieberman, 2013). However, a growing body of evidence suggests that the mirror system is involved in computing proximal (motor) intentions but not distal intentions necessitating (which, by their very nature, require more reflective processes) (de Lange et al., 2008; Rizzolatti et al., 2010). Nevertheless, the mirror system is likely to facilitate psychological inference by automatically decoding low-level intentional cues during the course of complex action plans. Our results indicate that damage to the cortical structures thought to subserve automatic processes in intention decoding can also alter the ability to understand intention (i.e. a higher-level process). Our findings suggest that both the mirror and the mentalizing system are engaged during the conscious attribution of intentions (as previously demonstrated in a functional connectivity study using the same task (Kana et al., 2014) and, more generally, during the ascription of psychological states (Spunt and Lieberman, 2012). In this respect, it is noteworthy that decreasing the threshold to P 5 0.005 (uncorrected) in the VLSM analyses revealed an additional cluster of voxels in the pars opercularis of the right posterior inferior frontal gyrus. Taken as a whole, our results fit well with the interactive (cooperative) dualprocess theory of mentalizing, in which it is posited that low-level embodied processes and high-level inferential processes are engaged during the ascription of psychological states (Uddin et al., 2007; Keysers and Gazzola, 2007; Lombardo et al., 2010a; Spunt and Lieberman, 2012). A plastic hodological mentalizing network One can legitimately wonder why our conventional voxel-wise analysis of a relatively large sample of patients failed to establish comprehensive statistical maps of the relationship between impaired mentalizing processes and the location of brain damage. One limitation of the current study relates to the fact that few patients had lesions in the right temporoparietal junction (as a result of natural spatial distribution of diffuse low-grade gliomas). However, this limitation did not apply to more anterior temporal and frontal regions. On the basis of data from lesion and multimodal imaging studies that featured the behavioural tasks used here (Brunet et al., 2000, 2003; Adams et al., 2010; Castelli et al., 2010; Moor et al., 2012; Herbet et al., 2013), one would A dual-stream model of mentalizing expect injury to the posterior inferolateral or medial frontal areas to impact task performance (at least to some extent) in conventional, quantitative lesion-symptom analyses. The absence of significant findings cannot be reasonably attributed to low statistical power. Firstly, we also performed VLSM analyses with higher voxel cut-offs (5 and 10 for resection cavity maps, and 5 for residual lesion infiltration maps) to improve the statistical power. Secondly, we sought to avoid false negative results by using a liberal threshold for statistical significance. One possible explanation relates to the lesions’ neuropathological. In contrast to acute lesions (such as vascular stroke), diffuse low-grade glioma slowly infiltrates brain tissues (Mandonnet et al., 2003) and can sometimes induce impressive functional remodelling (Desmurget et al., 2007) (as demonstrating by functional MRI and electrostimulation studies; Krainik et al., 2004; Duffau, 2005; van Geemen et al., 2013). This is why it is possible to surgically remove large brain regions, without inducing severe cognitive disorders (Duffau, 2012). That is not to say, however, that structures of the brain are equipotent. Functional plasticity in diffuse low-grade glioma is limited and some structures are clearly refractory to functional compensation. This is especially true for (i) white matter fibres (including large portions of the arcuate, superior longitudinal, inferior occipito-frontal and posterior inferior longitudinal fasciculi) that cannot be re-sected; and (ii) certain cortical epicentres with poor potential for functional compensation (Ius et al., 2011). One striking finding in the present study was that the degree of frontoparietal disconnection was correlated with poor mentalizing performance. This fully agrees with Ius and colleagues’ (2011) proposal of critical subcortical brain systems and, more generally, with the view that long-range connectivity is essential for cognition (Mesulam, 1998; Bressler and Menon, 2010; de Benedictis and Duffau, 2011; Knosche and Tittgemeyer, 2011). The underlying pathophysiological mechanism of ‘progressive disconnection syndrome‘ is probably related to disruption of cortical synchrony throughout the neurocognitive network, which is safeguarded under normal circumstances by the connectivity of subcortical white matter. In this respect, some studies have shown that damage to structural connectivity harms cognitive functions (Shinoura et al., 2009; Karnath et al., 2011; Kummerer et al., 2013), and induces major functional changes throughout the network (He et al., 2007a). These results have prompted some researchers to argue that structural alterations in white matter connectivity constitute the main obstacle to functional compensation, especially in the case of diffuse low-grade glioma (Duffau, 2013). We consider that our findings have potential clinical value and may enable prediction of the functional outcome of surgery (based on the preoperative degree of axonal connectivity infiltration) and the adjustment of individual cognitive rehabilitation programmes. Conceivably, VLSM analysis of residual lesion infiltration maps could be criticized if it does not confirm significant results generated with other methods. We do not share this scepticism. By definition, an interpretation based on a disconnection syndrome does not fit well with location-based models. In theory, a given neuropsychological manifestation can be induced by injury of any part of a fasciculus (Catani and Ffytche, 2005; Bartolomeo, 2012). Brain 2014: 137; 944–959 | 955 This may be one reason why VLSM analyses have been inconclusive (especially regarding the low-level mentalizing task). It may also be the case that impaired mentalizing accuracy is related to incremental damage to associative pathways. This would fit with the results of correlation analyses and also with the fact that our significant VLSM associations for the high-level mentalizing accuracy scores (attribution of intention and physical causality) with the white matter underlying the right temporoparietal region disappeared when residual lesion volumes was included as a covariate. Nevertheless, it is important to note that progressive damage to associative pathways does not completely explain the behavioural results. Although our study failed to fully describe mentalizing subnetworks at the cortical level, our qualitative and quantitative analyses demonstrate the importance of certain cortical epicentres (the pars opercularis of the right inferior frontal gyrus for the low-level mentalizing task and the supplementary motor area for the high-level mentalizing task). At a broader (conceptual) level, our findings support the network view of high-level cerebral processes. Cognitive functions should always be considered as the endpoint of sophisticated functional interactions within whole-brain neurocognitive networks and not as in classical neuropsychology (i.e. the byproduct of the neural activity of discrete, highly specialized cortical areas) (Bressler, 1995; Mesulam, 1998; McIntosh, 2000; Hickok and Poepple, 2004; Fuster, 2006; Just and Varma, 2007; Bressler and Menon, 2010; Meehan and Bressler, 2012). This opens up new opportunities for understanding neuropsychological impairments in non-focal disorders (i.e. neurodegenerative disease and neuropsychiatric disorders) as well as focal disorders (He et al., 2007b; Honey et al., 2010). In the latter case, the heuristic value of the networking hypothesis appears to be much higher, especially when impairments following neurological disorders are sometimes poorly explained by the lesion’s location. In this respect, researchers have started to use biomathematical approaches to model the effect of a focal lesion on the topology of wholebrain networks (Honey and Sporns, 2008; Alstott et al., 2009). It has been suggested that virtual lesions in certain cortical hubs can disrupt functional connectivity between distant cortical areas; this is in good agreement with studies of patients with focal damage (He et al., 2007a; Gratton et al., 2012; Tuladhar et al., 2013). Our findings fit well with a network paradigm of functional disturbances, by demonstrating that the associative axonal connectivity underlying large-scale networks is essential for cognitive functioning. This type of approach should prompt neuropsychological researchers to consider connectivity (rather than an oversimplistic, static, location-centred paradigm) when interpreting cognitive impairments, especially in the field of social cognition (Kennedy and Adolphs, 2012). In view of the above framework, our results must be considered in the broader context of neurodevelopmental disorders and especially autism spectrum disorders, for which impairments in social cognition (including mentalizing and empathy) are quintessential neuropsychological markers (Baron-Cohen et al., 1985, 2001; Lombardo et al., 2007, 2010b). It is now well established that autism spectrum disorders are accompanied by structural alterations through the brain (Cauda et al., 2010) and, in particular, to long-range white matter pathways (Ke et al., 2009; Fletcher 956 | Brain 2014: 137; 944–959 et al., 2010; Cauda et al., 2013; Aoki et al., 2013). It was recently reported that global white matter abnormalities were related to clinical measures of social and communicative abilities in patients with autism spectrum disorder (Gibbard et al., 2013). In view of the interconnections between key brain areas in social perception and cognition, structural disruption of the right arcuate/lateral superior longitudinal fasciculus complex has been posited as a possible substrate for the patients’ sociocognitive impairments (Cauda et al., 2013; Kana et al., 2014). Our present findings show how certain sociocognitive impairments can arise from structural alterations in specific associative pathways and shed light on the possible structural basis of related impairments in ASD populations. According to a prominent (Dapretto et al., 2006; Rizzolatti and Fabbri-Destro, 2010; Gallese et al., 2013) but controversial hypothesis (Hamilton, 2013), dysfunction of mirror mechanisms may account for the emotional empathy, imitation and basic emotion recognition disorders encountered in autism spectrum disorders. On the basis of our results, one can legitimately speculate that impaired low-level embodied representations in autism spectrum disorders are related to abnormalities in the arcuate/lateral superior longitudinal fasciculus. The current study has some potential limitations. We excluded patients with left posterior temporal/parietal lesions to minimize potential bias because of language impairments. Consequently, we were unable to study the potential impact of disconnection of left associative pathways in the occurrence of mentalizing impairments. Indeed, the low numbers of infiltrated patients prevented us from drawing unambiguous conclusions in this respect. On the methodological level, our use of a probabilistic diffusion tensor imaging-based atlas to estimate the degree of disconnection of each tract constitutes another possible limitation. Given that we used the full range of spatial coordinates of each fasciculus, the degree of the colinearity (Philippi et al., 2009) may have been greater than that induced by natural crossing (e.g. the inferior occipito-frontal fasciculus intersects with the arcuate fasciculus deep within the inferior frontal gyrus). In conclusion, the present study provided several new insights into the neural bases of mentalizing. As was expected from the literature data, mentalizing abilities may be functionally segregated into two neurocognitive systems devoted to the identification and attribution of mental states, respectively (Sabbagh et al., 2004; Spunt and Lombardo, 2012). A right dorsal stream may process the perceptual cues required for the rapid, pre-reflective identification of psychological states. This stream might connect the posterior part of the inferior frontal gyrus cortex and the inferior parietal lobule through two parallel pathways (the arcuate fasciculus and the lateral superior longitudinal fasciculus). A right midline stream may process more self-reflective and inferential aspects of mentalizing. The latter stream may connect the medial prefrontal cortex to the medial posterior parietal cortex through the cingulum. Mentalizing abilities may thus arise from these two physically distinct but functionally interdependent networks. Certain brain areas belonging to each network do indeed show effective connectivity during mentalizing (Lombardo et al., 2010a; Spunt and Lieberman, 2012). Taken as a whole and when combined with literature data, our results provide evidence of the previously hypothesized integrated, dynamic, dual-stream system G. Herbet et al. of mentalizing processes (Keysers and Gazzola, 2007; Uddin et al., 2007; Lombardo et al., 2010a; Spunt and Lieberman, 2012). This type of anatomic and functional model may offer new perspectives for understanding neuropathological/neurodevelopmental conditions characterized by sociocognitive impairments and structural changes in associative pathways. Funding G.H received a fellowship from the Association pour la Recherche sur le Cancer (grant number: DOC20120605069). The authors declare that they have no conflicts of interest. Supplementary material Supplementary material is available at Brain online. References Adams RB Jr, Rule NO, Franklin RG Jr, Wang E, Stevenson MT, Yoshikawa S, et al. Cross-cultural reading the mind in the eyes: an fMRI investigation. J Cogn Neurosci 2010; 22: 97–108. Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol 2009; 5: e1000408. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006; 7: 268–77. Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? Neuroimage 2010; 53: 78–84. Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism 2013; 4: 2040–392. Atique B, Erb M, Gharabaghi A, Grodd W, Anders S. Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage 2011; 55: 1899–911. Barbey AK, Colom R, Grafman J. Distributed neural system for emotional intelligence revealed by lesion mapping. Soc Cogn Affect Neurosci 2012; 6: 6. Baron-Cohen S, Klin A. What’s so special about Asperger Syndrome? Brain Cogn 2006; 61: 1–4. Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind"? Cognition 1985; 21: 37–46. Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 1999; 11: 1891–8. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 2001; 42: 241–51. Bartolomeo P. The elusive nature of white matter damage in anatomoclinical correlations. Front Hum Neurosci 2012; 6: 3. Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex 2007; 17: 2479–90. Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003; 6: 448–50. Bohl V, van den Bos W. Toward an integrative account of social cognition: marrying theory of mind and interactionism to study the interplay of Type 1 and Type 2 processes. Front Hum Neurosci 2012; 6: 11. A dual-stream model of mentalizing Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev 1995; 20: 288–304. Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 2010; 14: 277–90. Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 2001; 14: 486–500. Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 2000; 11: 157–66. Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia 2003; 41: 1574–82. Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp 2009; 30: 2313–35. Castelli I, Baglio F, Blasi V, Alberoni M, Falini A, Liverta-Sempio O, et al. Effects of aging on mindreading ability through the eyes: an fMRI study. Neuropsychologia 2010; 48: 2586–94. Catani M, Dell’acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, et al. Beyond cortical localization in clinico-anatomical correlation. Cortex 2012; 48: 1262–87. Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain 2005; 128(Pt 10): 2224–39. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008; 44: 1105–32. Cauda F, Costa T, Palermo S, D’Agata F, Diano M, Bianco F, et al. Concordance of white matter and gray matter abnormalities in autism spectrum disorders: a voxel-based meta–analysis study. Hum Brain Mapp 2013, in press. Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, et al. Functional connectivity of the posteromedial cortex. PLoS One 2010; 5: e13107. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129(Pt 3): 564–83. Coricelli G. Two-levels of mental states attribution: from automaticity to voluntariness. Neuropsychologia 2005; 43: 294–300. Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci 2006; 9: 28–30. De Benedictis A, Duffau H. Brain hodotopy: from esoteric concept to practical surgical applications. Neurosurgery 2011; 68: 1709–23. de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol 2008; 18: 454–7. Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slowgrowing lesions: a new door to brain plasticity. Brain 2007; 130(Pt 4): 898–914. Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex 2013; S0010-9452(13)00207–4. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 2005; 4: 476–86. Duffau H. Do brain tumours allow valid conclusions on the localisation of human brain functions? Cortex 2011; 47: 1016–7. Duffau H. The “frontal syndrome” revisited: lessons from electrostimulation mapping studies. Cortex 2012; 48: 120–31. Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer 2004; 100: 2622–6. Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain 2002; 125 (Pt 1): 199–214. Duval C, Piolino P, Bejanin A, Eustache F, Desgranges B. Age effects on different components of theory of mind. Conscious Cogn 2011; 20: 627–42. Brain 2014: 137; 944–959 | 957 Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 2010; 51: 1117–25. Frith CD, Frith U. The neural basis of mentalizing. Neuron 2006; 50: 531–4. Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol 2006; 60: 125–32. Gallese V, Rochat MJ, Berchio C. The mirror mechanism and its potential role in autism spectrum disorder. Dev Med Child Neurol 2013; 55: 15–22. Gibbard CR, Ren J, Seunarine KK, Clayden JD, Skuse DH, Clark CA. White matter microstructure correlates with autism trait severity in a combined clinical–control sample of high-functioning adults. Neuroimage Clin 2013; 3: 106–14. Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci USA 2012; 109: 14681–6. Gratton C, Nomura EM, Perez F, D’Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J Cogn Neurosci 2012; 24: 1275–85. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003; 100: 253–8. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009; 19: 72–8. Hamilton AF. Reflecting on the mirror neuron system in autism: a systematic review of current theories. Dev Cogn Neurosci 2013; 3: 91–105. Happé F, Malhi GS, Checkley S. Acquired mind-blindness following frontal lobe surgery? A single case study of impaired ‘theory of mind’ in a patient treated with stereotactic anterior capsulotomy. Neuropsychologia 2001; 39: 83–90. He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol 2007b; 20: 655–60. He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007a; 53: 905–18. Herbet G, Lafargue G, Bonnetblanc F, Moritz-Gasser S, Duffau H. Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow-growing lesion. Cortex 2013; 49: 2711–27. Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 2004; 92: 67–99. Honey CJ, Sporns O. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 2008; 29: 802–9. Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage 2010; 52: 766–76. Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 2006; 7: 942–51. Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol 2005; 3: e79. Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain". Neuroimage 2011; 56: 992–1000. Just MA, Varma S. The organization of thinking: what functional brain imaging reveals about the neuroarchitecture of complex cognition. Cogn Affect Behav Neurosci 2007; 7: 153–91. Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional Brain Networks and White Matter Underlying Theory-of-Mind in Autism. Soc Cogn Affect Neurosci 2014; 9: 98–105. 958 | Brain 2014: 137; 944–959 Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain 2002; 125(Pt 2): 350–60. Karnath HO, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain 2011; 134(Pt 3): 903–12. Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res 2009; 1265: 171–7. Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci 2012; 16: 559–72. Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci 2007; 11: 194–6. Kimberg DY, Coslett HB, Schwartz MF. Power in Voxel-based lesionsymptom mapping. J Cogn Neurosci 2007; 19: 1067–80. Knosche TR, Tittgemeyer M. The role of long-range connectivity for the characterization of the functional-anatomical organization of the cortex. Front Syst Neurosci 2011; 5: 00058. Krainik A, Duffau H, Capelle L, Cornu P, Boch AL, Mangin JF, et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 2004; 62: 1323–32. Kummerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Kloppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain 2013; 136(Pt 2): 619–29. Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol 2007; 58: 259–89. Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS One 2007; 2: e883. Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, et al. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci 2010a; 22: 1623–35. Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain 2010b; 133: 611–24. Mackinnon A, Mulligan R. The estimation of premorbid intelligence levels in French speakers [in French]. Encephale 2005; 31 (1 Pt 1): 31–43. Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr. et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 2005; 15: 854–69. Mandonnet E, Delattre JY, Tanguy ML, Swanson KR, Carpentier AF, Duffau H, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol 2003; 53: 524–8. Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol 2011; 62: 103–34. Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci 2012; 6 (189): 00189. Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, et al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fibre dissection and DTI tractography study. Brain Struct Funct 2013; 218: 105–21. McIntosh AR. Towards a network theory of cognition. Neural Netw 2000; 13: 861–70. Meehan TP, Bressler SL. Neurocognitive networks: findings, models, and theory. Neurosci Biobehav Rev 2012; 36: 2232–47. Mesulam MM. From sensation to cognition. Brain 1998; 121(Pt 6): 1013–52. Moor BG, Macks ZA, Güroğlu B, Rombouts SA, Molen MW, Crone EA. Neurodevelopmental changes of reading the mind in the eyes. Soc Cogn Affect Neurosci 2012; 7: 44–52. Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol 2010; 20: 750–6. Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science 1979; 205: 1401–3. G. Herbet et al. Parisot S, Duffau H, Chemouny S, Paragios N. Joint tumor segmentation and dense deformable registration of brain MR images. Med Image Comput Comput Assist Interv 2012; 15(Pt 2): 651–8. Parkinson C, Wheatley T. Relating anatomical and social connectivity: white matter microstructure predicts emotional empathy. Cereb Cortex 2012, in press. Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fibre tracts impairs recognition of the facial expression of emotion. J Neurosci 2009; 29: 15089–99. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 2001; 98: 676–82. Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci 2004; 27: 169–92. Rizzolatti G, Fabbri-Destro M. Mirror neurons: from discovery to autism. Exp Brain Res 2010; 200: 223–37. Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci 2010; 11: 264–74. Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 2004; 5: 813–9. Rudrauf D, Mehta S, Grabowski TJ. Disconnection’s renaissance takes shape: formal incorporation in group-level lesion studies. Cortex 2008a; 44: 1084–96. Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski TJ. Thresholding lesion overlap difference maps: application to categoryrelated naming and recognition deficits. Neuroimage 2008b; 41: 970–84. Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry 2000; 157: 2040–2. Sabbagh MA. Understanding orbitofrontal contributions to theory-ofmind reasoning: implications for autism. Brain Cogn 2004; 55: 209–19. Sarfati Y, Hardy-Bayle MC, Besche C, Widlocher D. Attribution of intentions to others in people with schizophrenia: a non-verbal exploration with comic strips. Schizophr Res 1997; 25: 199–209. Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind". Neuroimage 2003; 19: 1835–42. Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, et al. Introspective minds: using ALE meta–analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 2012; 7: e30920. Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn 2008; 17: 457–67. Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proc Natl Acad Sci USA 2010; 107: 9388–93. Schwartz MF, Kimberg DY, Walker GM, Brecher A, Faseyitan OK, Dell GS, et al. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proc Natl Acad Sci USA 2011; 108: 8520–4. Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009; 132(Pt 3): 617–27. Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol 2005; 18: 55–67. Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K. Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia 2009; 47: 2600–3. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default A dual-stream model of mentalizing mode: a quantitative meta-analysis. J Cogn Neurosci 2009; 21: 489–510. Spunt RP, Lieberman MD. An integrative model of the neural systems supporting the comprehension of observed emotional behavior. Neuroimage 2012; 59: 3050–9. Spunt RP, Lieberman MD. The Busy Social Brain: evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychol Sci 2013; 24: 80–6. Thiebaut de Schotten M, Kinkingnehun S, Delmaire C, Lehericy S, Duffau H, Thivard L, et al. Visualization of disconnection syndromes in humans. Cortex 2008; 44: 1097–103. Tuladhar AM, Snaphaan L, Shumskaya E, Rijpkema M, Fernandez G, Norris DG, et al. Default Mode Network Connectivity in Stroke Patients. PLoS One 2013; 8: e66556. Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci 2007; 11: 153–7. van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci 2008; 28: 10844–51. van Geemen K, Herbet G, Moritz-Gasser S, Duffau H. Limited plastic potential of the left ventral premotor cortex in speech articulation: Brain 2014: 137; 944–959 | 959 evidence From intraoperative awake mapping in glioma patients. Hum Brain Mapp 2013, in press. Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp 2009; 30: 829–58. Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 2009; 48: 564–84. Vistoli D, Brunet-Gouet E, Baup-Bobin E, Hardy-Bayle MC, Passerieux C. Anatomical and Temporal architecture of theory of mind: a MEG insight the early stages. Neuroimage 2011; 54: 1406–14. Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage 2006; 29: 90–8. Winner E, Brownell H, Happe F, Blum A, Pincus D. Distinguishing lies from jokes: theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain Lang 1998; 62: 89–106. Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci USA 2009; 106: 11382–7.