* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell type-specific activation of p38 MAPK in the brain regions of
Cognitive neuroscience of music wikipedia , lookup
Neurolinguistics wikipedia , lookup
Subventricular zone wikipedia , lookup
Brain morphometry wikipedia , lookup
Selfish brain theory wikipedia , lookup
Signal transduction wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neuropsychology wikipedia , lookup
Brain Rules wikipedia , lookup
Neurogenomics wikipedia , lookup
Human brain wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuroesthetics wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuroeconomics wikipedia , lookup
Haemodynamic response wikipedia , lookup
Limbic system wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Metastability in the brain wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuroanatomy wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
Optogenetics wikipedia , lookup
Aging brain wikipedia , lookup
Channelrhodopsin wikipedia , lookup
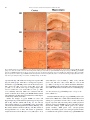
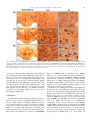
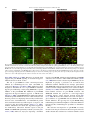
Neurochemistry International 51 (2007) 459–466 www.elsevier.com/locate/neuint Cell type-specific activation of p38 MAPK in the brain regions of hypoxic preconditioned mice Xiangning Bu a, Ping Huang a, Zhifeng Qi a, Nan Zhang a, Song Han a, Li Fang b,*, Junfa Li a,** a Institute for Biomedical Science of Pain, Beijing Key Laboratory for Neural Regeneration and Repairing, Department of Neurobiology, Capital Medical University, #10 You An Men Wai Xi Tou Tiao, Beijing 100069, China b Division of Neurosurgery, Department of Surgery, Neuroscience and Cell Biology, The University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555-0517, USA Received 23 December 2006; received in revised form 20 April 2007; accepted 25 April 2007 Available online 17 May 2007 Abstract Activation of p38 mitogen-activated protein kinase (p38 MAPK) has been implicated as a mechanism of ischemia/hypoxia-induced cerebral injury. The current study was designed to explore the involvement of p38 MAPK in the development of cerebral hypoxic preconditioning (HPC) by observing the changes in dual phosphorylation (p-p38 MAPK) at threonine180 and tyrosine182 sites, protein expression, and cellular distribution of p-p38 MAPK in the brain of HPC mice. We found that the p-p38 MAPK levels, not protein expression, increased significantly ( p < 0.05) in the regions of frontal cortex, hippocampus, and hypothalamus of mice in response to repetitive hypoxic exposure (H1–H6, n = 6 for each group) when compared to values of the control normoxic group (H0, n = 6) using Western blot analysis. Similar results were also confirmed by an immunostaining study of the p-p38 MAPK location in the frontal cortex, hippocampus, and hypothalamus of mice from HPC groups. To further define the cell type of p-p38 MAPK positive cells, we used a double-labeled immunofluorescent staining method to co-localize p-p38 MAPK with neurofilaments heavy chain (NF-H, neuron-specific marker), S100 (astrocyte-specific marker), and CD11b (microglia-specific maker), respectively. We found that the increased p-p38 MAPK occurred in microglia of cortex and hippocampus, as well as in neurons of hypothalamus of HPC mice. These results suggest that the cell type-specific activation of p38 MAPK in the specific brain regions might contribute to the development of cerebral HPC mechanism in mice. # 2007 Elsevier Ltd. All rights reserved. Keywords: P38 mitogen-activated protein kinases (p38 MAPK); Hypoxic preconditioning (HPC); Protein expression; Phosphorylation; Brain 1. Introduction It is well accepted that a sublethal ischemic/hypoxic exposure can improve the tissue or organ’s tolerance to a subsequent lethal ischemic/hypoxic insult; this protects cells against injuries and death, known as ischemic/hypoxic preconditioning (I/HPC) (Jones and Bergeron, 2004; Vannucci and Hagberg, 2004). Information regarding the signal transduction mediators of this phenomenon is complex and uncertain (Tsai et al., 2004). To investigate the molecular mechanism of cerebral I/HPC, we have developed a unique ‘‘auto-hypoxia’’-induced HPC mouse model that mimics clinical asphyxia (Li et al., 2005; Lu and Liu, 2001). * Corresponding author. Tel.: +1 409 772 2944; fax: +1 409 772 4687. ** Corresponding author. Tel.: +86 10 8391 1475; fax: +86 10 8391 1491. E-mail addresses: [email protected] (L. Fang), [email protected] (J. Li). 0197-0186/$ – see front matter # 2007 Elsevier Ltd. All rights reserved. doi:10.1016/j.neuint.2007.04.028 Using this model, we have demonstrated a series of protein kinases mediated the signalling cascades of cerebral HPC as follows: (1) increased membrane translocation of conventional protein kinase C bII and g (cPKCbII, g), novel protein kinase C e (nPKCe), and phosphorylation of its substrate, neurogranin (Li et al., 2005, 2006; Niu et al., 2005); (2) decreased phosphorylation levels and protein expression of extracellular signalregulated kinases 1/2 (ERK1/2) (Long et al., 2006); (3) enhanced phosphorylation of a nuclear transcriptional factor, cyclic AMP response element binding protein (CREB) (Gao et al., 2006). The p38 kinase is one of the mitogen-activated protein kinase (MAPK) family members that include extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAPK. They function as critical mediators of signal transduction pathways (Johnson and Lapadat, 2002). The activation of p38 MAPK requires dual phosphorylations of threonine 180 (Thr180) and tyrosine 182 (Tyr182) residues 460 X. Bu et al. / Neurochemistry International 51 (2007) 459–466 within the conserved TGY motif; this is reported to be involved in conveying extracellular stress to cellular response such as inflammation and the processes of cell differentiation, growth, and death (Widmann et al., 1999). Studies demonstrated that both global and focal cerebral ischemia caused neuronal death and activation of p38 MAPK in the brain of rat or gerbil (Irving et al., 2000; Piao et al., 2002; Sugino et al., 2000; Walton et al., 1998). Hypoxia increased cell death via a nPKCe-p38 MAPK pathway (Jung et al., 2004) and inhibition of p38 MAPK phosphorylation reduced cytotoxicity after hypoxia-reoxygenation procedures (Lee and Lo, 2003). In addition, inhibition of p38 MAPK activation during ischemia reduces injury and contributes to ischemia/reoxygenation preconditioninginduced cardioprotection in rat neonatal ventricular cardiocytes and isolated rat hearts, respectively (Marais et al., 2001; Saurin et al., 2000). However, little is known about the role of p38 MAPK in the development of cerebral HPC of mice in vivo. Both ERK1/2 and p38 MAPK pathways activate common transcription factors such as Ets-like transcription factor-1 (Elk-1) and CREB (Tan et al., 1996; Whitmarsh et al., 1997). However, we found that enhanced phosphorylation of CREB accompanied the decrease of ERK1/2 phosphorylation in the brain of HPC mice (Gao et al., 2006; Long et al., 2006). In this study, our goal was to investigate whether p38 MAPK was activated by observing the changes in phosphorylation and protein expression levels of p38 MAPK in the frontal cortex, hippocampus, and hypothalamus of mice following repetitive hypoxic exposure. 2. Experimental procedures 2.1. Materials Phosphatase inhibitors (okadaic acid, sodium pyrophosphate, and potassium fluoride), proteinase inhibitors (leupeptin, aprotinin, pepstatin A, and chymostatin), mouse monoclonal anti-b-actin antibody, and other reagents, such as EDTA, EGTA, SDS, dithiothreitol (DTT), and Nonidet P-40 were purchased from Sigma Company (St. Louis, MO, USA). Protein assay reagents, horseradish peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG, were purchased from Bio-Rad Company (Hercules, CA, USA). 2.2. ‘‘Auto-hypoxia’’-induced HPC mouse model Experiments were conducted on male BALB/c (Bagg albinos inbred ‘‘c’’ strain) mice at the age of 8–10 weeks (weighing 18–22 g) at room temperature (20–22 8C). The animal protocol was approved by the University Institutional Animal Care and Use Committee of Capital Medical University and is consistent with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23). ‘‘Auto-hypoxia’’-induced HPC mouse models that could mimic the clinical aspect of asphyxia were prepared as in our previous reports (Li et al., 2005; Lu and Liu, 2001). Briefly, mice were placed individually in a 125-ml airtight jar with fresh air and sealed with a rubber plug to mimic an environment of progressive auto-hypoxia. The mice were removed from the sealed jars as soon as the first gasping appeared, and tolerant time was recorded. A minimum of 30 min was allowed for recovery under normoxic conditions. Next, the mice were switched to another hermetically sealed jar with the same volume of fresh air after recovery from the previous hypoxic exposure. This procedure was repeated one to four times. It was subsequently designated as the normoxic control group (H0), which was kept in an open jar; hypoxic group (H1) was exposed to hypoxia once; and the repetitive hypoxic exposure became groups H2, H3, and H4. After four hypoxic exposures (H4), some mice were kept in the normoxic condition for 24 h for recovery before the fifth and sixth hypoxic exposures on the following day. Then, we made the experimental group of delayed HPC mice as group H5 and H6, respectively. At the end of the experiments, the mice were sacrificed. The frontal cortex, hippocampus, and hypothalamus regions of the brain were collected, frozen in liquid nitrogen, and kept frozen at 70 8C for later analysis. 2.3. Whole tissue homogenate preparation To determine the phosphorylation and protein expression levels of p38 MAPK, the frontal cortex, hippocampus, and hypothalamus were extracted as in our previous reports (Li et al., 2005, 2006). The whole tissues were homogenized at 4 8C in homogenizing buffer (50 mM Tris–Cl, pH 7.5, containing 2 mM DTT, 2 mM EDTA, 2 mM EGTA, 5 mg/ml each of leupeptin, aprotinin, pepstatin A, and chymostatin, 50 mM KF, 50 nM okadaic acid, 5 mM sodium pyrophosphate, and 2% SDS) and sonicated to dissolve the tissue completely. The protein amounts of samples were determined by BCA kit (Pierce Company, USA). 2.4. SDS–PAGE and Western blot analysis The phosphorylation and protein expression levels of p38 MAPK were analyzed by Western blot as reported previously (Long et al., 2006; Niu et al., 2005). Briefly, 50 mg of protein from the whole tissue homogenate of each sample was loaded in 10% SDS–PAGE gel. Then, the proteins were transferred onto the nitrocellulose membrane (NC membrane, Bio-Rad, USA) at 4 8C, 400 mA for 4 h. The transferred NC membrane was washed for 10 min with TTBS (20 mM Tris–Cl, pH 7.5, containing 0.15 M NaCl and 0.05% Tween 20) followed by the blocking solution with 10% nonfat milk in TTBS for 1 h. First, the blocked membrane was incubated with primary rabbit polyclonal antibodies against Thr180/Tyr182-phosphorylated p38 MAPK (p-p38 MAPK, Cell Signal Technology, USA) and total p38 MAPK (T-p38 MAPK), including phospho- and nonphospho-p38 MAPK (Santa Cruz Biotechnology Inc., USA) at a 1:1000 dilution for 4 h at room temperature, respectively. For the second antibodies, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad Company, USA) at a 1:5000 dilution for 2 h. The membranes were washed three times (each for 10 min) in TTBS after the incubation with the primary or secondary antibodies. Finally, immunoblotting signals were visualized using ECL-plus Kit (Chemicon International, USA). The ratio of p-p38 MAPK to T-p38 MAPK in H0 was recognized as 100%, and the values of groups H1–H6 were expressed as a percentage of the value of group H0. To analyze the protein expression levels of p38 MAPK, the expression of bactin was determined at the same time with the same protocol, and individual bands were quantified. Those membranes were blotted with antibodies against the T-p38 MAPK stripped using buffer containing 62.5 mM Tris–Cl, pH 6.7, 100 mM 2-mercaptoethanol, and 2% SDS, for 50 min at 55 8C following a period of constant shaking. Next, they were reprobed with primary mouse monoclonal antibody against b-actin (Sigma Company, USA) at a 1:1000 dilution for 3 h at room temperature. The value of the relative optical density of each band corresponding to T-p38 MAPK was normalized to the value of bactin to demonstrate protein expression level. The ratio of the values of relative units in group H0 was regarded as 100%, and data from groups H1–H6 were expressed as a percentage of the value of group H0. 2.5. Immunochemistry study Mice were anesthetized with 6% chloral hydrate (240 mg/kg, i.p.) and perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde. Brains were quickly removed and fixed in 4% paraformaldehyde for 24 h and cryoprotected with 20% sucrose solution. A 30-mm section of frozen mouse brain was placed in ice-cold 10 mM phosphate buffered saline (PBS). The slices were incubated with 3% H2O2 for 10 min to exhaust the endogenous peroxidase and washed in PBST (10 mM PBS, pH 7.4, containing 0.5% Triton X-100). Next, they were blocked with 10% goat serum for 1 h. The blocked slices were incubated with primary polyclonal antibodies against p-p38 MAPK at a 1:400 dilution for 12 h at 4 8C. The specimens were incubated in secondary antibodies X. Bu et al. / Neurochemistry International 51 (2007) 459–466 461 of biotinylated goat anti-rabbit IgG (ZYMED company, USA) at a 1:200 dilution for 2 h, and then were incubated with third antibodies of streptavidin-conjugated horseradish peroxidase (Sigma–Aldrich Company, USA) at a 1:200 for 2 h at room temperature. Finally, the slices were washed extensively in PBS, and a solution containing H2O2 (0.03%) and 3,30 -diaminobenzidine (DAB, 60%) was added to visualize the slices. The images were captured by a Leica microscope imaging system (Leica Company, Germany). For double-labeled immunofluorescent staining with p-p38 MAPK and neurofilaments heavy chain (NF-H, a neuron-specific maker), S100 (astrocytespecific marker), and CD11b (microglia-specific maker), sections were incubated with a mixture of primary rabbit polyclonal anti-p-p38 MAPK antibody with mouse monoclonal anti-NF-H antibody (Cell Sciences Company, USA), mouse monoclonal anti-S100 antibody (Chemicon International, USA), and mouse monoclonal anti-CD11b antibody (Chemicon International, USA) all at a 1:400 dilution overnight at 4 8C, respectively. Then, a mixture containing rhodamine-labeled anti-rabbit IgG at a 1:200 dilution (red color, Sigma Company, USA) and fluorescein isothiocyanate-labeled anti-mouse IgG at a 1:200 dilution (green color, Sigma Company, USA) was incubated for 30 min at 37 8C. The images were captured by fluorescent microscope (Leica Company, Germany). For negative control of the immunohistochemical staining (assessment of nonspecific immunostaining), the alternating sections from each experimental group were incubated with PBS without primary antibody. 2.6. Statistics analysis Images of immunostained sections captured by a Leica microscope imaging system were used for quantitative analysis. The number of immunoreactive cells was counted in a blinded fashion. Every second section was picked from a series of consecutive sections (30 mm), and six sections were counted for each region of the brain. We also performed a quantitative analysis for the immunoblot bands by using GelDoc-2000 Imaging System (Bio-Rad Company, USA). Presented values are expressed as means S.E. Statistical analysis was conducted by one-way analysis of variance followed by all pairwise multiple comparison procedures using the Bonferroni test. Significance was regarded as p < 0.05. 3. Results 3.1. Changes in phosphorylation and protein expression levels of p38 MAPK in the brain of HPC mice To determine the phosphorylation levels of p38 MAPK, the ratio of p-p38 MAPK to T-p38 MAPK in the frontal cortex, hippocampus, and hypothalamus of mice was quantified by using Western blot with primary antibodies against p-p38 MAPK and T-p38 MAPK, respectively. A typical result of Western blot in Fig. 1A and the quantitative analysis in Fig. 1B indicated that p38 MAPK phosphorylation levels increased significantly ( p < 0.05) in the frontal cortex, hippocampus, and hypothalamus regions of mice from the H2–H6 groups (n = 6 for each group) in comparison to the control group (H0 = 100%, n = 6, Fig. 1B). In contrast to p-p38 MAPK, we did not find significant changes in T-p38 MAPK protein expression levels in the frontal cortex, hippocampus, and hypothalamus of mice following repetitive hypoxic exposure (H1–H6 versus H0: 100%, n = 6 for each group) by using b-actin as internal reference (Fig. 1A, the negative data of quantitative analysis does not show). 3.2. Distribution of p-p38 MAPK in the brain of HPC mice To investigate the regional and cellular distribution of p-p38 MAPK in brain coronal slices of mice from H0, H3, and H6 Fig. 1. Increased phosphorylation of p38 MAPK in the brain of HPC mice. (A) Representative results of Western blot showed that the levels of p-p38 MAPK, not T-p38 MAPK, gradually increased in the frontal cortex, hippocampus, and hypothalamus of mice in response to repetitive hypoxic exposure (H1–H6). (B) Quantitative analysis indicated a significant increase of p-p38 MAPK in the frontal cortex and hippocampus of mice from H2–H6 groups, and in hypothalamus from H3–H6 groups (vs. H0: *p < 0.05, n = 6 for each group). H0: normoxic control; H1–H6: hypoxic exposure times; p-p38 MAPK: Thr180/ Tyr182-phosphorylated p38 MAPK; T-p38 MAPK: total protein of p38 MAPK including both phospho- and nonphospho-p38 MAPK. groups, we also performed immunostaining with primary rabbit polyclonal antibody against p-p38 MAPK. This method allowed us to visualize the phosphorylation levels as well as the localization of p-p38 MAPK inmmunoreactive cells in the brain regions affected by repetitive hypoxic exposure. Fig. 2 shows the immunostaining of p-p38 MAPK in cortex (Fig. 2A, B, and C) and hippocampus (Fig. 2D, E, and F) of mice from normoxic control H0 (Fig. 2A and D), three hypoxic 462 X. Bu et al. / Neurochemistry International 51 (2007) 459–466 Fig. 2. Immunostaining of p-p38 MAPK in the cortex and hippocampus of HPC mice. Brain slices (30 mm thickness) of frozen mouse brain from H0, H3, and H6 groups were cut in the coronal section and stained with primary rabbit polyclonal antibody against Thr180/Tyr182-phosphorylated p38 MAPK (p-p38 MAPK). Sparsely p-p38MAPK-immunostained cells were detected in both cortex (A) and hippocampus (D) of mice from the H0 group. Following three (H3) and six (H6) hypoxic exposures, the number of p-p38 MAPK immunostained glia-like cells increased significantly both in cortex (B and C) and hippocampus (E and F) of mice. Scale bars represent 50 mm in Fig. 3A, B, and C, and 100 mm in Fig. 3D, E, and F. exposures H3 (Fig. 2B and E) and six hypoxic exposures H6 (Fig. 2C and F) groups. After three or six hypoxic exposures, the number of p-p38 MAPK positive cells in the frontal cortex (H3: 43.0 3.6, H6: 35.3 0.9 versus H0: 18.1 2.0, p < 0.05, n = 3) and the CA1 region of hippocampus (H3: 32.3 2.1, H6: 34.1 9.4 versus H0: 9.3 4.2, p < 0.05, n = 3) markedly increased, which was consistent with the results of Western blots. Such p-p38 MAPK immunostained cells exhibited a glia-like phenotype with small, round-shaped, and intensely stained cell bodies. Unlike the changes of p-p38 MAPK in cortex and hippocampus, Fig. 3 indicated that repetitive hypoxic exposures (H3 in Fig. 3B, B1, and B2; H6 in Fig. 3C, C1, and C2) increased the numbers of p-p38 MAPK positive neuron-like cells in the dorsomedial hypothalamic nucleus (DM, Fig. 3A1, B1, and C1) and the zona incerta (ZI, Fig. 3A2, B2, and C2) of the hypothalamic area of mice. The results of quantitative analysis showed as follows: H3 (69.9 5.1), H6 (56.9 13.2) versus H0 (29.1 4.5) in DM ( p < 0.05, n = 6), and H3 (19.4 4.8), H6 (21.5 0.7) versus H0 (5.3 1.1) in ZI ( p < 0.05, n = 6). Fig. 3A, A1, and A2 showed the distribution of p-p38 MAPK positive neuron-like cells in the hypothalamic area—DM and ZI of mice from the normoxic control H0 group. 3.3. Determination of p-p38 MAPK positive cell type in the brain of HPC mice To further define the cell type of p-p38 MAPK positive cells in the brain of HPC mice, double-labeled immunofluorescent staining was performed on brain slices of mice after three hypoxic exposures (H3). We used primary rabbit polyclonal antibody against p-p38 MAPK (red color) and primary mouse monoclonal antibody against NF-H (green color, neuronspecific markers), S100 (green color, astrocyte-specific marker), and CD11b (green color, microglia-specific maker) to visualize the cell-specific localization of p-p38 MAPK in the X. Bu et al. / Neurochemistry International 51 (2007) 459–466 463 Fig. 3. Immunostaining of p-p38 MAPK in the hypothalamus regions of HPC mice. Brain slices (30 mm thickness) of frozen mouse brain from H0, H3, and H6 groups were cut in the coronal section and stained with primary rabbit polyclonal antibody against Thr180/Tyr182-phosphorylated p38 MAPK (p-p38 MAPK). The pp38 MAPK positive neuron-like cells increased in the dorsomedial hypothalamic nucleus (DM, 4A1, B1, and C1) and zona incerta (ZI, 4A2, B2, and C2) of mice following three (H3: 4B, B1, and B2) and six (H6: 4C, C1, and C2) hypoxic exposures, when compared to the normoxic control group (H0: 4A, A1, and A2). Scale bars represent 300 mm for A, B, and C, and 10 mm for A1–A2, B1–B2, and C1–C2. cortex (Fig. 4A, B, and C), hippocampus (Fig. 4D, E, and F) and dorsomedial hypothalamic nucleus (DM, Fig. 4G, H, and I) of H3 mice, respectively. From the merged images of Fig. 4C, F, and I (yellow color), we found that the p-p38 MAPK positive cells (Fig. 4A, D, and G, red) co-localized with the CD11b (Fig. 4B and E, green) not S100 (data not show) both in cortex and hippocampus, and the NF-H immunoreactive cells (Fig. 4H, green) in the DM area of mice from H3 group. This suggests that the increased p-p38 MAPK occurred in microglia of cortex and hippocampus as well as in neurons of hypothalamus of HPC mice. 4. Discussion Preconditioning with ischemia/hypoxia induces tolerance in the brain to protect against a subsequent lethal insult (Jones and Bergeron, 2004; Vannucci and Hagberg, 2004). For example, pre-treatment with hypoxia protects against injury caused by oxygen–glucose deprivation (OGD) in neuronal cell culture (Bruer et al., 1997). In adult rodent brain, a sublethal global ischemia insult protects against neuronal damage induced by a lethal insult and stimulates the activation of MAPK/ERK kinase 1/2 (MEK1/2) and its downstream target ERK1/2 (Shamloo et al., 1999). In addition, ischemic preconditionioning reduces the magnitude of MEK1/2, ERK1/2 and JNK activation that is normally observed after a lethal ischemic insult (Gu et al., 2001). Although the cellular mechanisms underlying cerebral HPC are still unclear, recent evidence suggests a role for the MAPK signaling pathways in the development of tolerance to brain injury. We used an in vivo HPC mouse model and found that the phosphorylation of p38 MAPK increased significantly in the frontal cortex, hippocampus, and hypothalamus regions of mice during the development of cerebral HPC both at the early and delayed phases. We also observed the activation of two substrates, CREB and mitogen- and stress-activated protein kinase 1 (MSK1) of p38 MAPK was involved in cerebral HPC (Gao et al., 2006). The activation of p38 MAPK may subsequently activate a group of substrates, such as MSK1, and then MSK1 phosphorylate the transcription factor CREB. CREB activation participated in the transcriptional activation of several immediate early genes, such as c-fos, junB, and egr1, and some antiapoptotic proteins expression requires the phosphorylation of CREB (Deak et al., 1998; Lonze and 464 X. Bu et al. / Neurochemistry International 51 (2007) 459–466 Fig. 4. Double-labeled immunofluorescent staining of p-p38 MAPK and CD11b or NF-H in different cerebral regions of HPC mice. Brain slices (30 mm thickness) of frozen H3 mouse brain were cut in the coronal section. We used primary rabbit polyclonal antibody against p-p38 MAPK (red color, A, D, and G), primary mouse monoclonal antibody against CD11b (green color, B and E) and neurofilament heavy chain (NF-H, green color, H) to visualize the cell-specific localization of p-p38 MAPK in the cortex (A and B), hippocampus (D and E), and dorsomedial hypothalamic nucleus (DM, G, and H) of H3 mice. From the merged images of C, F, and I (yellow color), we found that the p-p38 MAPK positive cells (red color, A, D, and G) co-localized with the CD11b and NF-H immunoreactive cells in the cortex (B, green color), hippocampus (E, green color) and DM area (H, green color) of mice from H3 group, respectively. Scale bars represent 20 mm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.) Ginty, 2002; Schiller et al., 2006). Therefore, our present study suggests that p38 MAPK signaling is involved in hypoxiainduced tolerance in brain of mice. The role of p38 MAPK in cardio- and neuroprotection induced by ischemia/hypoxia has been surrounded by controversy (Hausenloy and Yellon, 2006). Studies have been designed to determine the role of p38 MAPK in ischemic/ hypoxic preconditioning or injury using SB203580, a specific inhibitor of the p38 MAPK. The inhibition of p38 MAPK suppressed the hypoxia-induced apoptosis of HL-60 cells (Ikeda et al., 2006). p38 MAPK inhibition significantly reduced ischemia/reperfusion-induced elevation of left ventricular enddiastolic pressure accompanying decreased myocardial tumor necrosis factor, interleukin-1beta, and interleukin-6 protein levels, and reduced active myocardial caspase-1, caspase-3, and caspase-11 after myocardial ischemia (Wang et al., 2005). Cerebral endothelial cell death after hypoxia/reoxygenation was mediated by interactions between caspase-3 and p38 MAPK, and SB203580 significantly reduced cytotoxicity (Lee and Lo, 2003). Moreover, myocytes expressing a dominant negative p38a MAPK, which prevented ischemic p38 MAPK activation, were resistant to lethal simulated ischemia (Saurin et al., 2000). However, the activation of p38 MAPK may also be implicated in playing a protective role in neurons that underwent ischemic stimulation. For example, pretreatment with SB203580 could aggravate the infarction size of brain and cerebral vascular leakage induced by focal cerebral ischemia (Lennmyr et al., 2003). Limb ischemic preconditioning induced brain ischemic tolerance via p38 MAPK activation, and SB203580 blocked the protection to CA1 hippocampal pyramidal neurons against delayed neuronal death (Sun et al., 2006). However, these studies were lack of morphological evidences of p-p38MAPK and used different kind of models. Therefore, the opposing roles of p38 MAPK during ischemic/hypoxic stimulation might be a result of the difference in tissue-specific, ischemic/hypoxic procedures or stimulation intensity. The activation and expression of p38 MAPK are closely related to both the glia and neurons in brain regions. Previous studies showed that transient focal cerebral ischemia caused X. Bu et al. / Neurochemistry International 51 (2007) 459–466 intense immunoreactivity of p-p38 MAPK in astrocyte-like cells of the cortex, while increased p-p38 MAPK immunoreactivity was detected in neurons of Caudate nucleus (Irving et al., 2000). Using double-labeled immunofluorescent staining, activated p38 MAPK induced by permanent focal cerebral ischemia was identified both in neurons and in astrocytes of mice (Wu et al., 2000). In this study, we found that p-p38 MAPK localized differently with NF-H in both cortex and hippocampus, but colocalized with NF-H in DM area and CD11b (microglia-specific marker) in the cortex and hippocampus of HPC mice, respectively. This suggests that increased phosphorylation of p38 MAPK occurred in microglia of cortex and hippocampus as well as in neurons of hypothalamus of mice in response to repetitive hypoxia. Some studies showed that ischemia causes severe damages to the pyramidal neurons especially in the CA1 region of hippocampus. This kind of pyramidal cell death takes a delayed time-course, which called delayed neuronal death. This delayed neuronal death in the hippocampus appears to be accompanied by glial cell activation involving both astrocytes and microglia (Kirino, 1982). Activated microglia and astrocytes have been shown to release a variety of cytotoxic agents that lead to neuronal injury (Boje and Arora, 1992). Since the activation of p38 MAPK has been implicated in transcription of numerous genes involved in inflammatory processes, including proinflammatory cytokines (Beyaert et al., 1996; Matsumoto et al., 1998) or inducible nitric oxide synthase (Bhat et al., 1998; Da Silva et al., 1997), the induction of p-p38 MAPK in glial cells in the brain might be responsible for the synthesis of inflammatory mediators. In contrast, activation of p38 MAPK in neurons may be involvled in neuroprotection against ischemia. Isoflurane preconditioning induces the phosphorylation of p38 MAPK in neurons of different brain regions including cortex, hippocampus and subventricular zone (Zheng and Zuo, 2004). Our results showed that the activation of p38 MAPK was found in neurons in hypothalamus but not in cortex and hippocampus. The hypothalamus is considered a critical region of the brain for regulation of homeostatic process such as feeding and thermoregulation in the central nervous system (Schwartz et al., 2000). Studies have suggested that p38 MAPK was differentally activated in distinct regions of the hypothalamus depending on the condition of energy balance (Morikawa et al., 2004; Ueyama et al., 2004). However, the precise mechanisms of p38 MAPK phosphorylation in cortex, hippocampus, and hypothalamus regions remain unclear. We speculated that cell type-specific activation of p38 MAPK might attribute to the different cerebral regions, which have different sensitivity to hypoxia. In summary, our study demonstrated that the development of cerebral HPC is accompanied by increased phosphorylation of p38 MAPK without affecting its total protein expression levels in the frontal cortex, hippocampus, and hypothalamus of mice. These results are further supported by histological studies in which enhanced p38 MAPK phosphorylation in microglia and neurons occurred in the cortex, hippocampus, and hypothalamus of HPC mice, respectively. Further in vivo studies may be necessary to understand the role of p38 MAPK in neural protection during the development of cerebral HPC. 465 Acknowledgments This work was supported by the following grants: National Natural Science Foundation of China (30470650 and 30670782), Beijing Natural Science Foundation (5072008), China 973 program (2006CB504100), and NIH DE 15814. The authors thank Steve Schuenke for editorial assistance. Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neuint.2007.04.028. References Beyaert, R., Cuenda, A., Vanden Berghe, W., Plaisance, S., Lee, J.C., Haegeman, G., Cohen, P., Fiers, W., 1996. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 15, 1914–1923. Bhat, N.R., Zhang, P., Lee, J.C., Hogan, E.L., 1998. Extracellular signalregulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci. 18, 1633–1641. Boje, K.M., Arora, P.K., 1992. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 587, 250–256. Bruer, U., Weih, M.K., Isaev, N.K., Meisel, A., Ruscher, K., Bergk, A., Trendelenburg, G., Wiegand, F., Victorov, I.V., Dirnagl, U., 1997. Induction of tolerance in rat cortical neurons: hypoxic preconditioning. FEBS Lett. 414, 117–121. Da Silva, J., Pierrat, B., Mary, J.L., Lesslauer, W., 1997. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J. Biol. Chem. 272, 28373–28380. Deak, M., Clifton, A.D., Lucocq, L.M., Alessi, D.R., 1998. Mitogen- and stressactivated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426– 4441. Gao, Y., Gao, G., Long, C., Han, S., Zu, P., Fang, L., Li, J., 2006. Enhanced phosphorylation of cyclic AMP response element binding protein in the brain of mice following repetitive hypoxic exposure. Biochem. Biophys. Res. Commun. 340, 661–667. Gu, Z., Jiang, Q., Zhang, G., 2001. Extracellular signal-regulated kinase and c-Jun N-terminal protein kinase in ischemic tolerance. Neuroreport 12, 3487–3491. Hausenloy, D.J., Yellon, D.M., 2006. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc. Res. 70, 240–253. Ikeda, R., Che, X.F., Ushiyama, M., Yamaguchi, T., Okumura, H., Nakajima, Y., Takeda, Y., Shibayama, Y., Furukawa, T., Yamamoto, M., Haraguchi, M., Sumizawa, T., Yamada, K., Akiyama, S., 2006. 2-Deoxy-d-ribose inhibits hypoxia-induced apoptosis by suppressing the phosphorylation of p38 MAPK. Biochem. Biophys. Res. Commun. 342, 280–285. Irving, E.A., Barone, F.C., Reith, A.D., Hadingham, S.J., Parsons, A.A., 2000. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res. Mol. Brain Res. 77, 65–75. Johnson, G.L., Lapadat, R., 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912. Jones, N.M., Bergeron, M., 2004. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J. Neurochem. 89, 157–167. Jung, Y.S., Jung, Y.S., Kim, M.Y., Kim, E., 2004. Identification of caspaseindependent PKCe–JNK/p38 MAPK sigmaling module in response to metabolic inhibition in H9c2 Cells. Jpn. J. Physiol. 54, 23–29. Kirino, T., 1982. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 239, 57–69. 466 X. Bu et al. / Neurochemistry International 51 (2007) 459–466 Lee, S.R., Lo, E.H., 2003. Interactions between p38 mitogen-activated protein kinase and caspase-3 in cerebral endothelial cell death after hypoxiareoxygenation. Stroke 34, 2704–2709. Lennmyr, F., Ericsson, A., Gerwins, P., Ahlstrom, H., Terent, A., 2003. Increased brain injury and vascular leakage after pretreatment with p38-inhibitor SB203580 in transient ischemia. Acta Neurol. Scand. 108, 339–345. Li, J., Niu, C., Han, S., Zu, P., Li, H., Xu, Q., Fang, L., 2005. Identification of protein kinase C isoforms involved in cerebral hypoxic preconditioning of mice. Brain Res. 1060, 62–72. Li, J., Yang, C., Han, S., Zu, P., Wu, J., Xu, Q., Fang, L., 2006. Increased phosphorylation of neurogranin in the brain of hypoxic preconditioned mice. Neurosci. Lett. 391, 150–153. Long, C., Gao, Y., Gao, G., Han, S., Zu, P., Fang, L., Li, J., 2006. Decreased phosphorylation and protein expression of ERK1/2 in the brain of hypoxic preconditioned mice. Neurosci. Lett. 397, 307–312. Lonze, B.E., Ginty, D.D., 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. Lu, G.W., Liu, H.Y., 2001. Downregulation of nitric oxide in the brain of mice during their hypoxic preconditioning. J. Appl. Physiol. 91, 1193–1198. Marais, E., Genade, S., Huisamen, B., Strijdom, J.G., Moolman, J.A., Lochner, A., 2001. Activation of p38 MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. J. Mol. Cell Cardiol. 33, 769–778. Matsumoto, K., Hashimoto, S., Gon, Y., Nakayama, T., Horie, T., 1998. Proinflammatory cytokine-induced and chemical mediator-induced IL-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase-dependent pathway. J. Allergy Clin. Immunol. 101, 825–831. Morikawa, Y., Ueyama, E., Senba, E., 2004. Fasting-induced activation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus. J. Neuroendocrinol. 16, 105–112. Niu, C., Li, J., Cui, X., Han, S., Zu, P., Li, H., Xu, Q., 2005. Changes in cPKC isoform-specific membrane translocation and protein expression in the brain of hypoxic preconditioned mice. Neurosci. Lett. 384, 1–6. Piao, C.S., Che, Y., Han, P.L., Lee, J.K., 2002. Delayed and differential induction of p38 MAPK isoforms in microglia and astrocytes in the brain after transient global ischemia. Brain Res. Mol. Brain Res. 107, 137–144. Saurin, A.T., Martin, J.L., Heads, R.J., Foley, C., Mockridge, J.W., Wright, M.J., Wang, Y., Marber, M.S., 2000. The role of differential activation of p38mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 14, 2237–2246. Schiller, M., Bohm, M., Dennler, S., Ehrchen, J.M., Mauviel, A., 2006. Mitogenand stress-activated protein kinase 1 is critical for interleukin-1-induced, CREB-mediated, c-fos gene expression in keratinocytes. Oncogene 25, 4449–4457. Schwartz, M.W., Woods, S.C., Porte Jr., D., Seeley, R.J., Baskin, D.G., 2000. Central nervous system control of food intake. Nature 404, 661–671. Shamloo, M., Rytter, A., Wieloch, T., 1999. Activation of the extracellular signal-regulated protein kinase cascade in the hippocampal CA1 region in a rat model of global cerebral ischemic preconditioning. Neuroscience 93, 81–88. Sugino, T., Nozaki, K., Takagi, Y., Hattori, I., Hashimoto, N., Moriguchi, T., Nishida, E., 2000. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J. Neurosci. 20, 4506– 4514. Sun, X.C., Li, W.B., Li, Q.J., Zhang, M., Xian, X.H., Qi, J., Jin, R.L., Li, S.Q., 2006. Limb ischemic preconditioning induces brain ischemic tolerance via p38 MAPK. Brain Res. 1084, 165–174. Tan, Y., Rouse, J., Zhang, A., Cariati, S., Cohen, P., Comb, M.J., 1996. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 15, 4629–4642. Tsai, B.M., Wang, M., March, K.L., Turrentine, M.W., Brown, J.W., Meldrum, D.R., 2004. Preconditioning: evolution of basic mechanisms to potential therapeutic strategies. Shock 21, 195–209. Ueyama, E., Morikawa, Y., Yasuda, T., Senba, E., 2004. Attenuation of fasting-induced phosphorylation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus in response to refeeding. Neurosci. Lett. 371, 40–44. Vannucci, S.J., Hagberg, H., 2004. Hypoxia-ischemia in the immature brain. J. Exp. Biol. 207, 3149–3154. Walton, K.M., DiRocco, R., Bartlett, B.A., Koury, E., Marcy, V.R., Jarvis, B., Schaefer, E.M., Bhat, R.V., 1998. Activation of p38MAPK in microglia after ischemia. J. Neurochem. 70, 1764–1767. Wang, M., Tsai, B.M., Turrentine, M.W., Mahomed, Y., Brown, J.W., Meldrum, D.R., 2005. p38 mitogen activated protein kinase mediates both death signaling and functional depression in the heart. Ann. Thorac. Surg. 80, 2235–2241. Whitmarsh, A.J., Yang, S.H., Su, M.S., Sharrocks, A.D., Davis, R.J., 1997. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell Biol. 17, 2360–2371. Widmann, C., Gibson, S., Jarpe, M.B., Johnson, G.L., 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180. Wu, D.C., Ye, W., Che, X.M., Yang, G.Y., 2000. Activation of mitogenactivated protein kinases after permanent cerebral artery occlusion in mouse brain. J. Cereb. Blood Flow Metab. 20, 1320–1330. Zheng, S., Zuo, Z., 2004. Isoflurane preconditioning induces neuroprotection against ischemia via activation of P38 mitogen-activated protein kinases. Mol. Pharmacol. 65, 1172–1180.