* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Data Supplement
Protein moonlighting wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Immunoprecipitation wikipedia , lookup
Protein (nutrient) wikipedia , lookup
List of types of proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Proteolysis wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
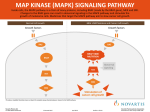
MS# 01-887R2 Increased Dietary Salt Activates Rat Aortic Endothelium Wei-Zhong Ying and Paul W. Sanders Nephrology Research and Training Center, Comprehensive Cancer Center, and Cell Adhesion and Matrix Research Center; Division of Nephrology, Department of Medicine, and Department of Physiology & Biophysics, University of Alabama at Birmingham, Birmingham, AL 35294-0007 and Department of Veterans Affairs Medical Center, Birmingham, AL 35233 Address correspondence to: Paul W. Sanders, M. D. Division of Nephrology/Department of Medicine 642 Lyons-Harrison Research Building 701 South 19th Street University of Alabama at Birmingham Birmingham, AL 35294-0007 Telephone: (205) 934-3589 FAX: (205) 975-6288 E-mail:[email protected] Ying and Sanders Page - 1 MS# 01-887R2 ONLINE METHODS Animal preparation Studies were conducted using 40 male Sprague-Dawley rats, 28 day of age, obtained from Charles River Laboratories (Wilmington, MA). Animals were chosen at this age because of our previous experience that showed normal renal function and blood pressure responses to dietary salt for up to two weeks of observation. 1 The rats were given 0.3% NaCl diet (AIN76A, Dyets, Inc., Bethlehem, PA) and water ad libitum for 4 d before initiating the experiment. The rats were then continued on a diet (AIN-76A, Dyets, Inc.) that contained 0.3%, 1.0%, 3.0%, or 8.0% NaCl. These formulated diets were identical in protein and electrolyte composition, except for NaCl content. On the fourth day of study, rats were anesthetized with pentobarbital sodium, 50 mg/kg, intraperitoneally. The aortas were perfused in situ with a cold isotonic heparinized perfusion solution that contained 90 mmol/L NaCl, 50 mmol/L sodium fluoride, 1 mmol/L Na3VO4, and 10 mmol/L sodium pyrophosphate. Fifty ml of solution was perfused over 2 min. In some experiments, five min prior to harvesting the aorta, a 1-ml bolus of either tetraethylammonium chloride (TEA) (Sigma Chemical Co., St. Louis, MO), 15 mmol/L in isotonic saline, or saline alone was injected intravenously in the tail vein over 5 minutes. This dose is less than the amount (2-6 mg/kg) administered intravenously to human volunteers with congestive heart failure; no toxic effects were reported in that study. 2 The aorta was perfused in situ with the perfusion solution that also contained 3 mmol/L TEA in those animals that received TEA intravenously. The aorta was harvested under sterile conditions. Ying and Sanders Page - 2 MS# 01-887R2 Western blot analysis (p38 MAPK, p42/44 MAPK, p46/54 JNK/SAPK, ATF-2, and Elk-1) Harvesting of aortic tissue, generation of protein lysates, and Western blotting proceeded as described previously, 3-5 with slight modifications. The lysis buffer contained sodium pyrophosphate, 2.5 mmol/L, and Na3VO4, 1 mmol/L. Protein concentration was determined using a kit (Micro BCA Protein Assay Reagent Kit, Pierce, Rockford, IL). Samples containing 60 µg of total protein were used in the immunoblot assays. The primary antibodies were used at 1:1000 dilution and recognized specifically total and phosphorylated forms of p38 MAPK, p42/44 MAPK and p46/54 JNK/SAPK (Cell Signaling Tech, Inc., Beverly, MA). Peroxidaseconjugated anti-IgG (Bio-Rad, Hercules, CA), 1:10,000 dilution, served as the secondary antibody. Bands were detected by ECL enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). Total and phosphorylated forms of the activating transcription factor, ATF-2, were detected using a kit (PhosphoPlus® ATF-2 (Thr71) Antibody Kit, Cell Signaling Tech.). The phospho-specific antibody recognized ATF-2 phosphorylated only at the threonine at position 71. Phosphorylated Elk-1 was detected using a monoclonal antibody directed against Elk-1 phosphorylated at the serine at position 383 (Phospho-Elk-1 (Ser383) 2B1 monoclonal antibody, Cell Signaling Tech.). Activity Assays for p38 MAPK and p42/44 MAPK Two hundred µl of aortic tissue lysate, 1 µg/µl, were incubated with 15 µl of suspended beads that bound anti-phospho-p42/44 MAPK (T202/Y204) or anti-phospho-p38 MAPK (T180/Y182) monoclonal antibodies, with gentle rocking overnight at 4ºC. Kinase assays were performed using 200 µmol/L ATP and 2 µg of substrate protein (ATF-2 or Elk-1 fusion protein). Substrate phosphorylation was detected and quantified using antibodies that specifically recognized phosphorylated ATF-2 or Elk-1. Ying and Sanders Page - 3 MS# 01-887R2 Immunohistochemical Staining for phospho-p38 MAPK and phospho-p42/44 MAPK Aortic tissue was isolated, fixed in phosphate-buffered 10% (vol/vol) formalin (Sigma Diagnostics, St. Louis, MO). Immunohistochemistry analysis proceeded in standard fashion, using affinity-purified rabbit polyclonal anti-phospho-p38 MAPK (1:25 dilution in TBS/Triton/BSA buffer) or anti-phospho-p42/44 MAPK (1:100 dilution in TBS/Triton/BSA buffer) (both from Cell Signaling Technology, Beverly, MA). Antibody binding was detected using a biotinylated anti-rabbit secondary antibody and avidin-biotin immunoperoxidase complex methodology (ABC-Vectastain Elite Kit, Vector Laboratories, Inc., Burlingame, CA). The slides were counterstained with hematoxylin. In vitro incubation studies Aortic tissue, which was harvested simultaneously from rats on the various NaCl diets, was cut into 2-3 mm ring segments. The samples were initially incubated with serum-free medium (RPMI 1640; Life Technologies, grand Island, NY) alone or medium that contained 50 µmol/L PD-098059 (2’-amino-3’-methoxyflavone), a potent and specific cell-permeable inhibitor of activation of MAPK kinase-1 (MEK1), 6 10 µmol/L SB-203580 (4-(4-fluorophenyl)2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole), a highly specific and cell-permeable inhibitor of p38 MAPK- 7, 8 or both inhibitors. The inhibitors (both from Calbiochem, San Diego, CA), were dissolved in dimethyl sulfoxide (DMSO) and were diluted in medium to a final concentration of 50 µmol/L immediately prior to use; the final concentration of DMSO was 0.1% (vol/vol). Control incubation medium contained 0.1% (vol/vol) DMSO without the inhibitors. After a 30-min incubation period, the medium was removed and refreshed with serum-free medium alone or medium that contained the same concentrations of PD-098059, SB203580 or both inhibitors. Incubation continued for 24 h at 37ºC. The medium was harvested Ying and Sanders Page - 4 MS# 01-887R2 and assayed for TGF-ß1 and NOx. Total and active levels of TGF-ß1 were determined by enzyme-linked immunoassay (TGF-ß1 Emax TM ImmunoAssay System, Promega Inc., Madison, WI), as performed previously. 3-5 Production of nitrite and nitrate was quantified in standard fashion using nitrate reductase and Griess reagent. 5, 9, 10 Experiments were also performed using aortic segments that had endothelium mechanically removed by gently rubbing the lumenal surface of each ring segment with a sterile wooden probe. 5 Statistical analysis Data were presented as mean ± standard error. Significant differences among data sets were determined using either unpaired t test or one-way analysis of variance using multiple comparisons by Fisher's protected least significant difference method, where appropriate. A P value less than 0.05 assigned statistical significance. REFERENCES 1. Chen PY, Sanders PW: L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest 1991;88:1559-1567 2. Relman AS, Epstein FH: Effect of tetraethylammonium on venous and arterial pressure in congestive heart failure. Proc Soc Exp Biol Med 1949;70:11-14 3. Ying W-Z, Sanders PW: Dietary salt modulates renal production of transforming growth factor-ß in rats. Am J Physiol 1998;274(Renal Physiol. 43):F635-F641 4. Ying W-Z, Sanders PW: Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-ß1. Am J Physiol 1998;275(Renal Physiol. 44):F18-F24 Ying and Sanders Page - 5 MS# 01-887R2 5. Ying W-Z, Sanders PW: Dietary salt increases endothelial nitric oxide synthase and TGF-ß1 in rat aortic endothelium. Am J Physiol 1999;277(Heart Circ. Physiol. 46):H1293-H1298 6. Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR: PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 1995;270:27489-27494 7. Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch RJ, Han J: Characterization of the structure and function of the fourth member of the p38 group mitogen-activated protein kinase, p38. J Biol Chem 1997;272:30122-30128 8. Gum RJ, McLaughlin MM, Kumar S, Wang Z, Bower MJ, Lee JC, Adams JL, Livi GP, Goldsmith EJ, Young PR: Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J Biol Chem 1998;273:15605-15610 9. Chen PY, Sanders PW: Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension 1993;22:812-818 10. Chen PY, Gladish RG, Sanders PW: Vascular smooth muscle nitric oxide synthase anomalies in Dahl/Rapp salt-sensitive rats. Hypertension 1998;31:918-924 Ying and Sanders Page - 6