* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neurotoxicology
Optogenetics wikipedia , lookup
Resting potential wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neuroscience in space wikipedia , lookup
Signal transduction wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Electrophysiology wikipedia , lookup
Biological neuron model wikipedia , lookup
Single-unit recording wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Action potential wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Synaptic gating wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neurotransmitter wikipedia , lookup
End-plate potential wikipedia , lookup
Nervous system network models wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Axon guidance wikipedia , lookup
Neuroregeneration wikipedia , lookup
Chemical synapse wikipedia , lookup
Neuroanatomy wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Synaptogenesis wikipedia , lookup
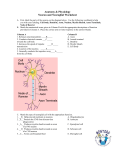
Introduction to Toxicology EV 460/660 & BI 460/660 Fall 2014 Toxic Effects on the Nervous System Brief Review of Nervous System Structure and Function 1. 2. 3. 4. 5. 6. Overview of nervous system, anecdotal comments Structural/functional divisions of nervous system -- central nervous system (CNS) vs. peripheral nervous system (PNS) -- sensory, integrative, motor systems -- somatic vs. visceral (autonomic – sympathetic and parasympathetic) Cell types of nervous system – neurons vs. glia Neurons -- structure – dendrites, soma (Nissl substance), axon (myelinated vs. non-myelinated), axon terminals, synapses -- resting membrane potential – Na+/K+ pump -- action potential -- Na+ and K+ voltage sensitive channels -- synaptic transmission, neurotransmitters (ACh, amines, amino acids, peptides), receptors, transmitter inactivation by enzymes and re-uptake, agonists & antagonists Glial cells -- astrocytes – blood-brain barrier, astrocytosis, phagocytosis, K+ buffering, transmitter metabolism, neuronal migration -- oligocytes – myelination in CNS -- Schwann cells – myelination in PNS -- microglia, ependyma cells Specialized sensory structures – eye and ear Toxic Effects on the Nervous System (neurotoxicity) 1. 2. 3. 4. 5. Direct vs. indirect effects on the nervous system Vulnerability of nervous system to toxic effects -- long pre- and postnatal developmental period, incomplete BBB and myelination at birth -- little or no regeneration of CNS -- high metabolic demands of CNS -- relatively large surface area and complex geometry of some cells -- high lipid content of some structures -- multiple potential mechanisms for toxic effects -- critical role in integration of bodily functions, multiple potential types of effects, subtle impairments Many plant and animal toxins developed as pharmacological agents and neurobiological tools Suffix “-opathy” General categories of toxic effects (see figure on back of Study Guide 3-3) -- neuronopathy -- axonopathy -- myelinopathy -- transmission toxicity – includes alterations in electrical conduction (membrane potentials and action potentials) and synaptic transmission Neuronopathies 1. 2. 3. 4. 5. Some toxicants specific for neurons, some of these target a particular group of neurons, the mechanism of specificity for a particular population of neurons is often not understood Neuron loss is irreversible, all parts of neuron degenerate, myelin sheath surrounding myelinated axon also degenerates Soma (cell body) is the target for the cytotoxic effects; there is a diversity of toxic mechanisms Some examples of toxicants inducing specific neuronopathies A. methylmercury (MeHg) – adult exposure -- neuron populations affected – small neurons of visual cortex and cerebellum -- multiple toxic mechanisms involved (may involve binding of MeHg to sulfhydryl groups) -- toxic mechanisms include impairments in glycolysis, nucleic acid synthesis, aerobic respiration, and protein synthesis) B. trimethyltin and triethyllead -- neuron populations affected -- limbic system (hippocampus, pyriform and entorhinal cortices) -- mechanisms are thought to be excitotoxic (excess excitation), energy deprivation, and cellular swelling C. manganese -- neuron populations affected – basal ganglia (striatum and globus pallidus) and cerebellum -- probable mechanism is manganese-catalyzed free radical oxidative damage Some examples of toxicant inducing encephalopathy (more diffuse and widespread neuronopathies) -- acute exposures to arsenic, lead, thallium, see also Casarett & Duoll Axonopathies 1. 2. 3. 4. 5. 6. 7. 8. Primary site of toxicity is the axon itself Axon degenerates as does the surrounding myelin sheath, however, cell body survives intact Has been termed “dying-back neuropathy”, but this is typically misleading (usually not begin at axon terminals and move toward the soma; rather the toxic effect results in a “chemical transection” of the axon and the axon distal to transection is functionally separated from the soma and degenerates Longer axons (more target area for toxic effects) are most vulnerable and most affected Initial signs and symptoms of axonopathy often show a “stocking-and-glove” distribution – e.g., impairment of motor and sensory function in feet and hands; effects may move more proximally depending on site of the toxic lesion Critical difference between axonopathies of CNS and PNS – PNS axons can regenerate (probably mediated by action of Schwann cells) while CNS axons cannot regenerate Large number of diverse toxicants can cause axonopathies (see Table 16-2, pages 641-642 of Casarett & Duoll) – toxic mechanism common to many is impairment of axonal transport processes Some examples of toxicants inducing axonopathies A. carbon disulfide -- toxic effect is due to blockage of axoplasmic transport by covalent cross-linking of neurofilaments within the axon -- toxic mechanism and pathological effect is similar to that of hexane-induced axonopathy; however, hexane requires metabolic activation to 2,5-hexanedione; CS2 does not require bioactivation B. acrylamide -- pathological effect resemble classic “dying back” pattern – e.g., begins at axon terminals and proceeds proximally -- multiple lines of evidence show impaired axonal transport, but exact mechanism not identified C. organophosphorus esters -- axonopathy-inducing mechanism is different than that of AChE inhibition (see section below on toxic effects on synaptic transmission) -- OPIDN (organophosphate-induced delayed neurotoxicity) signs and symptoms appear 7-10 days after exposure; damage to long, large diameter sensory and motor peripheral axons and in severe cases may involve spinal axons -- most serious incident involved TOCP (tri-ortho-cresyl phosphate) contamination of prohibitionera illicit beverage “Ginger Jake”, 20,000 individuals developed “Ginger Jake paralysis” -- very few organophosphate compounds used as pesticides produce axonopathy at acute doses that are survivable, exceptions are mipafox, leptophos, chlorpyrifos, and merphos -- mechanism is thought to involve inhibition of NTE (neuropathic target esterase) an enzyme with an unidentified role in neuronal lipid metabolism; however, some compounds that inhibit NTE do not result in OPIDN Myelinopathies 1. 2. 3. Three types of damage to myelin A. Myelin Swelling (AKA - intramyelinic edema) – splitting of the myelin lamella, reversible if early cessation of exposure to causative agent; if exposure continues will lead to primary demyelination B. Primary Demyelination – agent directly attacks myelin or the myelinating cell causing the loss of myelin content, myelin breakdown, and death of the myelin cell C. Secondary Demyelination – follows irreparable damage to the axon due to toxic axonopathies, degeneration and macrophage digestion of associated myelin Consequences of myelinopathies A. decreased conduction velocity of action potential, aberrant conduction, conduction block B. functional consequences depend upon extent and localization (CNS, PNS, or diffuse) of demyelination -- demyelination limited to PNS – peripheral neuropathy – frequently have remyelination by Schwann cells (though altered internodal distances) -- demyelination in CNS – little-to-no remyelination by oligocytes, persistent central deficits -- diffuse demyelination – more global neurological deficits, mixed pattern of recovery Some examples of toxicants inducing myelinopathies A. Hexachlorophene -- widely used in anti-bacterial soaps in the 60’s -- problems arose with bathing newborns, especially premature infants -- hydrophobic compound, high dermal absorption, enters both CNS & PNS -- resulted in intramyelinic edema in both CNS and PNS -- toxic mechanism thought to be uncoupling of oxidative phosphorylation, failure of membrane ion pumps and consequent edema -- severe cases – cerebral edema, increased intracranial pressure, seizures, coma, death B. Triethyltin -- anecdotal comments on organotin compounds, growing environmental concern -- highly lipophilic, (food chain concerns) -- results in intramyelinic edema, mechanism not clear C. Inorganic lead -- peripheral neuropathies typically associated with chronic, adult exposures -- motor nerves are most affected, wrist and ankle drop syndromes -- excess exposure produces both axonopathy and myelinopathy – may co-occur or may occur separately -- mechanism of myelinopathy is not clear Transmission Toxicity 1. 2. 3. Toxic effect may be on the axon or the synapse -- axon effects are due to interference with axonal ionic channels and do not result in axonopathies -- variety of mechanisms for effects on synaptic transmission Some examples of toxins/toxicants acting on axonal ionic channels A. DDT and DDT-like insecticides -- interfere with closure Na+ channels in axonal membrane, impair Na+ channel closure -- also thought to impair Na+/K+ - ATPase maintaining membrane potential B. Pyrethroid insecticides -- also interfere with closure of Na+ channels C. Toxins with specific actions on axonal Na+ channels -- have become useful tools in neurobiology research -- TTX and STX block opening of Na+ channels -- BTX enhanced opening of Na+ channels Mechanisms of action at the synapse -- variety of mechanisms for potential action of xenobiotics -- mechanism of action of several drugs of abuse – i.e., cocaine, opiates -- manipulation of these mechanisms is the current primary basis for much of neuropharmacology A. Mechanisms for alteration of synaptic transmission -- transmitter synthesis -- inhibition or stimulation of synthesis of neurotransmitter -- transmitter release – blockade or stimulation of transmitter release -- receptor interaction – postsynaptic receptor agonists and antagonists, autoreceptors -- transmitter inactivation – enzymatic degradation or reuptake inhibitors B. Some examples of toxins/toxicants acting at cholinergic synapses -- block of Ca2+ influx preventing exocytosis of vesicles-- -bungarotoxin -- block of ACh release – botulinum toxin -- stimulate ACh release/depletion – α-lactrotoxin -- ACh receptor agonists – nicotine & muscarine -- AChE inhibitors – OP and carbamate insecticides C. Some examples of toxins/toxicants acting at GABAergic and glycinergic synapses -- GABA receptor antagonists/blockers – picrotoxin, cyclodiene insecticides (dieldrin, aldrin), hexachlorocyclohexane, lindane, toxaphene -- glycine receptor antagonist/blocker - styrchnine -- tetanus toxin – antagonist for both glycine and GABA receptors