* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Heterochromatin-2015
Genome evolution wikipedia , lookup
Transposable element wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Genealogical DNA test wikipedia , lookup
Genomic imprinting wikipedia , lookup
United Kingdom National DNA Database wikipedia , lookup
DNA polymerase wikipedia , lookup
DNA damage theory of aging wikipedia , lookup
Genomic library wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Epitranscriptome wikipedia , lookup
Human genome wikipedia , lookup
Oncogenomics wikipedia , lookup
X-inactivation wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Histone acetyltransferase wikipedia , lookup
DNA vaccination wikipedia , lookup
Molecular cloning wikipedia , lookup
Nucleic acid double helix wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
DNA supercoil wikipedia , lookup
Transgenerational epigenetic inheritance wikipedia , lookup
Designer baby wikipedia , lookup
Neocentromere wikipedia , lookup
Epigenetic clock wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Point mutation wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Microevolution wikipedia , lookup
Genome editing wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
DNA methylation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Primary transcript wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Non-coding DNA wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Helitron (biology) wikipedia , lookup
Epigenetics wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
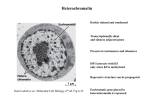
Heterochromatin Darkly stained and condensed Transcriptionally silent and silences adjacent genes Present at centromeres and telomeres HP1 interacts with H3 only when K9 is methylated Repressive structure can be propagated from Lodish et al., Molecular Cell Biology, 6th ed. Fig 6-33 Euchromatic gene placed in heterochromatin is repressed Histone Modifications Associated with Heterochromatin and Euchromatin from Lodish et al., Molecular Cell Biology, 6th ed. Fig 6-33 Initiation of Heterochromatin Assembly from Grewal and Gia, Nature Rev.Genet. 8, 35 (2007) Transcription factors and RNAi machinery bind to specific sequences or repetitive elements to recruit histone modifying enzymes Modified histones recruit HP1 HP1 recruits histone modifying enzymes to facilitate heterochromatin spread Boundary elements prevent further heterochromatin spread Mechanism of Heterochromatin Spreading HP1 binds to H3K9me3 HP1 recruits SUV39H1 methylase SUV39H1 methylates H3K9 on neighboring nucleosomes Heterochromatin spreading is restricted by boundary elements from Bannister et al., Nature 410, 120 (2001) H1 recruits Su(var)3-9 to heterochromatin resulting in transposon silencing Propagation of Heterochromatin from Maison and Almounzi, Nature Rev.Mol.Cell Biol. 5, 296 (2004) Passage of the replication fork releases parental modified nucleosomes Nucleosome binding sites are created by recruitment of CAF1 by PCNA CAF1-bound HP1 recruits Suv39h, Dnmt1, and HDAC Methylated histones provide new HP1 binding sites Structural RNA associates Heterochromatin Functions DNA or H3 methylation recruits adaptors such as HP1 Adaptors recruit effectors that are involved in chromosome segregation, gene silencing, transcriptional activation, and histone modification from Grewal and Gia, Nature Rev.Genet. 8, 35 (2007) Role of RNAi in Heterochromatin Formation in S. pombe dsRNA is transcribed from centromeric repeats or synthetic hairpin RNAs dsRNA is processed to siRNA siRNA promotes H3K9 methylation by Clr4 Methylated H3K9 recruits Swi6 to form silenced chromatin Transcription of the top strand of centromeric repeats is repressed Rdp1 activity ensures continuous dsRNA synthesis from Schramke and Allshire, Science 301, 1069 (2003) Recruitment of Clr4 by Swi6 chromatin leads to spread of heterochromatin Mechanism of Silencing at Telomeres Sir2 deacetylates histones Sir3,4 binds deacetylated histones and recruits additional Sir2 from Lodish et al., Molecular Cell Biology, 6th ed. Fig 7-35 CTCF Regulates Genome Architecture CTCF colocalizes with cohesin CTCF mediates long-range interactions between genomic sequences CTCF establishes domains in which genes are coregulated and targets regulatory sequences to their promoters from Ong and Corces, Nature Rev.Genet. 15, 234 (2014) Assays for Barrier Insulator Activity Barrier insulators protect transgene from position-effect silencing Functional borders may require several different mechanisms including CTCF binding The function of insulators is to maintain higher-order folding and establish topologically associating domains From Phillips-Cremins and Corces, Mol.Cell 50, 461 (2013) Assay for Enhancer-blocking Insulators Enhancer-blocking insulators block the action of distal enhancers Most CTCF binding sites do not act as enhancing-blocking insulators Enhancer-blocking may be more important in small genomes because of a greater need to regulate enhancer specificity From Phillips-Cremins and Corces, Mol.Cell 50, 461 (2013) gypsy Retrotransposon Contains an Insulator gypsy protects a transgene from position effects su(Hw) is necessary for enhancer blocking activity gypsy contains a su(Hw) binding site su(Hw) blocks the process that brings enhancer and promoter together Formation of insulator bodies at the nuclear periphery to divide the chromosome into looped domains Multiple su(Hw) binding sites can inhibit enhancer blocking activity Models for Heterochromatin Barrier Formation Stable block interrupts propagation of heterochromatin Active barrier recruits a complex containing chromatin remodeling activity from Donze and Kamakaka, BioEssays 24, 344 (2002) BRCA1 Modifies Pericentric Heterochromatin BRCA1 promotes enrichment of Ub-H2A in pericentric heterochromatin Loss of BRCA1 triggers transcription of satellite-DNA in pericentric heterochromatin Satellite-DNA transcription is sufficient to induce genome instability after loss of BRCA1 from Venkitaraman, Nature 477, 169 (2011) Epigenetics Heritable changes in gene function that cannot be explained by changes in gene sequences DNA methylation Histone variants and modifications Nucleosome positioning Epigenetic Modifications During Development Epigenetically imposed restrictions to plasticity are erased in the germ line Early mammalian development is characterized by progressive restriction of cellular plasticity accompanied by acquisition of epigenetic modifications Epigenetic modifications impose a cellular memory that accompanies and enables stable differentiation Epigenetic Modifications Within an Arabidopsis Chromosome Heterochromatin correlates with epigenetic marks from Zhang, Science 320, 489 (2008) DNA Methylation Methylation at CpG residues correlates with gene repression 5meC is involved in stable epigenetic repression Sites of methylation Inactive X Imprinted loci Transposon-derived sequences CpG islands are CpG-rich regions usually found at promoters Methylation patterns are reproduced at each round of cell division Methylated CpG Islands Inhibit Transcription from Portela and Esteller, Nature Biotechnol. 28, 1057 (2010) More than half of human promoters contain CpG islands Promoters are usually unmethylated Methylated DNA recruits methyl-CpG-binding domain proteins which recruit histone modifying and chromatin-remodelling complexes Unmethylated CpG islands recruit Cfp1 which associates with a histone methyltransferase creating H3K4me3 Methylation of Repetitive Sequences Stabilize Chromosomes from Portela and Esteller, Nature Biotechnol. 28, 1057 (2010) Unmethylated repetitive sequences cause reactivation of endoparasitic sequences RNA-dependent DNA Methylation in Plants from Matzge and Mosher, Nature Rev.Genet. 15, 394 (2014) Pol IV is recruited to chromatin and transcribes ssRNA which is converted to dsRNA by RDR2 siRNA is produced by DCL3 and loaded onto AGO4 Pol V transcribes a scaffold RNA that base pairs with AGP4-bound siRNA DNA is unwound and DRM2 is recruited and methylates DNA Histones are modified to reinforce the silenced state De Novo DNA Methylation in Mammals DNMT3L interacts with unmethylated H3K4 DNMT3A is recruited and activated and forms a tetrameric complex Active sites are separated by 8-10 bp and methylates opposite DNA strands from Law and Jacobsen, Nature Rev.Genet. 11, 204 (2010) Tetramer oligomerizes and results in 10 bp pattern of methylation on the same strand Establishment of DNA Methylation Pattern Most CpGs are unmethylated before implantation RNA pol II recruits H3K4 methyltransferase DNMT3L only binds unmethylated H3K4 and recruits DNA methyltransferases from Cedar and Bergman, Nature Rev.Genet. 10, 295 (2009) Propagation of DNA Methylation State Newly synthesized methylated DNA is hemimethylated NP95 binds hemimethylated DNA DNMT1 is a maintenance methyltransferase and binds PCNA NP95 links DNMT1 to hemimethylated DNA from Richly et al., BioEssays 32, 669 (2010) DNA is Demethylated by TET Proteins 5mC is oxidated iteratively by TET 5hmC is reverted to unmodified C by passive dilution during DNA replication Oxidative products are excised by thymine DNA glycosylase and repaired by BER from Kohli and Zhang, Nature 502, 472 (2013) Rett Syndrome Onset of symptoms in humans is 6-12 months of age Rett syndrome is a severe neurological disorder Abnormal head growth Decrease in speech function Breathing disturbances Repetitive hand movements Caused by mutation in the MeCP2 gene on the X chromosome MeCP2 Function and Rett Syndrome from Lyst and Bird, Nature Rev.Genet. 16, 261 (2015) MeCP2 binds methylated DNA, but also binds to unmethylated DNA via regions outside the MBD MeCP2 compacts chromatin structure MeCP2 recruits a corepressor complex that contains a histone deacetylase MeCP2 Regulates Gene Expression in Response to Neural Activity MeCP2 binds methylated DNA and silences target genes such as BDNF and corticotropin-releasing hormone Neural activity triggers MeCP2 phosphorylation and target gene activation from Bienvenu and Chelly, Nature Rev.Genet. 7, 415 (2006) Hippocampal neurons grow dendrites with fewer branches when MeCP2 is blocked Increase in mCH sequences after birth is enriched in genes with neuronal functions from Miller, Science 314, 1356 (2006) Many MeCP2-Repressed Genes Encode Proteins That Modulate Neuronal Physiology MeCP2 binds mCA Density of mCA is higher in long genes Frequency of mCA increases with age Long genes are selectively expressed in the brain Length- and mCA-dependent increase in gene expression in MeCP2 mutants from Gabel et al., Nature 522, 89 (2015)