* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Psilocybin Final Project-PDF
Functional magnetic resonance imaging wikipedia , lookup
Psychological effects of Internet use wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Human multitasking wikipedia , lookup
Nervous system network models wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neuroinformatics wikipedia , lookup
Human brain wikipedia , lookup
Neuroanatomy wikipedia , lookup
Selfish brain theory wikipedia , lookup
Neurolinguistics wikipedia , lookup
Biology of depression wikipedia , lookup
Brain morphometry wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Sports-related traumatic brain injury wikipedia , lookup
Neurotechnology wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuroplasticity wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brain Rules wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuropsychology wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Aging brain wikipedia , lookup
Time perception wikipedia , lookup
Metastability in the brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
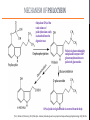
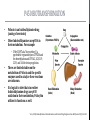
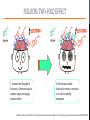
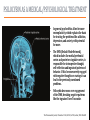
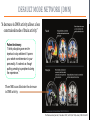
Psilocybin Jenna Hall Andres Garcia Azaria Brooks Nathan Brenner PSILOCYBIN Kekulé, skeletal formula of canonical Psilocybin • Psychoactive Alkaloid (amine-containing compound) • Found in 10 different genera of mushrooms • Frequently abused natural hallucinogen • Potential for Therapeutic Effects Tyls, F., Palenicek, T., Horacek, J. (2014) Psilocybin – Summary of knowledge and new perspectives European Neuropsychopharmacology 24(3) 342-356 Tyls, F., Palenicek, T., Horacek, J. (2014) Psilocybin – Summary of knowledge and new perspectives European Neuropsychopharmacology 24(3) 342-356 BRIEF HISTORY OF PSILOCYBIN • Ritualistic use of hallucinogenic mushrooms dates back 3000 years in Mexico A.D. 1960 1958 • Psilocybin was first isolated and identified by Albert Hofmann • Psilocybin was first synthesized by Albert Hofmann 1959 • Psilocybin widely used in the experimental research of mental disorders and psychotherapy 1990 • Psilocybin classified as a schedule I drug 1970 • Interest in human experimental research into Psilocybin and other psychedelics revived CHEMICAL STRUCTURES PSILOCYBIN IS RAPIDLY DEPHOSPHORYLATED TO PSILOCIN IN THE INTESTINAL MUCOSA BY ALKALINE PHOSPHATASE AND NONSPECIFIC ESTERASE. INGESTED MOLECULE FROM MUSHROOM METABOLIZED FORM (TOXIN) TORSTEN PASSIE, JUERGEN SEIFERT, UDO SCHNEIDER & HINDERK M. EMRICH. (2002, January 1). The pharmacology of psilocybin. Retrieved April 6, 2015, from http://www.maps.org/research-archive/w3pb/2002/2002_Passie_22704_1.pdf Mechanism of Action MECHANISM OF PSILOCYBIN Only about 50% of the total volume of psilocybin taken orally is absorbed from the digestive tract. Psilocin is glucuronidated by endoplasmic enzymes UDPglucuronosyltransferase to psilocin-O-glucuronide. 80% of psilocin-O-glucuronide is excreted from the body. Tyls, F., Palenicek, T., Horacek, J. (2014) Psilocybin – Summary of knowledge and new perspectives European Neuropsychopharmacology 24(3) 342-356 MECHANISM OF PSILOCIN Psilocin undergoes a demethylation and deamination to 4-hydroxyindol-3-yl-acetaldehyde (4-HIA). 4-HIA gets oxidized, believed to be by hepatic aldehyde dehydrogenase and monoamine oxidase, to 4-hydroxyindol-3acetic acid (4-HIAA) and 4-hydroxytryptofol (4-HT). However, only about 4% of psilocin gets degraded in this way. If dephosphorylation of Psilocybin is inhibited no psychotropic effects are perceived, thus Psilocin is the main active metabolite of Psilocybin. Another possible pathway for psilocin is oxidation by hydroxyindol oxidases to a product with an oquinone or iminochinon structure. Metabolism of Psilocin P450 BIOTRANSFORMATION • • Psilocin is an Indolealkylamine drug (analog of serotonin) Other Indolealklyamines use p450s in their metabolism. For example: • 5-MeO-DIPT aka “foxy methoxy” (a psychedelic tryptamine)uses CYP2D6 and for demethylation and CYP1A2, 2C8, 2C9, 2C19, and 3A4 for deisopropylation. • • There are limited studies on the metabolism of Psilocin and the specific enzymes used to catalyze these reactions are unknown. It is logical to infer that since other Indolealkylamine drugs use p450 oxidation in their metabolism, Psilocybin utilizes its functions as well. Yu, A. (2008) Indolealkylamines: Biotranformations and Potential Drug-Drug Interactions AAPS Journal 10(2) 242-253 PHARMACOKINETICS • The image on the left shows the biotransformation of Psilocybin to Psilocin and the four other metabolites • Psilocin is the main pharmacologically active substance. • Dephosphorylation of Psilocybin creates Psilocin. • Psilocybin is completely converted to Psilocin before entering the blood stream. • The first pass effect is significant, converting the majority of psilocybin to psilocin in hepatocytes • In humans, Psilocin was detectable in plasma at 20-40 minutes and had psychological effects at plasma levels of 4-6 mg/ml PHARMACOKINETICS Subjective impressions from individuals Plasma concentration after initial dose of 0.224 mg/kg These graphs show a correlation between the subjective effects and the plasma concentrations. The concentration and effects increase rapidly than plateau for almost an hour before decreasing. After 5-6 hours, the plasma Psilocin concentration will decrease to a level (in this case ~2 ng/ml) in which the effects are mostly gone. Desired effects also depend on individual susceptibility. Using the same dose as the last graph, this graph shows the excretion rate of Psilocin. After 3 hours, about twothirds of the toxin has been excreted by the kidneys. Mean elimination half-life of Psilocin is 50 minutes. ABSORPTION AND EXCRETION OF PSILOCYBIN ORAL: In humans, Psilocybin and psilocin can be found in blood Plasma 20–40 min after oral administration of psilocybin Maximum levels of psilocin are achieved between 80 and 105 min The half-life• ORAL INGESTION: 2.5 hours • INTRAVENOUS: 1.23 hours 80% of Psilocin in plasma was found to be in a conjugated form with glucoronic acid. Both are detectable in human urine • Effective Dose of oral Psilocybin is 0.045–0.429 mg/kg Effects of Psilocin TOXICITY OF PSILOCYBIN • • • No toxicity in heart or intestines (mice and rabbits) Not neurotoxic Fatalities associated with psilocybin ingestion have been described Therapeutic Index Comparison LD50/ED50 LSD A human would have to 19 g of the pure drug to bring about death Aspirin Vitamin A 641 Psilocybin 21 Nicotine 4816 • However the only victims were not linked to toxicity of psilocybin but due to suicide • 9637 199 Tyls, F., Palenicek, T., Horacek, J. (2014) Psilocybin – Summary of knowledge and new perspectives European Neuropsychopharmacology 24(3) 342-356 Symptoms No Effect Dizziness Heart Rate Weakness/ Drowsiness Body Temperature Tremor Ionic Balance Nausea/ Vomiting Blood Glucose Tendon Reflexes Cholesterol Levels Increase in levels of cortisol Plasma Concentration BIOCHEMICAL EFFECTS Similar to LSD: In theory, hypertension and tachycardia may occur in predisposed individuals. Extremely high doses of Psilocybin could cause coma, hyperthermia and respiratory failure (serotonin syndrome), however no cases have been documented. PSILOCYBIN IN YOUR BRAIN TWO-FOLD EFFECT SIMILAR CHEMICAL STRUCTURES • Target: serotonin receptors in the brain • Only 20% of the Psilocybin actually reaches the target • Effect: • Prevents reuptake of serotonin • Binds to serotonin receptors (due to similarity in chemical structures) Serotonin Psilosin Psilocybin PSILOCIN: TWO-FOLD EFFECT 1. Prevents the Reuptake of Serotonin = Serotonin stays in synapse longer, prolonging euphoric effects 2. Psilocin has a similar chemical structure to serotonin so it is able to amplify stimulation Moffit, M., & Brown, G. (2015, March 11). Your Brain On Shrooms. Retrieved April 24, 2015, from https://www.youtube.com/watch?v=F5kqThVON18 HOW DOES PSILOCYBIN WORK? Normal Neural Network Scientists suggest the brain may temporarily rearrange itself by inhibiting normal brain activity and immediately creating new, biologically stable brain connections Able to experience moments without any actual stimuli; difficulty in determining fantasy from reality Activation of the anterior cingulate cortex and hippocampus occurs- brain regions associated with dreaming Communication Between Brain Networks The dots and colors correspond to connection-rich networks Non-Psychedelic Compound • Well-ordered correlation state • Not much cross-linking between networks Psilocybin • Substantial increase in number of network cross-links • Newly wired neural pathways emerge Keim, B. (2014, October 13). Science Graphic of the Week: How Magic Mushrooms Rearrange Your Brain | WIRED. Retrieved April 24, 2015, from http://www.wired.com/2014/10/magic-mushroom-brain/ ACTION OF PSILOCYBIN ON THE BRAIN • A study by scientists at Johns Hopkins University found that a single experience with psilocybin in a controlled environment altered their personalities, making people more open to new experiences on a long term scale. • The Results: • Psilocybin never increased activity in the brain • Psilocybin only decreased activity in the thalamus • Knocking out this key hub with Psilocybin appears to allow information to travel more freely in the brain, probably explaining why people's imaginations become more vivid and animated and the world is experienced as unusual. Eduardo E. Icaza, M.D., George A. Mashour, M.D., Ph.D.; Altered States: Psychedelics and Anesthetics. Anesthesiology 2013;119(6):1255-1260. doi: 10.1097/01.anes.0000435635.42332.ee. PSILOCYBIN AND PSILOCIN SEROTONIN RECEPTORS Both psilocybin and psilocin have agonist activity on serotonin 5HT2A/C and 5HT1A receptors. PSILOCYBIN PSILOCIN Psilocybin interacts mainly with serotonergic neurotransmission Psilocin binds to many different receptors including dopamine in the following order: 5-HT1A, 5-HT1D, 5HT2A and 5-HT2C receptor subtypes. 5HT2B>5HT1D>D1>5HT1E>5 HT1A>5HT5A>5HT7>5HT6> D3>5HT2C>5HT1B>5HT2A. Passie, T., Seifert, J., Schneider, U. and Emrich, H. M. (2002), The pharmacology of psilocybin. Addiction Biology, 7: 357–364. doi: 10.1080/1355621021000005937 Future of Psilocybin PSILOCYBIN AS A MEDICAL/PSYCHOLOGICAL TREATMENT • In general psychedelics allow for more neuroplasticity, which explains the basis for treating the problems like addiction, depression, and anxiety, with potential for more. • The DMN (Default Mode Network), which includes the medial prefrontal cortex and posterior cingulate cortex, is responsible for introspective thought, self-reflection and ingrained patterns of behavior. If this becomes overly engaged with negative thoughts or cravings it can lead to the previously mentioned problems. • Psilocybin decreases over engagement of the DMN, breaking negative patterns like the ingrained ‘need’ to smoke. The Pharmaceutical Journal, 1 November 2014, Vol 293, No 7834, online | URI: 20066899 DEFAULT MODE NETWORK (DMN) “A decrease in DMN activity allows a less constrained mode of brain activity.” Patient testimony: “I think psilocybin gave me the impetus to stay abstinent. It opens up a whole new dimension to your personality. It is almost as though quitting smoking is peripheral during the experience.” These MRI scans illustrate the decrease in DMN activity. The Pharmaceutical Journal, 1 November 2014, Vol 293, No 7834, online | URI: 20066899 SCHEDULE 1 DRUG • Schedule 1 drugs are extremely regulated, making the process of researching them difficult, tedious and expensive. • Putting potentially useful compounds under this category is a huge disservice to health science research. • If the complete therapeutic effects of Psilocybin are to be harnessed and fully understood it is essential that the classification of this drug be reconsidered. The Pharmaceutical Journal, 1 November 2014, Vol 293, No 7834, online | URI: 20066899 REFERENCES [1] Eduardo E. Icaza, M.D., George A. Mashour, M.D., Ph.D.; Altered States: Psychedelics and Anesthetics. Anesthesiology 2013;119(6):1255-1260. doi: 10.1097/01.anes.0000435635.42332.ee. [2] Keim, B. (2014, October 13). Science Graphic of the Week: How Magic Mushrooms Rearrange Your Brain | WIRED. Retrieved April 24, 2015, from http://www.wired.com/2014/10/magic-mushroom-brain/ [3] Moffit, M., & Brown, G. (2015, March 11). Your Brain On Shrooms. Retrieved April 24, 2015, from https://www.youtube.com/watch?v=F5kqThVON18 [4] Passie, T., Seifert, J., Schneider, U. and Emrich, H. M. (2002), The pharmacology of psilocybin. Addiction Biology, 7: 357–364. doi: 10.1080/1355621021000005937 [5] The Pharmaceutical Journal, 1 November 2014, Vol 293, No 7834, online | URI: 20066899 [6] TORSTEN PASSIE, JUERGEN SEIFERT, UDO SCHNEIDER & HINDERK M. EMRICH. (2002, January 1). The pharmacology of psilocybin. Retrieved April 6, 2015, from http://www.maps.org/research-archive/w3pb/2002/2002_Passie_22704_1.pdf [7] Tyls, F., Palenicek, T., Horacek, J. (2014) Psilocybin – Summary of knowledge and new perspectives European Neuropsychopharmacology 24(3) 342-356 [8] Yu, A. (2008) Indolealkylamines: Biotranformations and Potential Drug-Drug Interactions AAPS Journal 10(2) 242-253