* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Morphological and F`unctional Identifications of Catfish Retinal
Response priming wikipedia , lookup
Neural modeling fields wikipedia , lookup
Signal transduction wikipedia , lookup
Metastability in the brain wikipedia , lookup
Axon guidance wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Electrophysiology wikipedia , lookup
Single-unit recording wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Subventricular zone wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Central pattern generator wikipedia , lookup
Multielectrode array wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural coding wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuroanatomy wikipedia , lookup
Nervous system network models wikipedia , lookup
Synaptic gating wikipedia , lookup
Optogenetics wikipedia , lookup
Efficient coding hypothesis wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Morphological
and F’unctional Identifications of
Catfish Retinal Neurons. III. Functional Identification
KEN-ICHI
NAKA,
Divisions of Applied
Pasadena, California
PANOS
2. MARMARELIS,
Science and Biology,
91109
TWO MAIN QUESTIONS arise naturally
CaZifornia
for
publication
April
8, 1974.
RAYMOND
Institute
Y. CHAN
of
Technology,
input does. In practice, of course, the system is tested with a great variety of inputs
(instead of all), because of the finite duration of the experiment,
and the resulting
functional characterization is a statistically
averaged one. Thus, in this sense, a whitenoise stimulus becomes the universal probe
for testing and identifying
a system functionally, and the resulting characterization
is global, as it describes the system over its
entire stimulus-response function space.
In spite of the power and generality of
the white-noise “universal” experiment, no
attempts except one (39) were made to
apply it to biological systems until recently,
when we made an extensive study on the
application
of the theory (properly formulated and extended) to the systematic description of the dynamic characteristics of
certain neuron chains in the vertebrate
retina (16, 18-21). In that series of studies
we concluded that the theory could be successfully applied to the functional identification of neuron chains and that the resulting characterizations could predict the nonlinear response of these neuron chains with
a good degree of accuracy. Inasmuch as the
major objective of these studies was the
exploration
of the potentialities
of the
“white-noise methods,” the biological scope
of these studies was limited. Accordingly,
the application of the methods was confined
to the horizontal
cell responses (a slow
potential) and the spike discharges of the
ganglion cells (a discrete signal), thus examining the two representative classesof neural
signals. Although
the great advantages of
the method, especially when applied to intracellular
recording, were recognized at
this earlier stage of the development,
we
also realized that for such a functional
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
in the
study of neural systems: “What does the
system do?” and its logical companion and
does the system do this?”
sequel, “How
Answering the first question involves the
ability to predict the system response to any
stimulus, and it is therefore usually carried
out through the performance
of suitable
stimulus-response experiments. As this endeavor is directed to the discovery of the
system function (in its processing of stimuli
signals into response signals), we term it
functional identification
of the system.
In this series of papers (parts I, II, and
III) we attempt to answer both of these
questions for the neural systems in the catfish retina. While part I (24) dealt with
answering the structural question and part
II (28) dealt with answering the functional
question by traditional
methods, part III
(this paper) concentrates on answering the
functional
question with the white-noise
analysis technique and with pooling the
results of parts I, II, and III for the comprehensive identification
of the vertebrate
retinal neurons.
Twenty years ago, approximately,
Wiener
(49) proposed that a nonlinear system could
be identified functionally
by stimulating it
with a Gaussian white-noise input, i.e., a
random signal containing
all frequencies
within the system bandwidth
with equal
power (and whose amplitude is distributed
in Gaussian fashion). Wiener’s proposal
was based on the idea that, since the principle of superposition
does not hold for
nonlinear
system, we would need to test
such a system exhaustively with all possible
inputs; this is exactly what a white-noise
Received
AND
WHITE-NOISE
identification
to be of much biological significance it had to be conducted together
with the structural
identification
of the
neural system. It is to this latter task that
part I (24) of this study is directed-structural characterization. Part II (28) attempts
to correlate these findings with traditionally
oriented functional studies.
In this paper (part III of the series) we
apply the nonlinear
analysis technique
(through
white-noise
stimulation
of the re-
Y3
it morphologically.
The best such example
is the argument presented to identify a class
of neurons as “amacrine cells” by observing
their responses (11, 12, 44, 47, 48).
We will conclude that I) the nonlinearanalysis technique can be applied successfully to the intracellular
responses of the
retinal neurons; 2) their nonlinear responses
can be predicted by a small set of characterizing kernels which may include, for some
neuron types, up to the third-order
nonlinear kernel; 3) identification
of the horizontal and bipolar cells (as classes of neurons) is straightforward;
4) it is not possible
to classify the neurons in the proximal
layers
into two distinct classes, namely,
amacrine and ganglion cells; and 5) instead,
we propose to classify them into three functional types, types N, C, and Y, which have
no strict correspondence (but rather a loose
one) to morphologically
established
classes.
Finally, in the APPENDIX
we attempt a preliminary classification analysis of the neurons in this vertebrate retina to assess the
objectivity and validity of the classification
we propose
in this paper.
METHODS
Experimental
The retina of the channel
catfish, Ictalurus
$~nctatus, in eye cup preparation,
was used for
the experiments.
The apparatus
used and the
general experimental
conditions
have been described previously
(18, 26, 27). In this experiment the moist oxygen was not supplied as in
the previous series but we did not find any
adverse effects (compare with results in part II
(28)). The optical bench used in previous experiments
(26) was modified
by placing glow
modulator
tubes (R-l l?llC, Sylvania Electric) in
appropriate
places in the apparatus. The glow
tubes were driven by amplifiers with large negative feedback in order to linearize
the current
through them. The spectral composition
of the
light produced by the glow modulators
remains
unchanged
for the range of intensities used in
the experiments
of this study (18).
The white-noise
signals were generated
digitally and converted into analog form by a D/A
converter and appropriate
low-pass filtering. Subsequently, these signals were stored on analog
magnetic tape to be used in the experiments,
to
modulate
the glow-tube
light sources. The resulting white-noise
signals have an amplitude
dynamic range of about 40 to 1 and a flat power
spectrum from 0 to 60 Hz. In the experiments
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
ceptive-field components) to the intracellular responses of the vertebrate neurons.
Following the white-noise test, dye is intracellularly
injected into the unit being
tested. The objective is to identify both
functionally
and structurally the classes of
neurons in the catfish retina. When applied
to intracellular
responses, the white-noiseanalysis technique has distinct advantages
in that a) it allows us to gather a large
amount of diverse stimulus-response data in
a short span of time; and b) unwanted contaminating
noise, such as may arise from
the electrode, can be eliminated (because of
the cross-correlating process employed (18,
21)). These are important
factors to be
considered when one tries to record intracellular responses from retinal neurons and,
at the same time, tries to establish the morphological type of the neuron through intracellular
dye injection.
Particularly,
in
this part, we will attempt to correlate the
functional characteristics of the neurons, as
derived from the nonlinear
white-noise
analysis, to their morphological
characteristics which we have described in parts I
and II: a series of kernels (18,2 1) will define
functionally the neurons, and a majority of
these neurons will also be identified morphologically by Procion dye injection.
The central theme of this paper is to
answer the question, “Can we reconcile the
morphological classification of neurons with
the classification of similar neurons based
on their functional
traits?” So far it has
been commonly assumed that there is a oneto-one correspondence between a morpholog
ical class of neurons and a characteristic class
of responses. That is, it is commonly asserted that a given class of morphologically
defined neurons gives rise to characteristic
responses or, conversely, that knowing the
response of a given neuron one can identify
ANALYSIS
94
NAKA,
MARMARELIS,
Analytical
For a time-invariant
system with input x(t)
and o utput y(t), both of which are functions of
SPOT
ANNULUS
FIELD
i
i
tc- 5.0+
FIG. 1. Sketches of the approximate
shape
size of the three standard stimulus* patterns
throughout
this experiment, spot, annulus, and
stimulations.
In two-input
experiments both
and annulus
of lights
were given together.
and
used
field
spot
CHAN
time t only, Wiener (49) showed that the relationship between y(t) and x(t) can be written as
a series
where {Gi} is a complete set of orthogonal
functionals with respect to a Gaussian white-noise
input x(t). The first four functionals
in the
series are:
G,P,~ WI = h,
G,[h,, x(t)] = j&(x)x@ - x)dt
0
h,(t,, t2)x(t - +(t
G,[h,, x(t)] = Jl
- Z2)dtIdtz
- P s”h2(t, z)dz
0
G&9 x(t)] = J 7 J h&9 X2’Z&q - zJx(t - z2)x
0
(t - t3)dtldt2dt3
- 31’7 h3(tl, z2, z2)x(t - QdTldt,
(2)
0
where P is the power level of the white-noise
stimulus; i.e., P = @,,(f) (where f is the frequency in hertz) and Q&(f) is the power spectrum of the stimulus white noise. In practice, of
course, Q,,(f) becomes less than P for frequencies much higher than the system bandwidth.
The system is characterized
functionally
by
the set of kernels {h,, h,(x), h2(t1, TV), h&r, z2,
.}. That is, if we have knowledge of these
kernel functions, we are able to describe quantitatively
the system response to any stimulus
x(t) by carrying out the integration
indicated by
equation 2 and summing. Each kernel is a symmetric function
of its arguments. Kernel ho, a
constant, indicates the DC response value to the
white-noise
stimulus signal and it plays a significant
role in the signal processing by the
retinal neurons, as we will see later. Kernel h,(z)
is the “impulse
response” if the system is approximated
as a linear system. That is, for example, if the system is linear, the response to
a brief flash of light (at the mean intensity level
of the white-noise signal) would be given exactly
by h,(z) as a function
of time t. Similarly,
the
nonlinear
kernels [h2(z1,z2), h&
z2, zs) . . .I
“crosstalk“
between difquantify
the nonlinear
ferent portions of the past history of the stimulus
as it affects the system response at the present;
i.e., how much the response to (two, three . . .)
$)’
l
l
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
described here, two light-stimulus
patterns were
used; a spot of light (0.30 mm in diameter)
placed at the center of the receptive field of the
neural unit under study, and a concentric
annulus of light (0.35-mm inner diameter and 5.0mm outer diameter). Three kinds of stimulation
(experiments)
were utilized: 1) the spot area and
stimulated by
annulus area were simultaneously
separate light sources whose intensities
were
modulated
by separate, statistically independent,
white-noise
signa ls; 2) the spot area alon .e was
stimulated
light signal while
bY a white-noise
the ann ulus area was kept at dark; and 3) the
annulus area alone was stimu lated bY a whitenoise light signal while the spot area-is kept at
dark. In addition,
in a particular
series of experiments, a single input “field”
(spatially uniform) stimulus, covering nearly two-thirds of the
entire retinal surface, was used (Fig. 1). In this
case, the maximum
intensity of the light input
was similar to that of the spot input and decreased by neutral-density
filters. In all two-input
experiments
the intensity of the annular
light
was dimmer by about 0.8 log units than the
spot light. In some experiments
the intensities
of two inputs were decreased by interposing
neutral-density
filters after the two
were
combined
in to one.
After the functional
identification
experiment
(through
white noise), Procion yellow dye (M4RAN) was injected
iontophoretically
into the
cell by pulses of current (about 10 nA and duty
cycle of 0.5) for 30 s. In the earlier part of these
experiments
the sections were made according to
the method described by Matsumoto
and Naka
(Z?), but later the flat-mount
method developed
and described in part II was used to detect injected neurons. All pictures of Procion neurons
shown in this paper were from radially or tangentially sectioned prepara tions.
AND
WHITE-NOISE
different
impulses deviates from the superimposed responses due to each impulse separately.
For example, for a second-order nonlinear system
(i.e., h, = 0, K > S), kernel h,(t, t - to) denotes
this deviation,
at time t, from linear superposition (for t > to) between an impulse input at
t = 0 and an impulse
at t = to. Thus this kernel, in some cases, can be interpreted
to signify
effects such as saturation, facilitation,
refractoriness, etc.
Lee and Schetzen (15) showed that the kernels
{hi} can be readily obtained through the use of
cross-correlation
techniques. Specifically, the n thorder kernel would be given by
l
.
.
on)
=
-
E
n!Pn
n-
-
1
m= 1
z
YW
K
G,Jh,,
0
x(t)]
x(t
-
01>
.
l
l
x(t
-
a,)
95
where Pa, Pu, are the power levels of (independent)
white-noise
inputs x(t) and u(t), respectively. Kernels h&tl,
h2e&l,
t2) and h2&r1,
zZ)
z2)
(we call them self-kernels) along with h,,(z) and
h&),
are symmetric functions
of their arguments, while h22u(tl,
h2,&,
t2) (we call a cross kernel)
is, in general, asymmetric with respect to its
arguments.
The
cross kernel
describes
the
(second order) nonlinear
interaction
of the two
inputs as it affects the system response, while the
self-kernels
describe the individual
nonlinear
contribution
of each input
to the response.
These kernels can be estimated through the use
of cross-correlation
techniques. They are given
by the following
equations, where z(t) is either
x(t) or u(t) and it has zero mean (18, 21):
>(3)
where x(t) is the Gaussian white-noise
stimulus,
y(t) is the corresponding
system response, P is
the stimulus power level, and E{.) signifies a
statistical average over the entire record length,
i.e., statistical “expected value” of the quantity
inside the brackets.
The method was extended
to systems with
more than one input and output (18, 21). This
extension
alleviates greatly some of the persistent difficulties
in dealing with neural systems,
such as the short lives of the experimental
preparations over which the identification
process
must be carried out. As an example, let us consider a system with two inputs, x(t) and u(t),
and one output y(t). The two inputs used for
the identification
are statistically
independent
Gaussian white-noise
processes. Then,
hO
= ECYW
h&j
= wP,)E~Ywt
h&l
h&!&$9 $1
$1 == (1
(1 /q2Y3
/q2Y3
- 41
[r(t)
[r(t)
- h()W - QP - $))
h&&p
02)
Because
=
(w&p{Yw(t
- o&G
- 62)) P-9
of the orthogonality
of the terms of
5 and the independence
of the inputs,
it is possible to describe the transfer characteristic due to each input separately. Thus, a twoinput identifying
experiment
provides the inr(t) = I?4 G,Kh>,~ w 491
(4) formation of two one-input experiments and, in
addition,
the information
about the interaction
7Z=0
between the two inputs. In consideration
that
where {h}, is the set of kernels of degree n.
experimental
life times are very limited in the
Terms of different degrees as well as those that
case of neural systems (in particular
in this
arise from each input exclusively
are mutually
study, where both intracellular
recording
and
orthogonal
and normalized.
The first three terms
dye injection
are done on the same unit) the
of this series are given by
two-input
characterization
through
white-noise
stimulation
is critically
efficient. Thus, the sysG,KhlO~x(t), WI = h,
tem response to two inputs can be “separated”
G,[{h},, x(t), u(t)] = 7 h,&)x(t - t)dt
into three components,
two of them each de0
scribing
the
effect
of
each
input to the response
00
and the third describing the interaction
between
+ J h,&)u(t - t)dt
0
the two inputs. This is easily seen from equation 4 and 5 as follows. Without
loss of generality we assume that inputs x(t) and u(t) have zero
from
means (i.e., these signals are measured
their average values). Then, if we set u(t) = 0
(i.e., u(t) is constant at its average value) we
- pm7 &(t, t)dt
easily
see from equations 4 and 5 that
0
equation
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
1
h&r1
ANAM&
NAKA,
96
MARMARELIS,
00
Y#)
= $h&)x(t
- tldz
0
Y,(9 = J h,&>u(t
0
+ s”s h2&1’
0
- ddt
QJP
- z2)dt,dt,
- Q+
- Pu 7 h2&,
z)dt
(8)
0
Subtracting
r,(t) and mu
from the total response r(t) we can obtain the interaction
signal
between inputs x(t) and u(t):
v,(t)
= r(t) -Y,(t)
-Y,(t)
which is a measure of the dynamic nonlinear
crosstalk between the two inputs x(t) and u(t)
(note that this term depends on the product of
x(t) and u(t)). In all the catfish two-input
(spot and concentric
annulus)
experiments
this
term was very small, signifying
that there is no
dynamic interaction
between these two inputs.
However, the self-kernels for each input (component)
were different
for one-input
experiments (in which the complementary
component
is totally absent) and two-input
experiments
(in
which the complementary
component
is present
but equal to a constant, unmodulated
DC value).
This indicates that there is interaction
between
the two receptive-field
components
(as excited
by the spot and annulus stimuli) but that this
interaction
takes place only for DC or very low
frequencies.
Computational
Prior to the execution
of a white-noise
experiments several preliminary
measurements and
analyses must be made in order to achieve an
optimal
functional
identification.
These include a) choice of the stimulus mean level and
amplitude
range, b) choice of the stimulus
white-noise
bandwidth
so as to minimize
undesirable
effects but still evaluate
the system
over its entire bandwidth,
c) measurement
of
the system “memory”
(settling time) to be used
in estimating the times up to which the system
kernels should be evaluated, d) the number of
terms (kernels) to be identified
for a desirable
accuracy of the system characterization,
e) the
length of the identifying
experiment
required
by the types of noise, the specific system features
(nonlinearities,
etc.), and other issues. All these
preliminary
steps have been described in detail
and the experimental
and analytical procedures
to deal with them established (18, 21). In addition, the effect on the kernel estimates of many
types of contaminating
noise as well as stimulus
deviations
from gaussianness and whiteness has
been analyzed. For the present studies, a series
of preliminary
experiments
and analyses were
made and these parameters
were settled and
fixed for all subsequent experiments.
The mean intensity
level in the two-input
experiments
was about 5 X 10-S pW/mm2 for
the spot input and 0.56 X 10-s pW/mrn” for the
annular input without attenuation.
In the oneinput experiments the mean intensity level without attenuation
was the same as the spot input.
Generally
the mean intensity
level of whitenoise inputs was attenuated
by interposing
the
appropriate
neutral density filter (Kodak type
M carbon), the value of which is given in the
text. Naturally
the depth of modulation
remained
constant throughout
the experiment.
The amplitude
range of the input in each case
was about 40 to 1. The white-noise
bandwidth
extended flat from DC to about 100 Hz. The
first-order
kernels h,(z) were measured up to
at least z = 0.4 s and the second-order
kernels
up to at least z1 = z2 = 0.32 s. For most neural
systems the kernels only up to and including
the second order were measured. For some neurons the third-order
(nonlinear)
kernels were
also measured, as they contributed
significantly
to the response. The length of the white-noise
experiments
varied, depending
on conditions,
from 10 to 50 s.
The data were initially
stored on magnetic
tape and subsequently
transmitted
through
a
special-purpose
multichannel
A/D
converter
onto the disc memory of a digital
computer
(IBM 370/ 135). The large spike discharges present in the recording
from ganglion
cells were
filtered out by selecting appropriate
low-pass
filters but, as will be seen from the computed
kernels, it was not possible to eliminate
completely
these spike components.
The signals
(white-noise
stimuli
and recorded
responses)
were sampled at 0.00% or 0.004-s intervals, depending
on the frequency-response
characteristics of each neural system. Subsequently,
the
stimulus-response
data were processed and the
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
where we have also subtracted
the average
value of the response (h,) from the data. Thus
we obtain by equation 7 the contribution
of input x(t) to the response when input u(t) is held
at zero (i.e., its constant average value). Similarly
we obtain the contribution
*of input u(t) to the
response
AND CHAN
WHITE-NOISE
system kernels were computed
following
procedures outlined
above (equations 3 and 6) and
discussed elsewhere (ref 18, Fig. 3).
Evaluation
of functional
identification
00
Y#) = J h,(dx(t - 4dz
0
is computed
(with x(t) the white-noise
signal
used in the experiment)
and its mean square
deviation
from the experimental
response is
measured and normalized
in the same units.
This number
gives a measure of goodness of
the linear representation
of the system as a percentage (since the error of the zeroth-order
model is 100 units). The smaller this number,
the better is the agreement. Subsequently,
the
second-order
nonlinear
response term
is computed
and the mean square deviation
from the experimental
response of this nonlinear
model
Y#)
+ Y#
is computed and normalized
in the same units.
Thus, a quantitative
measure of the system nonlinearity
is obtained
by comparing
this reduction in MSE with that due to the linear kernel
alone, in addition
to assessing the predictability
of the characterization
at each (linear,
nonlinear) stage.
97
A similar
process of MSE measurement
is
carried out for the case of a two-input
system.
In this case, in addition
to assessing the predictability
of the model, the MSE gives an
indication
of the relative contribution
of each
input
component.
For example,
with knowledge of MSE for the spot and annular inputs to
a bipolar cell, it is possible to assess the relative
contribution
of each input to the bipolar cell
responses (in percent).
This is, of course, in
reference to the MSE of the zeroth-order
model.
The MSE (or more exactly the difference
between the MSEs of the linear and nonlinear
models) is again used to indicate the degree of
nonlinearity
involved
in the generation
of responses from a given class of neurons.
The spectral density
functions
(or power
spectra) for the inputs and various responses
(linear, nonlinear,
experimental,
etc.) were computed by estimating
the autocorrelation
function and Fourier transforming
it following
the
various numerical procedures discussed in Blackman and Tukey (4). The spectral density function is a measure of the energy present at each
frequency (Hz) in the signal. The relationship
between input x(t) and output r(t) and transfer
function H(jj of a linear system is
where Q(fl
and a&f)
are the spectral density
functions of response r(t) and input x(t), respectively. Since, in our case, the input x(t) is broadband white noise, a,&)
= 1 over all frequencies of interest. Then,
and therefore
the power spectrum of the response is a direct measure of the linear system
transfer function.
Scaling of kernels
Due to computational
requirements
the input
and response signals were multiplied
by an arbitrary constant factor, thus scaling the kernel
amplitude by a certain constant. However, for a
given series of experiments
this multiplying
factor remains the same so that the relative contribution
from each input
component
(spot or
annulus) can be compared. We feel that in the
intracellular
recordings from smaller neurons in
the retina, the absolute amplitude
of the kernel
is a far less reliable
indicator
of the neuron
functional
characteristics
than, say, its latency or
peak response time. Nevertheless for the linear
neurons, the amplitude
of the kernels is such
that the response was calculated
to range between 5 and 15 mV, depending
on the class of
neurons and recording
conditions.
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
The “model”
responses (linear,
nonlinear,
due to a particular
input, etc.) were computed
by estimating
the integrals
depicted
by equations 4, 5, 7, 8, and 9 and using the measured
kernels. Wiener
(49) showed that two systems
are equivalent
if and only if they respond identically to white-noise
input. Consequently,
the
criterion
of “goodness”
of the functional
identification and predictability
of the measured kernels is how well the model response mimics the
actual experimental
response to the same whiteThis
comparison
of
the
noise
stimulus.
and functional
model
system
experimental
(manifested by the measured system kernels) is
carried out, in this study, by quantizing
the
agreement
in waveshape of the two responses
in terms of the mean square deviation. Consider,
for example, the case of a one-input
system. The
zero-order model (h,) is a constant equal to the
average value of the response over the entire
record. The mean square error (MSE) for this
model is computed and normalized
to 100 (arbitrary) units. Subsequently
the response, as predicted by h,(z), i.e.,
ANALYSIS
NAKA,
98
Definition
MARMARELIS,
of terms
For the efficient presentation
of the results
the f&lowing
notation and terms are used:
h,(t) Orh,
h,(t,* t2) Orh,
h,(t,, t2, t3>or h,
his* h2s
%ash2a
h2,/a
h la/s'
h2,/s
LM,,
LMa
NM,,
NM,
or
MR
monotonic
receptive
field
biphasic
receptive
field
CHAN
underdamped
overdamped
complementary
component
(of
receptive
field)
cutoff frequency
band
pass
low pass
high-frequency
asymptote
exhibiting
overshoots
or undershoots (in response)
exhibiting
no
overshoots
or
undershoots
(in response)
“other”
of two components
(e.g.,
complementary
component
of
spot is annulus)
frequency
at which
system
response
starts
to
attenuate
rapidly
system
response
attenuates
significantly
for both low and high
frequencies
sys tern response
attenuates
significantly
only
for
high
frequencies
and
remains
rather
unchanged
for low frequencies
rate
of attenuation
of system
response
for high frequencies
(in
dB/octave)
RESULTS
In this series of experiments, the vertebrate retinal neurons were functionally
identified through white-noise stimulation
and the subsequent estimation of a small set
of kernels for each neuron; in addition,
these neurons, in the majority of cases, were
also identified morphologically
through intracellular
dye injection.
To avoid any
possible bias from morphological
clues we
classified responses (neurons) based solely on
only)
functional
traits, such as waveform and
respectively,
nonlinear,
linear,
polarity
of
kernels,
frequency response and
model
responses
for spot
compower contribution
of each component,
ponent
(or annulus
component)
in two-input
(spot and annulus)
degree of nonlinearity
involved, and preby
experiments
as predicted
dictability of model responses. From these
h Is/a and LhIe/a
and h2s/al Or functional clues it was possible to classify
h
and [hl /s and h2a/J
about 75a/, of the neurons (responses), extl?%onlinear
qnteraction
model
cept the receptors, into five distinct types:
response
as predicted
by has in
a two-input
experiment
two of them were identified as the horiresponse in a onetotal model
zontal and bipolar cells while the remaining
or two-input
experiment
(i.e.,
three could not be correlated to well-desum
of
NM,/,,NM,,,,
and
fined morphological
types. To avoid any
N"aJ
mean
square
error
deviation
of
structural
implications
we will refer to
model
iesponse
(cf. section on
them simply as types N, C, and Y responses
evaluation
of functional
identi(neurons). All these three types were refication)
corded from neurons in the proximal parts
receptive
field of a neuron
for
of the retina.
which
a stimulus
anywhere
in
field
evokes
rethe receptive
As discussed in METHODS,
the functional
sponses
of same polarity
in cell
identification of each neuron by a two-input
potential
(e.g., receptive
field of
(spot and concentric annulus) white-noise
horizontal
cells)
experiment results in a set of six kernels for
receptive
field of a neuron
for
which
center
(spot)
and
surthis neuron: ho, h Is/a(t)9 h2s/a( h, t2)9 hla/f3(t)p
round
(annulus)
stimulation
h2a,s(tl, t2), and h,(tI,
tz>. These kernels
evoke responses
of opposite
poare
interpreted
as:
kernel
ho (the zerolarization
in cell potential
(e.g.,
order kernel) is simply the average value
receptive
field of bipolar
cells)
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
h Is/a
DC or zero-order
kernel
linear
or first-order
kernel
quadratic
or second-order
kernel
or second-order
nonlinear
kernel
cubic
or third-order
kernel
or
third-order
nonlinear
kernel
first- and second-order
kernels
in
one-input
experiments
with spot
only stimulus
first- and second-order
kernels
in
one-input
experiments
with
annulus
only stimulus
firstand
second-order
(self)
kernels
for spot component
in
two-input
experiments
and
second-order
(self)
firstkernels
for annular
component
in two-input
experiments
crosstalk
in two-input
experiments
representing
dynamic
interaction
between
two spot and
annulus
inputs
linear
model
response
(as preexdicted
by h, in one-input
periments)
(LM,
for spot only,
LM,
for annulus
only)
nonlinear
model
response
(as
predicted
by h, and h2) in oneinput
experiments
(NM,
for
annulus
spot
only,
NM, - for
AND
WHITE-NOISE
P-
h,,,,.,(t),
if linear
h,,,(t)
+bs/a
if nonlinear
(1,,1),
hrojs (11, if linear
-;1,AVERAGE
---m-e--t DARK
I
J
t-
h,/,(t)
+ b,,r(trt),
if nonlinear
FIG. 2.
Schematic
representation
of the two-input
white-noise
experiment
in which
the set of kernels
predicts
the r&ponse
of the system
to an impulse
input
which
is superposed
on the DC i .nput whose
amplitude
corresponds
to the average
mean intensity
input,
while
the 0th .er input
of the white-noise
is
held at a DC level which
corresponds
to the average
mean intensity
level of the other
white-noise
input.
99
indicates that there is interaction between
these two receptive-field components but
that this interaction
takes place only for
DC or very low-frequency
signals. Naturally, because of the limited duration of a
white-noise experiment
(and record) the
kernels do not reflect this very low-frequency behavior.
The average length of the white-noise
records analyzed in this experiment
were
15-25 s for horizontal, bipolar, and type N
neurons and 2045 s for types C and Y neurons. Although
longer records were desirable for a more accurate kernel computation, the need for performing three sets of
white-noise input experiments and injection
of the dye into the same neuron limited the
practical length of each white-noise experiment.
Horizontal
cells
In part I of this study (24) we showed
that there are three subclasses of horizontal
cells in the catfish retina: external, intermediate, and internal All these three subclasses of horizontal
cells produced slow,
hyperpolarizing
responses to photic stimuli.
Examples of Procion dye-injected horizontal
cells are shown in Fig. 3, in which the external and intermediate
horizontal
cells
are shown in radial sections and the internal cell in a semitangential
section. As
we have already mentioned in part I, the
three horizontal cells share morphological
features common to those found in other
fish (11, 14, 40).
In this part, the analysis will be limited
to the external and internal horizontal cells
because the frequency response of the intermediate horizontal
cell is so slow that
within the normal length of 15-25 s of the
white-noise test, the power content in the
low frequencies was so limited that this
neuron seemed to produce only sustained
(DC responses (cf. ref 14)). A typical response
of an internal horizontal cell to a two-input
(spot and concentric annulus), white-noise
stimulus is shown in Fig. 4 in which record
A is by a white-noise input of unit average
intensity (0 log), while record B is by a
similar input whose average intensity and
also depth of modulation
are decreased by
0.8 log units.
Some of the nonlinear characteristics of
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
(DC) of the response to the white-noise
stimuli. Kernel hlSia (the linear spot kernel
in the presence of an annular white-noise
input) is the impulse response of the best
linear system approximation
if an impulse
is delivered to the spot input while the
annular input is kept constant (unmodulated) at its average value, as shown schematically in the upper diagram in Fig. 2.
In other words, it denotes the linear responses of the neuron if a brief flash of light
is given on top of the average intensity of
the spot white-noise signal, while the annulus of light is kept at a constant value equal
to the average intensity of the annulus
white-noise signal. Similarly, the annular
first-order kernel is the best linear response
of the system to a brief flash of light superposed on the constant input in the presence
of similar spot input, as shown in the
lower diagram in Fig. 2.
The nonlinear crosstalk kernel, h,,, for
all the two-input (spot and concentric annulus) experiments in the catfish retina was
of the model
very small; i.e., improvement
performance by the interaction (model) response, NM,,, was always less than 3%.
This signifies that, for this stimulus configuration, there is no dynamic interaction because of changing signals between these two
receptive-field components as excited by the
spot and annulus of light. However, the
“self-kernels”
for each input were quite
different
for one-input
experiments
(in
which the complementary
input stimulus
is totally absent or kept at dark), and twoinput experiments
(in which the complementary input is present but equal to a
constant, unmodulated,
DC value). This
ANALYSIS
100
NRKA,
MARMARELIS,
the external and internal horizontal cells
have been described previously (19, 20).
There, it was shown that these cells are
fairly linear and act essentially as low-pass
filters. In this paper we describe the response characteristics of the horizontal cells
in order to facilitate a comparison with
similar responses from other types ot neu-
CHAN
rons in the retina. Specifically, tile analysis
has been pcr-lorrnctl
on all cells which were
identifietl
morphologically
through intracellular dye injection. As seen frorn records
in Fig. 4, the horizontal cell responses were
characterized by a large IX component on
which modulation
due Co white-noise was
superposed; the horizontal cells were responding mainly to the rnagnitutle and less
to the faster changes of the level of input
signal. As already noted, the horizontal
cells are essentially low-pass filter devices
wllicli detect the DC level of the input
signal. This characteristic of the horizontal
cells, together with the fact that they form
a monotonic receptive field, enabled Naka
and Kushton (30) to derive the log-stimulus
intensity versus resl)orise-;~~~l~~lit~~~fe
curves
(V-log I curve) and show that the relationship between these two quantities is the
tanh-log curve. ‘I‘his relationship has since
been found as a general stimulus-response
transfer characleristic in the horizontal and
receptor cells, which equally exhibited the
low-pass filter characteristics (1, 3, 6). However, as will be discussed lacer, any conclusion drawn from a similar analysis on
neuron responses exhibiting
a bandpass
frequency charactcrislic
(a transient response) must be interpreted carefully.
The four first-order
(linear) kernels
shown in Fig. 5 wcrc computed from the
data shown in Fig. 4 which were obtained
from an internal horizontal cell; curves 1
and 2 are hIr,:, and h,:,,, at 0 log mean intensity, while curves
3 and 4 arc the corresponding kernels from the same neuron at
-0.8 log units rnean intensity. From these
kernels we observe the following traits of
the horizontal cell responses: I) The annular
response component, as exhibited by lilt,,,,
is much larger than the spot component
L/w a fact which can be predicted from
the assumption that the horizontal cells
form a laminar layer (or S space) of low
intercellular
resistivity (17, 30, 37). However, the relative amplitude
of the spot.
component was larger in the external cells
than in the internal cells due to a difference
in spatial decay characteristics of the two
types of cells (17, 37). 2) The annular
kernels are slightly underdamped.
3) The
latency and peak response time become
shorter as the average intensity of the in-
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
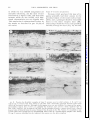
FIG. 3.
Procion
dye-injected
horizontal
cells seen
in the radially
sectioned
preparations.
A: external;
B: intermediate;
C: internal
horizontal
cells. In C
the section
was at some angle to the radial
plane to
show the larger
part of the cell. Responses
shown
in Fig. 4 were recorded
from
the cell shown
in C
in this figure.
R, receptors;
EH, external
horizontal
cells; IH, internal
horizontal
cells; and ISL, inner
synaptic
layer.
AND
WHITE-NOISE
ANALYSIS
101
put signal is increased; i.e., at higher intensities the response becomes faster. At 0 log
average intensity the latency was 25 ms and
the peak response time was 70 ms. At a
given intensity and under similar adaptation conditions
those parameters of the
horizontal cell h, were surprisingly consistent and they did not differ significantly
from cell to cell, a conclusion which is
consistent with our hypothesis that the horizontal cells form a laminar layer, a structure which
would
tend to minimize
individual
cell differences (see APPENDIX).
This observation is in contrast with the
responses from other types of cells which
showed a large variation in the response
parameters although all responses were recorded under similar experimental
conditions.
Two sets of horizontal
cell response
power spectra from the same unit are shown
in Fig. 6, one obtained at 0 log (A) and the
other at -0.8 log (I?) average intensity.
These spectra were calculated from the responses ihown in Fig. 4 and the kernels of
Fig. 5. In the figure are shown the power
Frequency
5. First-order
kernels
from
the horizontal
cell response
in Fig. 4. Trace
1 for h,s,a
and trace
* for hl,El are for 0 log unit, while trace 3 for h,s,a
and trace 4 for hra,s are for -0.8
long units mean
in tensi ty level.
Ordinates
are for volts/(photons/
mm2).
Upward
deflection
is for hyperpolarization
of
the membrane
potential.
The
amplitude
of the
kernels
with
log filters
was scaled
down
by the
value
corresponding
to the optical
density
of the
filter?
FIG.
(Hz)
FIG. 6.
Power
spectra
of the horizontal
cell responses shown
in Fig. 4. Power spectra
in A are for
the upper
record
A in Fig. 4, and power
spectra
in
B are for the lower
record
B in the figure.
Curves
are so scaled
that the power
level of the system
response,
R, in A is approximately
at 0 dB. R,
system
response;
MR,
model
response;
LM,
and
NM,,
linear
and nonlinear
model
responses
for the
annular
input;
and LMs and NMS, linear
and nonlinear
model
responses
for
the spot
input.
For
definition
of terms refer to the text,
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
FIG. 4. Oscilloscope
recording
of the response
from the internal
horizontal
cell to two-input
white-noise
stimulation.
Record
A was by 0 log unit and B by -0.8
log units average
mean intensity.
In this and all
subsequent
oscilloscope
recordings
the lower traces are the white-noise
signals for spot and annular
inputs.
Amplitude
of the response
in A was about 40 mV. Upward
deflection
is for hyperpolarization
of the membrane potential.
NAKA,
102
MARMARELIS,
spectra of a> the system experimental
response, R; b) the system model response,
MR, as predicted by hlB,B, h18,s, h2a,s, and
h 2s/a; c) the model response of the annular
component:linear
(LM,) by hl,,, and nonlinear (NM,) by hl,,, and haa,s; and d) the
model response of the spot components:
linear (LM,) by hl,,a and nonlinear NM,
by
b/a
and
h29/a.
CHAN
more direct experiments
turtle retina (3, 10).
Bipolar
reported
in the
cells
As already described in part II (28), the
catfish bipolar cells produce only slow potentials, an observation similar to that already made in other vertebrate retinas (11,
12, 22, 43, 46, 48). In contrast with the horizontal cells, which have a monotonic
receptive field, the catfish bipolar cells form
a field which is referred to as a biphasic receptive field; i.e., the spot and concentric _
annulus of light give rise to responses of
opposite polarity (part II).
Responses from two bipolar cells to twoinput white noise are shown in Fig. 7 in
which one produced a hyperpolarizing,
Bb
(A>, and the other a depolarizing response,
Ba (B). The former cell is apparently what
is known as an off-center bipolar cell and
the latter cell an on-center bipolar cell (11,
12, 43).
As in the horizontal
cells: bipolar cell
responses have a DC response component
on which modulation
due to the whitenoise input is superposed. In some bipolar
cells we observed a large on-transient due to
the response of the cell to a sudden increase
in the level of input, practically the same
on-response having been observed with a
step input. In others these initial transient
responses were less prominent
(Fig. 7A),
while in still others no such initial transient
l
FIG. 7.
2 set
I
Oscilloscope
recordings
of responses
from
two bipolar
cells to white-noise
inputs.
One off center
cell response
in A and the other
on-center
response
in B were recorded
from
the bipolar
cells
shown
in Fig. 1OF and H, respectively.
In B the
bandwidth
of the white-noise
inputs
is limited
to
10 Hz. The
amplitude
of the response
in both
A
and B is approximately
10 mV. Upward
deflection
is for hyperpolarization
of the membrane
potential.
Average
mean intensity,
-0.8
log units.
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
A decrease of the average mean intensity
level by 0.8 log units resulted in a decrease
of the response power level by 10 dB and
the cutoff frequency shifted from 12 to 8
Hz. At the same average mean intensity
level, the common response characteristics
as seen from these power spectra are that:
I) Agreement between experimental
and
model power spectra is extremely good
(MSE of about 10%) indicating the small
amount of noise present in the system. 2)
The system is a low-pass filter and has a highfrequency attenuation
at about 24 dB/octave of frequency; for the stimulus of less
than 3 Hz the system gain is almost constant. 3) The responses are dominated
by
the annular component
as seen by close
agreement between the power spectra of
the total response and those of the annular
component.
These observations, made on
the horizontal cell power spectra, augment
as well as confirm the similar observations
made on the system response (Fig. 4) and the
system kernels (Fig. 5).
Practically the same results have been
obtained from the external horizontal cells
even though the gain of the spot component increased in the presence of the annular
input as already described in a previous
paper. This analytical study suggests that
such an increase in the gain of the spot
component is due to an increase in the space
decay constant of the potential in the laminar structure formed by the external horizontal cells (17).
Another
feature of the horizontal
cell
response is the speeding up of the spot component in presence of the annular input
(20). We have hypothesized that this is due
to the feeding of the horizontal cell potential back to the receptors in order to improve the frequency response of these initial
stages in the processing of the visual signal.
This hypothesis is in accord with results of
AND
WHITE-NOISE
FIG. 8.
First-order
kernels
from
the bipolar
cell
which
is shown
in Fig. 1OC. One set of kernels
are
from
two-input
and the other
set are from
oneinput
white-noise
stimulation
performed
on the cell
under
the same experimental
conditions.
Trace
1
is h la,s; trace 2, h,,,,;
trace 3, hlB; and trace 4, h,,.
Average
mean intensity
level is - 0.8 log units.
All
four records
are scaled by the same factor
and ordinates are for volts/(photons/mm2).
Upward
deflection
is for
hyperpolarization
of the
membrane
potential.,
103
presence of a constant spot input). These
four kernels, obtained under the same experimental conditions, indicate the following characteristics of the catfish bipolar cell
responses: I) The hl’s are only slightly underdamped,
suggesting that bipolar cells,
like the horizontal cells, detect mainly the
level of the stimulus signal. 2) The annular
kernels (Fig. 8, traces 1 and 4) have both
longer latency (40 ms for annular response
versus 24-30 ms for spot response) and
longer peak response time (80 versus 70 ms).
3) In the presence of an annular input, both
the latency and peak response time of the
spot hI (Fig. 8, trace 2 versus trace 3) becomes shorter but no such speed up of the
annular kernel is observed in the presence
of the spot input. 4) The amplitude (and,
therefore, the dynamic gain) of both the
spot and annular kernels becomes larger in
the presence of the complementary member
of these two inputs (Fig. 8, trace 1 for annulus and trace 2 for spot). 5) The amplitude of the spot kernels (traces 2 and 3) is
comparable to that of the annular kernels
(traces 1 and 4) but the polarity is opposite,
an observation which is characteristic of
the bipolar cell kernels and which is in
sharp contrast with the horizontal
cell
kernels for which the amplitude of the spot
hl is much smaller than that of the annular
hl, but of the same polarity (Fig. 5).
The response power spectra of one of the
slower bipolar cells are shown in Fig. 9.
They are the power spectra of the experimental response (R), of the spot NM,,, and
annular NM,,, components in the two-input experiment and of the spot and annular
experimental
responses in the one-input
experiments. In a large number of spectra
obtained from bipolar cells we note the
following
frequency-response
characteristics of the bipolar cells: 1) Some have a
low-pass characteristic while others exhibit
a band-pass characteristic (corresponding,
respectively, to the “slow” and “fast” bipolar cells) but they do not segregate into
two well-defined groups. 2) Both the spot
and annular response components increase
their gain in the presence of the complementary member of these two inputs. 3) In
the presence of the annular input, the frequency response of the spot component
t
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
be observed (Fig. 7B). In some of the
published records from bipolar cells a similarly large transient response could be seen
(cf. Fig. 2, ref 43).
The response characteristics of the catfish bipolar cells are not at all consistent
and a considerable variability was observed:
some bipolar cells had very fast-frequency
responses (as judged from the latency of h1
and the cutoff frequency of their response
power spectra), while others were very slow.
Although the most obvious explanation
is
that the former responses are from the
smaller (cone) bipolar cells and the latter
ones are from the larger (rod) bipolar cells,
the dye-injection results could not confirm
or deny this possibility.
In part II (28) we have shown that all
the catfish bipolar cells had a biphasic receptive-field organization in which a spot of
light and concentric annulus of light produced responses of opposing polarity. It
further was observed that when the two
inputs were given together, the DC response
level was set somewhere between the two
DC response levels produced either by a
spot or by an annulus of light alone (part
II, Fig. 1C). Toyoda (43) reported a similar
observation in a teleost retina, that of the
carp.
The first-order (linear) kernels of a bipolar cell, as evaluated from these stimulusresponse data, are shown in Fig. 8. They
are: h,, (spot stimulus alone), hl, (annulus
stimulus alone), hlgia (spot component in
the presence of a constant, DC, annular input), and l&/s (annular component in the
could
ANALYSIS
NAKA,
FIG. 9.
responses.
are shown
Hz
Power
spectra for the bipolar
cell
Power
sp&tra
of the white-n&se
inputs
as annular
and spot and are flat up to
5o IGo Itspot + annulus’ Rspor and RanIlulus
indicate
the system
responses
to three
stimulus
modes,
spot
and annulus
&en
together
(two input)
or spot or
annulus
given
alone
(one input).
NMa
and NM8
responses
for the annular
and
are nonlinear
model
spot inputs
in two-input
experiments.
Notice
the
appreciable
increase
in the power
levels of the spot
and annular
responses
in two-input
experiments
compared
with the power levels of the two responses
in one-input
experiments.
For definition
of terms
see the tekt. Curies
are all scaled by the same factor
so that the power
level for the two-input
system
response
is close to 0 dB.
shows an im provemen t; a similar effect is
not observed for the annular corn ponent.
@Ve have already reached the same conclusion by observing the first-order kernels.)
4) In the particular bipolar cells analyzed
in Fig. 9 the difference in the power levels
of the spot and annular components
is
about 4 dB at 5 Hz, while in the horizontal
cells the corresponding difference is about
12 dB. 5) The bipolar response power
spectra have a steep asymptote (about 2430 dB/octave) and no high-frequency
component is present.
Of the observations made above the most
interesting is the increase in the dynamic
gain of each component
response in the
presence of the complementary
member of
the two-input stimulus; no other types of
retinal neurons showed such a mutual enhancement of the two components. In the
horizontal cells, Marmarelis and Naka (17)
have observed an increase in the gain of the
spot component in the presence of an annular input, but not vice versa, and in
other neurons, as will be described later,
mutual depression was commonly observed.
AND
CHAN
In part II (28) we showed that, in the
catfish bipolar cells, the step responses to
(low intensity) spot and annulus inputs
had opposite polarities and that when two
inputs were given simultaneously,
the DC
level of the resulting response was settled
somewhere between the two opposi te-polarity DC levels resulting from stimulation
by each input alone (part II, Fig. 1C). In
interpreting
the self-kernels (hl,/B, hl,,,,
etc.) of the two-input
white-noise experiments we noted that the complementary
input (e.g., the spot for hl,,,) can be treated
(as seen from the other input) as a DC input
whose amplitude
is the average mean intensity level of this particular input, a fact
illustrated in Fig. 2. This is a direct consequence of the orthogonality
of the response
terms arising from the two inputs. For example, hl,,, is the annular (linear) kernel
describing the dynamics of the annulus contribution
to the response resulting from
modulation
at the mean intensity level of
the annular white-noise signal, while the
input to the spot is a constant (DC) light
equal to the mean intensity of the spot
white-noise signal. Thus, in the bipolar
cells, the effect of the complementary
input
(due to the presence of white-noise stimulation) in the two-input experiments, is to
bring the DC level of the cell potential
closer to the resting (dark) level. We also
note that the bipolar cell response to a
spot or an annulus of light, when given
alone, has a very small dynamic range and
the response shows an amplitude saturation
even with a small increase in the stimulus
intensity (13, 45, 46). The increase of the
dynamic response gain in the two-input experiments can best be interpreted as due to
a shift of the operating point (DC response
level) of the bipolar cell from points near
the saturation level back toward the middle
of the range (near the dark level).
By analyzing the ganglion cell discharges
resulting
from
extrinsic polarization of the
horizontal cells, Naka and Nye (27) and
Naka and Witkovsky (31) have concluded
that both catfish and dogfish bipolar cells
must be comparing two signals, a local signal coming from the spot (center of receptive field) and an integrating signal coming
from the annulus (surround of receptive
field). Similarly, Marmarelis and Naka (17)
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
Frequency,
MARMARELIS,
IVHITE-NOISE
105
teristic of the bipolar cells With their dendrites in the outer synaptic layer and with
their axons in the inner synaptic layer. We
also note that some of the bipolar cells
have their smaller and round somata in the
proximal layer of the inner nuclear layer
while some others had their larger vaseshaped somata in the proximal layer of the
inner nuclear layer. So far, we have not
been able to correlate such morphological
subclasses to functional subclasses.
The results obtained from the bipolar
cells lead us to conclude that those neurons
with biphasic receptive-field organizations
FIG. 10. Examples of Procion dye-injected bipolar cells on which white-noise analysiswas performed.
All neurons are seen in the radial section. In E, a receptor, probably a cone, is also stained although injection
was limited
to the bipolar
cell.
Letters
D and
A are for
dendrites
and
axons.
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
have concluded from white-noise analysis of
the horizontal and ganglion (spike) cell responses that the following relationship must
exist: (bipolar cell response) E (horizontal
cell input) - (receptor cell input). Thus,
the present results of the nonlinear analysis
on the bipolar cells give further and more
direct evidence to support this conclusion
drawn in previous studies.
Examples of Procion dye-injected neurons
of the type classified functionally as bipolar
cells (from the functional traits described
above) are shown in Fig. 10. We note that
these neurons exhibit a geometry charac-
ANALYSIS
106
NAKA,
MARMARELIS,
in which the two subfield components are
mutually enhancing, are the class of ncurons known as bipolar cells, and that those
neurons which do not exhibit such functional characteristics are not bipolar cells.
This conclusion is substantiated further by
the results we described in part II (28) of
this series.
AND
CI-TAN
Neurons whicll l~orlucetl this type of rcsponse generally had their somata in the
proximal region of the inner nuclear layer
(INL) and a principal dendrite descended
down lo the inner synaptic layer (ISL)
where longer dendrites were seen spreading
laterally through the layer (Fig. 11). In some
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
11~. 11, Protion
tl!e-idcntilictl
cvamplcs
of type N ncuxon~
scrn in radial
srctions.
A, IS, and C are
typical
type N neurons
with their
somata
in the INL with
a descending
principal
dcndritc
from
which
$plcatl
the holirontal
dcndritcs.
Although
the flat-mount
views arc not axailablr,
the shapes of the principal dendrites
suggest
that A and C arc probably
the starbmst
type\
and B, a thick
spaghetti
type in
part I (24). Neuron
I; has its soma in the INL,
but its principal
tlcndritc
i? much
thinner
and a lateral
process
takes off directly
from the soma. Neuron
D has its soma in the ISI,. Neuron
I; is classified
as type
N from white-noise
analysis
but its morphological
trait is that of a spindle-shaped
ganglion
cell. Among
this nemon
‘L\BS the only cxccption.
25 type N nrulons
identified
by dye injection,
WHITE-NOISE
B
Oscilloscope
recordings
of
responses
FIG. 12.
from
types Na, shown
in A, and Nb, shown
in B,
neurons
to
two-input
white-noise
stimulation.
Amplitudes
of both responses
are about
15 mV. Upward deflection
is for hyperpolarization
of the membrane potential.
Average
mean intensity,
0 log unit.
107
,
0.1 set
,
FIG. 13.
First-order
kernels
from
type Na shown
in A and Nb shown
in B neurons
which
are shown
in Fig. 11B and F, respectively.
Two sets of kernels,
one set from
two-input
experiment
and the other
set from
two one-input
experiments,
are from
the
responses
recorded
under
the same conditions.
In
A traces 1 and 3 are hla/s
and h,,, and traces 2
and 4 are hls,a and hrs. Note the complete
suppression of his in the presence
of the annular
input.
In B, traces 1 and 3 are hla,a and hr8, and traces 2
and 4 are hlR/8 and h,,. In both records
responses
are scaled
by the same
factor
with
ordinates
as
volts/(photons/mmz).
Upward
deflection
is for
hyperpolarization
of the membrane
potential.
Average mean intensity,
0 log unit.
form and amplitude of the annular kernels
are little affected by the presence of the
spot input.
The spot kernels, on the other
hand, are slower latencywise and peak response timewise and they are overdamped.
In the type Na neurons, the presence of
the annular input completely depresses the
spot response component, as shown by curve
2, h Is/a, in Fig. 13A; while in the type Nb
response, the presence of the annular component increases slightly the spot response,
as shown by curve 3 in Fig. 13B. The latenties of the annular and spot responses are
25 and 35 ms and peak response times are
65 and 85 ms, respectively.
The MSEs of Na and Nb model responses, as predicted by the system kernels
in Fig. 13, are tabulated in Table 1. They
indicate: 1) Types Na and Nb responses
are linear either for one- or for two-input
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
neurons the principal
dendrite was very
short and in others it was long. The latter
type of neurons corresponds to those classified as the spaghetti type (part I (24), Fig.
6E-H) and the former to the starburst
type (part I, Fig. 5). However, exceptions
could be found and some of those neurons
classified as type N had their somata in the
ISL with their lateral dendrites spreading
throughout
the layer (see part II (28), Fig.
4) .
Responses from the type N neurons are always depolarizations
(type Na) or hyperpolarizations
(type Nb) of the membrane
potentials. Examples of types Na and Nb
responses are shown in Fig. 12 in which we
observe that the responses, regardless of
their polarity, are composed of a small DC
component on which modulations
due to
the white-noise input are superposed. Lack
of a high-frequency
component in the response indicates that spike activities or regenerative slow potentials
are absent in
this system. Thus the responses from type N
neurons are strikingly similar to those from
the bipolar cells shown in Fig. 7. In part II
we have already noted that the step responses of the type N neurons were very
similar to those from the bipolar cells.
Figure
13 shows the four first-order
kernels of types Na and Nb neuron responses; hl,, hlsr hla,s, and hl,,, were obtained by the three standard white-noise
stimulus modes. We note that the annular
kernel, whether obtained by single or twoinput stimulation,
are either depolarizing
or hyperpolarizing
and underdamped.
This
suggests that the annular component of this
system responds to changes in the annular
signal (slightly differentiating).
The wave-
ANALYSIS
108
NAKA,
MARMARELIS,
AND CHAN
TABLE
1.
MSEs for model responses predicted
from Na and Nb neurons
by sets of kernels
Two-Input
Annulus
and Spot
One-Input
N”da
and
Na
Nb
Values
are
L”s/a
L”a/f3
99
86
35
31
percentages.
For
spot
MR
NMa/t3
definitions
terms
2
Summary
of MSES
for
see the
18
29
Type C neurons (response)
In part II (28) of these series, it was
shown that, in the catfish retina, there is a
class of neurons which give rise to transient
in this paper
Improvement
and
External
horizontal
Internal
horizontal
Ba
Bb
Na
Nb
C
Y
Values
90 k 6
95 & 3
57 2
47 t
91 &
94*3
92 z!z
92 t
are
percentages.
2
12
5
4
7
15 t
MR
L”Zl,S
6
20
28
power spectra of these responses, as shown
in Fig. 14. We note, in addition to the
points made above, the following: I) In the
two-input
experiment
the system exhibits
a low-pass characteristic with a small peak
at 4 Hz. 2) In the one-input experiments the
annular response shows something of a
band-pass characteristic, while the spot response shows a low-pass characteristic (Fig.
14B). 3) In the presence of the annular
input the power level of the spot response
is depressed to nearly 20 dB below the level
of the response to a spot input alone (Fig.
14A, NM, and B, RPOt).
Thus, in summary, the functional traits
of the type N neuron (response) are in
marked contrast with those of the bipolar
cells in which the annular and spot components have opposite polarity (biphasic
receptive-field organization), and these two
components are mutually enhancing.
catfish neurons obtained
L”a/B
18
32
text.
L”S,a
L”8/a
MR
L”a
12 2 4
11+5-
9&3
lo&3
12
62 k 2
68 + 8
31 ;8
27 2 6
91+5
57-t-11
25 +
25 :
30 2
26 &
84 +
51211
6
7
6
6
5
-+
1924
20 +
2725
23 2
46k
33 &
bY h,
2
4
6
12
8
No. of
Neurons
1
7
6
4
3
3
38
18
5
20
8
11
13
37
7
-2
--
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
stimulation because of the improvement
in
the performance of the model response by
the introduction
of the second-order model
response (computed from h,‘s) is less than
6%. 2) In the two-input
experiment
the
response is largely due to the annular component, particularly
in the type Na response. 3) The introduction
of the interaction term (NM,,) or the difference in
MSES between (NM,,, + NM,,, and MR)
improves the predictability
by less than 3%,
indicating
the absence of any significant
dynamic interaction
between the two inputs. As seen in Tables 1 and 2, the MSEs
of the model responses from the type N
neurons, therefore, are comparable to those
of the linear and more distally located neurons, such as the bipolar and horizontal
cells. It is interesting that neurons which
belong to the ISL have a linear characteristic, a strong indication of the absence of
any regenerative activity.
The functional
traits of the type Na response, as observed from the first-order
kernels and also from the performance of
the model responses, are confirmed in the
TABLE
16
35
24
of
MR
L”s
28
30
25
Annulus
WHITE-NOISE
I
2
FIG. 14. Power
System
responses
terns, one two-input
bY R4qmt + annulus’
5
IO
Frequency
0-k)
20
50
spectra
from
a type Na neuron.
to three
standard
stimulus
patand two one-inputs,
are shown
Rf3pot’
and
Rannulus*
MR*
mode1
response;
NM,,
nonlinear
model
response
for the
annular
component
in two-input
experiment.
NMR,
similar
model
response
for the spot
component.
Responses
are scaled
by the same factor
to bring
the power
level of the system response
to two-input
stimulation
close to 0 dB. Note the absence
of any
high-frequency
component
as seen from
the steep
asymptotic
slope and also the depression
of the spot
component
in the two-input
experiment.
on-off responses very similar to those obtained from a class of neurons identified as
amacrine cells in the mudpuppy
(47, 48),
carp, and goldfish (11, l&44). In a majority
of cases, the somata of those neurons giving
rise to type C responses could be found in
the proximal regions of the INL. In part
II we reported that this response originated
from the spindle-type neurons (part II, Fig.
6). Examples of Procion dye-injected type
C neurons are shown in Fig. 15 in which
three neurons (A, 23, and C) fit the morphological critera proposed in part II for the
neurons which produced transient depolarizing responses.
Two examples of type C responses to
109
white-noise input are shown in Fig. 16 in
which A shows the response including the
initial transient and B is a part of the response from another unit. We observe that
the response is composed of high-frequency
transients and no DC component. We also
notice that some of the transients are much
larger in their amplitudes relative to others,
suggesting a strong nonlinearity
in the system. In the type C neurons, spike discharges can be seen occasionally to superpose on the depolarizing phase of the response, but such cases are the exception
rather than the rule. Although not so conspicuous, a closer observation of B in Fig. 16
reveals the presence of spike discharges of
very small amplitude.
Type C responses produce consistently
very small and noisy h,‘s and two such examples are shown in Fig. 17. These noisy
first-order (linear) kernels indicate that the
response is highly nonlinear (as we have already noted from the records in Fig. 16)
and that this type of neuron is responding
to more complex stimulus parameters than
those taken care of by a linear transformation., On the other hand, the type C responses
produce consistent and characteristic nonlinear kernels h,; four such examples are
shown in Fig. 18. We note that the secondorder (nonlinear) kernels of type C neurons
have a large negative peak on the diagonal
at about 65 ms and two off-diagonal positive peaks at t1 = 65 ms, and t2 z 130 ms.
In consideration that hI is very small, the
second-order kernel is interpreted
as follows: two pulses of light given in close succession (within 50 ms) would produce, 65
ms later, a negative (depolarizing) response,
whereas, if the two pulses are separated by
about 65 ms, they would produce, 65 ms
after the occurrence of the second pulse, a
positive (hyperpolarizing)
response. Strictly
speaking, of course, these responses due to
h2 are in addition to the responses (to the
two pulses) due to h, (but h1 is very small
in this case, as we have seen). Although the
positions of the peaks in h2 are slightly
different among type C neurons, the number, size, extent, and relative locations of
the peaks are very consistent and can be
used as a reliable functional
identifier (a
signature) of type C neurons (responses).
The responses from a type C neuron to
annular white-noise inputs, together with
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
B
ANALYSIS
110
NAKA,
MARMARELIS,
AND
CHAN
the predicted model responses, are shown
in Fig. 19, in which are also shown photographic records from a part of the response
from the same neuron (but not the same
L
2 set
I
Oscilloscope
recordings
of the type
C
responses
to two-input
white-noise
stimulation.
Neuron
A did not produce
any spike discharges
but
neuron
13 had small spike
discharges.
Record
B is
a portion
of an experiment
approximately
10 s after
the beginning
of the white-noise
stimulation.
In
both records
the largest
transient
peak is about
20
mV. IJpward
deflection
is for hyperpolarization
of
the membrane
potential.
Average
mean
intensity,
0 log unit.
FIG.
16.
portion). As we have already mentioned, the
response of the type C neuron is characterized by the presence of large, discrete de-
FIG. 17.
First-order
kernels
from
two
type
C
neurons;
one of them, B, is from
the same neuron
which
is shown
in Fig. 19. Traces
1 through
4 are
h la/s’ hld hls,a’
The
scaling
of
kernels
is
and his.
such that
their
amplitude
is expanded
approximately
10 times compared
with kernels
from other
neurons
such as those shown
in Figs. 8 or 13. All
traces are scaled by the same factor
with the ordinates for volts/(photons/mmz).
Upward
deflection
is
for hypcrpolarization
of the membrane
potential.
Average
mean intensity,
0 log unit.
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
Procion
dye-identified
examples
of the type C neurons.
Neurons
A and B arc seen in the radial
FIG. 15.
sections.
Note that in A the soma has a shape of a fish with a descending
process, P, giving
the neuron
a
bistratified
dendritic
field. Neuron
B showed
a descending
dendrite
arising
from
its soma. In C and D
neurons
are vicwcd
in tangential
sections
(not in flat mount).
Neuron
C has the characteristic
spindleshaped soma, but that of neuron
D is more round.
In both neurons
the dendrites
extend
horizontally
from
the somata
which
are in the INL.
Due to the tangential
sectioning,
any possible
bistratified
dendritic
structure
is not seen.
WHITE-NOISE
oy
+
ANALYSIS
T, (msec)
$4
+
158
+
I?2
o
+
111
T, (msec)
6,4
58
+
+
‘9?
+
226
B
l
1
-0
6?
+
l
128
+
~
192
+
Of
+
6,4
+
‘2+8
+
19+2
+
2?6
1921
Type
FIG. 18.
Four
second-order
positive
peaks which
are seen
axes are in milliseconds
with
ANNUAL
SYSTEM
RESPONSE
hl,
MODEL
h2
CH?DER MODEL
hl, h2,h3
CRT
h, (T,,T~)
Note
the characteristic
from
type C neurons.
Kernels
are for annular
input
in two-input
scaled by volts/(photons/mm2)?
INPUT
2nd ORDER
3td
(self) kernels
in the kernel.
units
of ha’s
C response:
RECORD
System
and model
responses
from a type
FIG. 19.
C neuron
to annular
white-noise
input.
Model
responses are computed
from sets of kernels
shown
in
the figure. For detail see the text. Upward
deflection
is for hyperpolarization
of the membrane
potential.
The oscilloscope
recording
is from the same neurons
but not from
the same portion
of the record
used
Peaks of the response
are
for white-noise
analysis.
about
20 mV.
negative
experiments
and
and
polarizing responses which are evident in
the records shown in this figure.
The linear model (predicted by h,) of the
type C neurons performed poorly, as expected from the noisy first-order kernels,
two examples of which are shown in Fig.
17, and the MSE of such a model is of the
order of SO-90% (for average figures see
Table 2). The introduction
of the secondorder nonlinear term improved the predictability of the model considerably (with a
MSE of about 45%); still the performance
of the second-order model is not satisfactory
when compared with the system response
(Fig. 19). The addition,
however, of the
third-order
nonlinear
term reduced the
MSE of the model response to 15-20%, the
main improvement
seen as a sharpening of
the large depolarizing transient peaks which
are characteristic of the type C response
(Fig. 19).
The fact that a third-order
nonlinear
term has to be introduced to describe the
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
256
112
NAKA,
MARMARELIS,
Type 1’ neurons (responses)
Neurons (responses) classified functionally as type Y exhibit the following characteristics: 1) they produce well-defined linear
kernels, and 2) they have a large secondorder nonlinearity.
Those responses classified as type Y, based on these two criteria,
originate from neurons of various morphological types (as could be expected from the
great morphological
variation of neurons
in the proximal region of the retina).
Examples of typical type Y neurons in the
Procion preparation
are shown in Fig. 20
in which we observe that the somata of the
type Y neurons lie close to the inner limiting membrane or in the layer of the classical
ganglion cells. Some of the type Y neurons
are observed having round somata in the
flat-mount
preparations,
suggesting that
they correspond to one of the polar (ganglion) cells described in part I (24); other
type Y neurons have elongated and more
complex somata.
Oscilloscope recordings of the type Y responses are shown in Fig. 21 in which three
examples are shown; in the first example,
shown in A, the neuron produces a spike
discharge of large amplitude (about 30 mV);
in the second example, shown in B, the amplitude of the spike discharges are less than
the amplitudes of the postsynaptic potentials; and in the third example, shown in
C, the amplitude of the spike discharge is
very small and barely visible in the record.
It was often observed that penetration
of the electrode resulted in a gradual loss of
the spike activity without
any apparent
effect on the slow potential activity of the
CHAN
impaled neuron. Rarely did we encounter
type Y responses without any trace of spike
activity. As we have already discussed in
parts I and II, the amplitude and/or time
course of the spike discharge is not thought
to be an indication of the type of neurons,
but rather depends on such incidental factors as the distance between the spike-generating site and tip of recording electrode.
In part I, we showed that in some neurons
the axons took off from one of the principle
dendrites, some distance away from the
soma. In Fig. 21 we also notice that the
slow potentials (synaptic or generator potentials) look as if they are half-wave rectified and that no appreciable DC component
is present in the response.
The type Y responses produce well-defined but somewhat noisy first-order kernels
(probably because of the presence of the
spike discharges which could not be filtered
out completely due to the overlap in the
frequency ranges of the spike discharges and
the slow synaptic potentials). In Fig. 22
are shown two such sets of hl’s, one producing depolarizing
(type Y,) and the other
producing
hyperpolarizing
(type Yb) responses. Although the polarity is reversed,
the two sets of kernels are, otherwise, very
similar and display common features: 1)
The annular kernels hl, are overdamped
and faster than the spot kernels latencywise
and frequency responsewise. 2) The spot
kernels, hl,, are underdamped
and slower
latencywise and frequency responsewise. 3)
In the two-input experiments the presence
of the annular input depresses completely
the spot compo,nent, hr,,a, while the presence of the spot component either depresses
or enhances the annular components, hlalfi,
although such changes are not as drastic as
in the case of the spot component. Thus, the
interaction
between the spot and annular
components is rather unilateral for type Y
responses, while in the bipolar cells such an
interaction
is mutual.
In analyzing the
spike discharges in the catfish retina, Marmarelis and Naka (20) reported that the
annular input gave rise to a slow, overdamped kernel and that the faster component depressed the slower component. In
fact the set of kernels (derived from spike
discharges) shown in Fig. 7 of Marmarelis
and Naka’s paper corresponds exactly to
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
sharp peaks is probably an indication
of
some threshold mechanism followed bv a
regenerative slow potential. The time scale
of the response is about 30 ms and this does
not warrant us the description of this phenomenon as a “spike” and, as we have already shown, fast spike discharges are
clearly present in the type C neurons in
addition
to the transient depolarization.
It is conceivable that in large neurons, such
as those found in the catfish retina, the
need for a regenerative slow potential might
arise if signals are to be transmitted over a
large distance without involving spike generation.
AND
WIII’I‘E-NOISE
ANALYSIS
113
those in Fig. 22 shown here. If we assume
that the frequency of the spike discharge is
proportional to the amplitude of the depolarizing (intracellular)
slow potentials, the
close agreement between the results of the
two analyses (one, on the spike discharges
and the other, on the intracellular
slow
potentials) is what one could expect if both
responses are recorded from the same class
of neurons.
Although indirect in approach, it might
thus be possible to identify morphologically
the origin of the extraccllularly recorded
spike discharges by comparison with the
intracellular
recordings in which morphological identification is possible through the
intracellular
dye-injection
technique. For
example, the set of kernels shown in Fig.
22B were recorded from a three-polar
ganglion cell, and the fact that this set of
kernels is very similar to those shown in
Fig. 7 of Marmarelis and Naka (20) suggests
that the extracellular
spikes observed in
their experiment
might have originated
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
FIT.. 20. Examples
of Procion
dye-itlcntified
type Y nwrons
seen in the radial
(‘4 through
II) ant1 tangential
(E and F) sections.
Neurons
A through
U are probably
the polar neurons
in part I (24), to judge
from their round
somata.
Neuron
D is apparently
a spindle
type, while
neurons
E and F are two-polar
and kite-type
ganglion
cells, respectively.
Optic
fiber bundle
is shown
by “on.”
114
NAKA,
MARMARELIS,
CHAN
from a neuron
of similar
morphologicd
origin.
A second-order
kernel
obtained
from a
type Y response is shown in Fig. 23; it is for
the annular
component
in a two-input
experiment
(the corresponding
spot kernel is
not shown since it is almost zero). The kernel has three peaks; one at the diagonal
at
t, = t2 = 64 ms, and the other two off the
diagonal
at tl = 64 ms ad
t2 = 128 ms.
The general
features
of the second-order
kernel
of type C neurons
bear very close
resemblance
to those from the !qnglion
cell
discharges
(rcf 19, 20) and those from type
Y respoiiscs
(although
the type C secondorder kernels were always less noisy than the
type Y second-order
kernels).
Typical
response
power
spectra of the
type Y neurons
are shown in Fig. 24. We
observe the following:
I) The spectra have
some characteristics
of band-pass
filter. 2)
The spectra have large high-frequency
components
as seen from the slower asymptote
at frequencies
2040
Hz and the secondary
peaks at very high frequencies.
3) The discrepancy
between
the system response and
model response (computed
from 11, and Ii,)
or between tile linear and nonlinear
annular model responses becomes larger at the
higher frequency
range, intlicaling
the pres-
T, (msec)
I28
64
OP
,
O.Isec
’
+
’
*
’
192
+
+
256
’
,
1 I(.. 22. 1 ilst-order
hcinelc
fion~ typri
Yb, shown
in ‘4, ant1 Ya, \hou n in B, neurons.
In both sets of
kclnrl\,
traces 1 and 3 ale h,n,s
and hl,, and trace?
4 and 2 are Ir15,T and h,\. All trace5 a;e xalcd
by
the same factor
;\ith
ortlilrate5
f’or volt\/(photon\/
mmz).
A lowpa\s
hltcr
WI\ 11w1 to \uppre~s
the
higll-frcclw~~~y
components
tluc
to the spike
di\thargra.
IJpr\ard
deflection
is fol 1iypcrpolarir;rtiorI
of the mcmbiatlc
potential.
Alciagc
mean inlcnGly,
0 log unit.
Type
Annul<w
SC( olltl-Ol
a type
Y rrcurc~n
are shown
in Fig. 22.1.
ant1 units of h, arc scaled
11.):,,s’ from
kcr&ls
:ccontls
lIlf112j”.
Y response
h, (r,,
tlnr
(wlr)
7‘2)
kc1 nrl,
Ivhosc
first-order
Axes are in milliby volts/(photons/
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
Ix. 21. Samplr
responw
from
type Y neurons
obt.~inctl
by IT\O illput
!\hitc
noise stimulations.
In
A the :Implitmle
of the spike tlischargc
i* about
30
m\‘, arltl ilr 11 and C amplitutlrs
of the synaptic
potential5
al c about 10 ml’. Notice
the difference
in
heights
of spike distllargcs
and alto the half-wake
rectified
qnaptic
potentials.
IJpwartl
deflection
is
for hypelpolari/ation
of the mcmlnanc
potential.
,\I crage mc’d~~ intensity,
0 log unit.
AND
WHITE-NOISE
0
ANALYSIS
r
2
5
100
FIG. 24.
Power
spectra
of responses
from
a type
Y neuron.
R, system response;
MR, model
response,
LMB and NM,,
linear
and
nonlinear
model
responses
from
the spot input;
and LM,
and NM,,
linear
and nonlinear
model
responses
from
the annular input.
Responses
are so scaled that the pourer
level of the system response
is close to 0 dB. Notice
the presence
of the high-frequency
component.
ence of high-frequency
components in the
response. Such high-frequency
components
seem to originate from two sources: one,
the intracellular
slow potentials and the
other, the remnant of the spike discharges
left unfiltered. However, it is worth mentioning here that despite the presence of
the high-frequency
component,
the model
response based on hI and h2 can predict the
system response with reasonable accuracy,
in contrast with the type C response in
which h3 had to be introduced
to predict
the system response with comparable accuracy (see Table 2).
Com,pa rison of responses
retinal
neurons
from
catfish
In the preceding sections we ha ve described the function al traits of the catfish
retinal neurons as revealed by morphological (through intracellular dye injection) and
functional
(through
white-noise
analysis)
experimen ts, and classified
identification
the responses into five major categories
based on a few selected ex.amples which we
believed to be typical of a given class of
neurons. As we will amplify in the APPENDIX,
such a traditional
classification aPpreach fails to indicate how objective the
classification is or how clearly each cla ss of
response is separated from the rest. To
overcome partially this difficulty and also
to give some objectivity to the classification
scheme which we propose for the catfish
retinal neurons, the responses from about
100 neurons on which two-input
whitenoise analysis have been performed
are
classified into five types based on their functional (mainly through
the kernels) and
morphological characteristics, and the MSEs
for each class of neurons are tabulated in
Table 2. In the table are shown five sets
of values: MSE for LM,,,, LMa,s, LM,,, -ILM,/S? and MR, and the difference between
the last two MSEs or the improvement
in
the model performance
by the addition
of the second-order terms predicted by hS’s.
In the table we notice that both the external and internal horizontal cells (which
were identified by dye injection) are linear
because the introduction
of the secondorder term improves the MSE of the external cells by 1% and worsens the MSE of
the internal cells by 2%. Such worsening
of the model response by the addition of
the second-order term is due to the fact
that the term is composed mainly of the
high-frequency
noise because of the nearperfect fit of the annular
linear model
(MSE of 9%). This fact also indicates that
in the horizontal cells the total response
MR is almost entirely due to the annular
inputs, a fact which is predictable from our
assumption that the horizontal cells form a
laminar layer (17, 30, 37).
The bipolar cells, both types Ba and Bb,
are linear, but less so than the horizontal
cells, as the second-order model showed an
improvement
in the MSE of about 5%
over the linear model. However, contrary to
what we have seen in the horizontal cells,
the MSEs for the spot and the annular
linear models are quite comparable, indicating almost equal contribution
to the
total response from the two inputs. Together with the fact that the polarities of
the spot and annular h,‘s are opposite,
this table indicates that the bipolar cells
form a biphasic receptive field. We notice
that in the bipolar cells the MSEs for the
total MR models are about 20%, while in
the horizontal
cells it is about 10%. To
judge from the absence of the high-frequency components
in the bipolar cell
of the
responses, the poor predictability
second-order model seems to reflect more
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
Freqiky
-I
20
(Hz)
115
116
NAKA,
MARMARELIS,
CHAN
sponses to step inputs. In this study they
showed that the relationship
between the
amplitude of the light input and the amplitude of the resulting horizontal
cell response (V-log I) could be fitted by a tanh-log
curve. In some other retinas, such as those
of the carp and mudpuppy,
similar curves
were constructed for the responses arising
from neurons other than the horizontal
cells (13, 4547). However, such a relationship can be unambiguously established only
if the system under study shows a constantgain low-pass frequency response; i.e., if
the system detects only the magnitude of
the stimulus. Otherwise it is a strong function of frequency. Another condition for
the meaningful
interpretation
of such a
static V-log I curve is a monotonic receptivefield organization;
if the receptive fields
are organized in a more complex fashion,
such as those found in the bipolar cells,
the interpretation
of the resulting V-log I
curves becomes problematic. In the catfish
retina the analysis performed
so far has
shown that, except for the horizontal cells
and probably also the receptors, the retinal
neurons have complex receptive-field organizations and, often, strong band-pass characteristics. Therefore, their input-output
relationships must be established based on
the dynamics of their responses, preferably
by the use of two-input white-noise analysis,
so that both receptive-field components are
accounted for. This is due to the fact that
the dynamics of a given system can be efficiently described in terms of a small set of
kernels and that the two-input
analysis
technique is capable of separating the responses from the two components.
In the present study, however, we limit
our analysis to one-input experiments which
could, although not as completely as twodescribe the dynamic
input experiments,
response range of the catfish retinal neuDynamic response ranges of catfish
rons. In this study we confine the stimulus
retinal neurons
to field illumination
(Fig. l), which covered
Thus far the study of the relationship
about two-thirds of the entire retinal surexisting between input and output in the
face and whose average mean intensity level
neurons in vertebrate retinas has been ex- was controlled by interposing neutral denplored mainly through stimulating the sys- sity filters (while keeping the modulation
tem by step or sinusoidal functions. The
depth constant). To make interpretation
most ubiquitous
relationship
found so far easier, the analysis was performed on the
was originated in Naka and Rushton’s (29) linear neurons in which the magnitude of
treatment of the tenth horizontal cell ‘re- the first-order kernels can be taken as an
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
difficult recording conditions rather than
the presence of the higher order terms.
The second-order nonlinearity in the type
N neurons is also small; an improvement
in the model performance of 3% is seen
by the addition of the second-order term.
In the type N neurons the annular linear
model predicts the system response with a
MSE of about 30%, while a similar spot
model predicts the system response with a
MSE of 90%, thus indicating that in the
type N neurons, as in the horizontal cells,
the total response mainly arises from the
annular input.
The type C neurons are characterized by
the fact that the first-order models perform
very poorly, thus indicating the highly nonlinear characteristics of the neuron. The
improvement of the model response by the
introduction
of the second-order term is
about 40%, the largest in the catfish retinal
neurons, a fact which further substantiates
the high nonlinearity
in the type C response. We have already mentioned that the
addition of the third-order term improves
the predictability
of the model response by
nearly 20%.
In the type Y responses the improvement
in the MSE by an addition of the secondorder term is IS%, a value halfway between
the similar improvements
in the linear
(horizontal
and bipolar cell) and in the
highly nonlinear (type C) responses. Under
the present experimental
conditions
the
type Y responses are largely due to the annular inputs and only a small part is due to
the spot inp ut.
Those observations we made in Table
2 agree fully and augment further the classification scheme we proposed earlier of the
catfish retinal neuronsA and also the conclusions we have drawn on the functional
traits of each class of neurons.
AND
WHITE-NOISE
0.8 log units
step
,
IOOmsec
113
cussed later. However, all types of neurons
show a common feature: a shorter peak response time as the mean intensity level is
increased and a similar decrease in latency
(although
the latter cannot be seen as
clearly as the former).
Clearly, the response range (or sensitivity)
of a neural system, unless it shows a constant-gain low-pass characteristic, is a function of the stimulus frequency. Therefore,
a natural way to study this matter is
through the response (to white noise) power
spectra, which in effect measures the power
(or amplitude) of the response at each frequency. These spectra, of course, would
have to be measured for different mean intensity levels (while the modulation
depth
is kept constant). Incidentally,
such an approach to measuring the dyna .mic response
range of a neuron circumvents the problems
of nonlinearity
as these are also accounted
for in the spectrum.
One such&attempt is shown in Fig. 26 in
which are shown the power spectra of bipolar cell responses recorded at two different mean intensity 1evels, one at 0 log and
the other at -0.8 log units. From the re-
,
25. First-order
kernels
from
horizontal,
A, two bipolar,
B and C, and type N, 0, neurons
obtained
white-noise
input.
Curves
marked
1 are obtained
by inputs
with average
mean level at 0 log unit.
For subsequent curves marked
2 through
5 the average
intensity
levels are decreased
by 0.8 log unit steps by
interposing
neutral-density
filters.
Upward
deflection
is for hyperpolarization
of the membrane
potentials.
The amplitude
of kernels with log filters was scaled down by the factor corresponding to the optical density of the filter.
FIG.
by field
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
indication of their dynamic sensitivity. In
Fig. 25 are shown results from one horizontal
(A), two bipolar (B and C), and one type
3J (D) cells; those records marked 1 were
obtained by inputs whose average intensity
level was 0 log units (without any neutraldensity filter) and for the records 2 through
5, the average intensity levels were decreased
by a decrement of 0.8 log units. In the
figure we notice that the amplitude of the
horizontal cell h1 decreases rapidly as the
level of mean intensity is decreased and
such a decrease is roughly proportional
to
the decrease in b; i.e., the DC component
of the response. Thus the dynamic range of
the horizontal cell is comparable to the response range when it is explored by step inputs. The response from the other types of
neurons, however, has a much larger dynamic range and a decrease in the mean intensity level by one or two log units does
not produce a marked change in the amplitudes of the first-order kernels (cf. Fig. 25).
In some cases a decrease in the mean intensity level results in an increase in the
dynamicgain
of the system. A possible explanation for this observation will be dis-
ANALYSIS
NAKA,
MARMARELIS,
CHAN
due to the annular component; 2) as the
mean intensity level is decreased the system
becomes overdamped, due to predominance
of the response by the spot component; and
3) decreases in the level of intensity result
in a faster decrease in the power level of
the spot than the annular component.
Similar characteristics have been observed
consistentIy in the power spectra of the
linear neurons and they were not confined
to the bipolar cell responses. We conclude
that the dynamic response range of the horizontal cells (and probably the receptors)
is the smallest (for the range of intensities
used) and is comparable to the static V-log
I relationship
of these cells, while other
proximal neurons such as the bipolar cells,
have much larger dynamic ranges, well over
34 log units, although their static response
range, as probed by the step inputs, is reportedly very limited (13, 45).
DISCUSSION
Classification
Frequency
(Hz)
FIG. 26.
Bipolar
cell power
spectra
obtained
at
two mean
intensity
levels,
A at 0 log and B at
-0.8
log units.
R, system
response
to two-input
white-noise;
MR,
model
response;
LMa
and NMa,
linear
and nonlinear
model
responses
for the annular
input;
LMS and NM,,
linear
and nonlinear
model responses
for the spot input.
In both records
responses
are scaled by the same factor
so that the
power level of the system response
at 0 log in tensi ty
is close to 0 dB.
sults of each two-input
experiment
six
power spectra are computed for each set:
the power spectra of the system experimental response, R, of the model response,
MR, the nonlinear
annular-component,
NM,, the linear annular-component,
LM,,
the nonlinear spot-component,
NM,, and
the spot linear component, LM,. In addition to the features we have already described these power spectra show that 1)
there is a very close agreement between
model and system responses, and 2) a similar close agreement exists between the linear and nonlinear model responses. Comparing sets of power spectra, we notice 1)
at higher mean intensity, the system exhibits
a band-pass characteristic which is mainly
of neurons
During the late 19th and early 20th century, vertebrate retinal neurons were classified into taxonomical
sets based on such
morphological
traits as the degree of dendritic expansion, location of somata, or the
presence or absence of axons. As already
recognized by Ramon y Cajal (5), such a
morphological
classification was necessarily
tentative, as any class of neurons requires
functional as well as structural definition.
The introduction
of the intracellular dyeinjection
technique,
particularly
of Procion dyes, offers now the possibility of testing the validity of the classical classification
of the retinal neurons. Pioneering studies
by Dowling and Werblin (7, 48) and especially, Kaneko (1 l), followed by Matsumoto
and Naka (22), have shown that intracellular recordings, as well as dye injection, can
be performed in the vertebrate retinal neurons. Although these studies have brought
forth
valuable
information,
they were
unsatisfactory in four respects:
1) No appreciable effort was made to establish the classical morphology
of the
neurons in the retina in which intracellular
dye injection was attempted. For successful
structural identification
of a class of neurons through intracellular
dye injection,
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
B
AND
WHITE-NOISE
119
distal layers of the retina agrees fully with
the conclusions reached in the earlier
studies, our identifications
of the neurons
in the proximal layers deviate from the
previously accepted views. However, we
also realize that our identification
of the
proximal neurons is not yet complete, and
to circumvent any further complications,
we have assigned them the noncommital
designations, types N, C, and Y neurons, reflective of their response characteristics. A
more quantitative categorization procedure,
such as the one attempted in the APPENDIX,
might be able to establish a more definitive
classification of these neurons.
Functional identification
The functional identification
of a neural
system involves the complete determination of the input (stimulus) versus output
(response) dynamic relationship of the system in the form of a compact mathematical
representation. For a linear system the identification
procedure
is simple and well
established; a step or a series of sinusoidal
stimuli of different
frequencies are well
suited for this purpose because the principle of superposition holds for these systems.
Except for a few cases where certain linearization techniques have been used (38), a
step or sinusoidal stimulus has been used
almost exclusively to identify functionally
the response characteristics of a given neuron. Here we mention an attempt by Schellart and Spekreijse (35) to study the dynamics of the retinal ganglion
cells by
cross correlating the noise input and the
resulting spike discharges.
When applied to the study of sensory
systems, testing (in order to identify functionally) by these traditional inputs is very
disadvantageous
in that such stimuli are
unnatural, inefficient in gathering data over
a short period of time and, most of all, unsuitable for the nonlinear
quantitative
description of the system stimulus-response
behavior. In a series of studies we have successfully applied Wiener’s theory (49) of
nonlinear
analysis to the horizontal
and
ganglion
(spike) cell responses from the
catfish retina and have shown that this
method is applicable and well suited to the
study of the retinal ne ural systems. Specifically, the white-noise method of testing
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
the classical morphology
has to be established through the Golgi silver-impregnation and/or the methylene blue vital-staining technique. The apparently premature
identification
of amacrine cells by Toyoda
et al. (44) exemplifies the danger inherent
in basing conclusions on incomplete data.
As already pointed out by Stell (40), a considerable amount of variation in the morphology of the retinal neurons exists even
among the teleost retinas.
2) The histological procedure employed
by the earlier investigators
reduced the
number of recovered neurons, due to the
tedious sectioning involved, and eliminated
virtually any characterization
of the neurons based on the lateral spread of their
dendrites. We have already shown in part
I (24) that morphological
classification of
retinal neurons in radial section (or side
view) is of limited value in the catfish retina; some of the drawings by Ramon y Cajal
(ref 5, plate III, Fig. 4e) indicate the importance of viewing the neurons in flatmount preparation.
3) The scope of the functional identification of the neurons was severely restricted
by being based only on the responses to
step (pulse) input. In part II (28) of this
series we have already described the difficulties involved when such step inputs are
used as a functional probe.
4) Due to the diversity in shape and size
of the retinal neurons (cf. ref 5, 24, 40),
morphological
and functional
identifications have to be performed on the same
neurons; any conclusion on the correlation
between structure and function
must be
statistically based on the results of a large
number of such dual identification
experiments. Unfortunately,
in the intracellular
dye-injection experiments so far carried out
in the vertebrate retina, such conditions
have not been fully met, except for the
excellent studies on the turtle horizontal
cells (23, 34, 37).
In this series of studies we have avoided
these obstacles found in the earlier attempts
to correlate structure and function in the
vertebrate retina. Moreover, each paper in
this series was developed independently,
so
that results from one part would not bias
any conclusion drawn from any other parts.
While our analysis of the neurons in the
ANALYSIS
120
NAKA,
MARMARELIS,
CHAN
white-noise analysis of spike discharges requires a recording time of 5-10 times longer
than for slow potentials (in order to achieve
similar statistical accuracy) and produces
less satisfactory results.
Horizontal
cells
The morphological
and functional identification of the horizontal cells is straightforward. The horizontal
cells, both external and internal,
have independent
monotonic receptive fields (cf. ref 17, 30,
37). As shown above, their dynamic as well
as static range of responses to the light
stimulus is limited. The horizontal
cells
have a large DC response component (h,)
which together with the first-order kernels
is sufficient to describe the response of the
cell with very good accuracy. Although
there is a certain dynamic, “small signal”
nonlinearity
associated with the cell, i.e.,
asymmetric rising and falling phases, besides the usual saturating nonlinearity,
the
horizontal cell is, with the depth of modulation used, essentially a linear device with
a constant-gain, low-pass filter characteristic.
The results of the nonlinear analysis so
far performed on the cell, as well as those
from other mathematical
analyses (17, 30,
37), lead to the conclusion that the cell’s
function is simply to detect the magnitude
of the input signal and integrate it by
means of its laminar structure. We have
also noted a remarkable consistency in the
responses from horizontal
cells, a feature
easily attributable
to a group of cells acting as a syncytium (see APPENDIX).
Bipolar cells
The identification
of the bipolar cells as
a class of neurons did not pose any difficulty, morphologywise
(part I (24)) or functionwise (part II (28)). However, the patterns of responses from individual
cells
encompassed a large range in which certain response parameters varied continuously (see APPENDIX).
Thus, it was not
possible from the present analysis to classify
them into two classes which might correspond to the smaller and larger bipolar
cells. Such classification of the bipolar cells
can perhaps be obtained in the near future
by an experiment in which the diameter of
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
a sensory neural system (in order to identify
it functionally)
has four major advantages
over the traditional
methods of testing by
pulses and/or sinusoidal inputs: 1) It allows a concise quantitative
description of
the dynamics and nonlinearities of the system. 2) The white-noise stimulus, by its
nature, nearly maximizes the rate of information gathering
about the stimulus-response behavior of the system as compared
with a sinusoidal stimulation
or a brief
pulse given once in a few seconds. This is
a very important point in the study of the
vertebrate retina (or any other part of the
central nervous system), where stable intracellular recording time is limited to less
than a few minutes, during which a given
neuron must be identified morphologically
(through dye injection) as well as functionally. 3) Most types of unwanted,
contaminating noise are eliminated through either
the cross-correlation process involved or the
orthogonali ty of the white-noise characterization. This is also important
in intracellular recordings from smaller neurons
where noise is indeed a problem. 4) When
expanded to multi-input
systems, necessary
because of the biphasic or concentric nature of retina1 receptive fields, the whitenoise analysis allows us to identify
the
dynamics of each field component separately
as well as their interaction
in a single experiment.
For example, we have shown
that a 20. to 40-s-long experiment
on a
bipolar cell is sufficient to give information
on the contributions
to the total response
from each compnent, spot or annular, and
also on the nature of their mutual dynamic
interaction (which was found to be rather
small in all the catfish retina1 neurons).
Moreover, the effect of each receptive-field
component
on the other is manifested
clearly and completely by the change of
the kernels from the one-input experiments
to t.he two-input
experiments
(e.g., the
change of hl, and hZa to hl,,, and h2a,s).
Thus white-noise analysis is an ideal tool to
identify functionally
the retinal neurons
which have either concentric, biphasic, or
monotonic receptive-field organizations (in
which the spot and annular stimuli play
important
roles) and which transmit signals primarily
through analog potentials.
As we have discussed elsewhere (18, 2 1) the
AND
WHITE-NOISE
I
121
cell predicts
nicely the increase
in the dynamic gain of each component
observed
in
the two-input
experiments:
we have shown
that the horizontal
cells have a large DC
response
component;
the results obtained
in the receptors
of other retinas indicate
a
similarly
large DC component
must exist in
the catfish receptor
signal (1, 3, 6, 41, 48).
With
only one input
active, such a large
DC component
easily
saturates
the cell
driven
at the next stage (bipolar
cell), thus
limiting
the dynamic
gain of the system.
Steeper
V-log
I curves were seen in the
mudpuppy
bipolar
cells (45, 46) and in the
presumed
carp
bipolar
cells (13). However, if both inputs
are active with similar
DC components
but of opposite
polarity
(as in the case here), the bipolar
cell potential
is set to an intermediate
level in its
range from which it can be swung over a
large rangea characteristic
of a comparator or differential
amplifier.
This is clearly
evidenced
by the larger gain of hIa,s and
h Is/a? respectively
(see Fig. S), since in the
former case the system is “biased”
nearer the
middle
of its range.
The analysis
so far made, directly
and
indirectly,
on the function
of the catfish bipolar cell still supports
the original
contention
(27) to the effect that its main function is to compare
two signals, one local
(center)
and the other
integrating
(surround.)
Neurons
in proximal
layers
The functional
and morphological
identifications
of neurons
in the proximal
layers
of the catfish retina were far more difficult
and ambiguous
than for the neurons
in the
distal layers. This is due to the complexity
of the response
patterns
as well as the diverse morphology
of the neurons
in the
former
lavers.
Based on their
functional
traits obtained
through
white-noise
analysis,
we have classified
the neurons
(responses)
into three types, N, C, and Y. However,
such a classification
cannot
be taken
as
unique
nor every neuron
(response)
classified into one of these three categories
without any ambiguity.
We rather feel that the
neurons
in the proximal
layers constitute
a continuous
spectrum,
structurewise
and
functionwise,
and that the three
neuron
types (or any other
classification
scheme),
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
the spot of light as well as its intensity
are
modulated
in white-noise
fashion.
The DC response
component
was very
large in some of the bipolar
cells and small
in others.
Within
the depth
of modulation
used in these experiments
the bipolar cells behaved
linearly
and the firstorder
(linear)
model
describes
the system
response
with
a fair degree
of accuracy.
The addition
of the second-order
(nonlinear) kernels improves
the MSE only slightly
(about
5%). An interesting
feature
of the
bipolar
cell kernel is that the annular
component
always had both longer latency and
the
frePeak response time, al though
quency
response
of this corn .ponent
was
faster than that of the spot compon .ent.
We have also noticed that ihe spot componen t became faster frequencywise
and that
the latency
and peak response
time of the
component
became shorter
in the presence
of the annular
input.
From results of previous
experiments
in
which
current
was injected
into the horizontal cells (of dogfish
and catfish retinas)
it has been proposed
that a bipolar
cell receives two inputs:
one representing
the
local signal
and coming
directly
from
a
small number
of receptors
and the other
realizing
the integrating
signal by the horizontal
cells and reflecting
the average
intensity level of the visual environment
(26,
27, 31). As both the receptors
and horizontal
cells hyperpolarize
in the dogfish
and catfish retinas,
while
the bipol&
cell hyperpolarizes
for one of these inputs
and depolarizes
for the other, it was further
argued
that one of these two inputs must invert its
polarity.
Marmarelis
and Naka
(19) have
further
stipulated
that when a large number
of receptors
are activated,
as in the case of
a field or annular
input,
the resulting
horizontal
cell activity
is fed negatively
back
to the receptors
in order to improve
(speed
frequency
response.
Such
a
up)
t .heir
speedup of the receptor
response must also
result in a faster spot response
in the bipolar
cells, while
the longer
latency
and
peak response
times of the annular
bipolar
cell response
can be attributed
to the delay involved
in the three-stage
transmission
of the annular
input as compared
with the
two-stage transmission
of the spot input.
The function
proposed
for the bipolar
ANALYSIS
122
NABA,
MARbdARBLiS,
represent only the peaks in a mountain
range formed by the entire population
of
the neurons. The degree of complexity of
any classification scheme depends simply
on the level of threshold set to separate
the (imaginary) individual peaks in such a
continuous range.
CilAN
apses) must be based solely on the hyper- or
depolarization
of the potential, since this
is the only available information
at this
stage about the origin (spot or annulus) of
the signal. A .lternatively, if type N respon ses
are produced by inputs from the two different types of bipolar cells there must be a
“switching”
mechanism such that the inputs from the bipolar cells always produce
either a depolarization
or hyperpolarization response. The fact that both bipolar
cell and type N responses are linear seems
to exclude the possibility of a complex
signal transmission between the two cells.
-Here we recall that the horizontal cells
form a monotonic receptive field giving rise
to responses of the same polarity to any form
of inputs. In consideration of the fact that
the internal horizontal cells and the neurons giving rise to the type N responses face
each other directly at the junction of the
inner nuclear layer and inner synaptic
layer, the possibility cannot be excluded
that there is a direct-signal transmission between these two classes of neurons. However, they are distinguished
from one another functionally
by different
response
patterns: 1) although both neurons form
monotonic receptive fields, the spot response
is not depressed by an annular input in the
horizontal cells, while in the type Na neurons the presence of an annular stimulus
completely depresses the spot response component; and 2) the dynamic gain of the type
N response covers a far larger range than
the horizontal cell response.
Type C responses
The type C response, evoked by step inputs, is a transient depolarization
at the
on- and offset of the stimulus. Similar depolarizing
transient responses have been
seen in the mudpuppy
and goldfish retinas
by Werblin
and Dowling
(48) and by
Kaneko (11, 12), who ascribed them as
originating
from amacrine cells. Procion
dye injection performed concurrently with
white-noise analysis has revealed that the
majority of the type C responses was produced by a class of neurons referred to as
the spindle-type (ganglion) neuron in part
I (24) of this series; the observations made
in part II (28) substantiate this conclusion.
The most striking feature of the type C
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
Type N responses
Procion dye injection performed concurrently with white-noise analysis has revealed that the majority of type N responses
originated from neurons with their mitershaped somata in the proximal layer of the
INL. In part I (24) of this series these neurons were referred to as starburst or spaghetti, the latter neurons being characterized by their thick dendrites which expanded nearly 1 mm through the ISL. Identification of the type N neurons in this
paper agrees well with the identification
through the step inputs in part 11 (28) in
which these neurons produced sustainedtype responses. A morphological
correspondence (not necessarily functional)
of type
N neurons can be found in Ramon y Cajal’s
amacrine cells (such as those in the perch
retina; ref 5, plate I, Fig. 5), in the frog
(plate II, Fig. 3), in the green lizard (plate
III, Fig. 4), and in the chick (plate V, Figs.
7 and 8). Ramon y Cajal never located
axons associated with these neurons; we
are still not sure, however, that in fact these
cells do not possess any.
Al though their en tire dendri tic spread
lies in the ISL, type N neurons have linear
responses and lack any higher frequency
components.
Their
functional
traits are
very similar to those of the horizontal or
bipolar cells. However, in type N neurons
(responses) both the spot and annular inputs give rise either to depolarizing
(Na)
or hyperpolarizing
(Nb) responses, while in
all the bipolar cells these stimuli produce
responses of opposing polarity. If type N
neuron responses result from inputs coming
from one type of bipolar cell, then the signal due to one of these two inputs must be
inverted somewhere along the chain of
processing. However, it should be noted
that the “decision” (for the inversion of this
signal) by an observer sitting at the output
of the bipolar cell (which must be the site
of the decision by the type N neuron syn-
AND
WHITE-NOISE
Type Y responses
Responses were classified as type Y based
on two criteria: a noisy but well-defined
first-order kernel and a large second-order
nonlinearity.
These were produced by a
large variety of neurons which fit best the
definition of “classical” ganglion cells. In
123
the methylene blue preparations
it was
always possible to find a group of neurons
with axons which had a close morphological
resemblance to the Procion neurons which
gave rise to type Y responses. Apparently,
neurons which produce type Y responses
constitute .a large population
of neurons
which may include many morphological
as
well as functional subclasses. In one of the
companion papers (part I (24)) we showed
that the morphology of the ganglion cells
encompasses a whole gamut of structural
variation. A further study has to be undertaken to correlate the rich variety of morphological shapes with the response characteristics of these neurons; for example, it
is interesting to compare the dynamics of
simpler (or more primitive) neurons, such
as one-polar cells, to those of more complex
(or more advanced) neurons, such as- the
multipolar
cells. Probably spacewise whitenoise input, combined with quantitative
morphological
identification,
holds the key
to the classification of the type Y neurons.
It has been reported that ganglion cell
discharges showed a strong rectifying nonlinearity which was commonly believed to
be simply a manifestation
of the fact that
there are no “negative”
spike discharges
(38). However, in a recent paper Marmarelis
and Naka (18, 19) have suggested that such
rectification takes place at the ganglion cell
stage, probably at the ganglion-bipolar
cell
synapses, and the results of more direct
observations made in this study on the intracellular potentials have shown that this
rectification
takes place when the signals
are transmitted from bipolar or type N cells
to types Y and C neurons. Apparently in the
catfish retina, then, initiation
of spike discharges is not the main site of rectification.
Morphological
and functional characteristics of the catfish retinal neurons studied
in this trilogy are summarized in Table 3.
Comparison with results obtained
other retinas
in
The morphological
and functional identification of the horizontal cells agrees fully
with the results in earlier studies (11, 14,
40). Although the identification
of bipolar
cells as a class of neurons is largely in accord
with the conclusions drawn in other earlier
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
response is the absence of well-defined firstorder (linear) kernels, suggesting the existence of a high nonlinearity
involved in the
response. Another characteristic feature of
the response is the large, spikelike depolarization which apparently is produced by
some thresholdlike mechanism. This feature
requires the introduction
of the third-order
nonlinear kernel in order to describe the
response with a reasonable degree of accuracy. Similar depolarizing
(regenerative)
slow potentials
which bear close resemblance to the type C response were seen in
the turtle photoreceptors
(ref 10, Fig. 9;
ref 33, Fig. 3). It has been shown in the
catfish retina that some neurons have very
large dendritic fields, reaching nearly 1 mm
in diameter, and it is conceivable that some
sort of a regenerative mechanism is set up
in order to transmit signals over such a
large distance. In the horizontal cells this
type of problem is overcome by the formation of a laminar layer in which the potential decays much more slowly than in a
simple tubular structure (cf. ref 17, Fig. 2).
In their studies on the discharge patterns
of the catfish ganglion cells Naka and Nye
(26, 27) have reported that these cells form
concentric receptive fields in which a spot
and an annulus of light produce responses
of opposing modes; i.e., transient versus
sustained discharges. We have found that
the pattern of the type C response to a large
extent is invariant of the stimulus parameters (part 11). Therefore
there are two
possibilities: a) Naka and Nye failed to
record spikes from a class of ganglion cells,
if indeed the type C neurons are ganglion
cells; or b) type C neurons feed signals into
the ganglion cells or type Y neurons. However, the fact that type C neurons do not
exhibit any clearly defined first-order kernels indicates that the noisy but well-defined
first-order kernels seen in type Y neurons
are likely due to signals from other neurons,
such as type N or bipolar cells.
ANALYSIS
124
TABLE
catfish
NAKA,
MARMARELIS,
3. Summary of morphological
retinal neurons
AND
CHAN
and functional
characteristics of
Response
Response
polarity
Classification
Part
I
Part
II
Part
III
Spot
AnnuIus
Receptive
field
Characteristics
spotannulus
in teraction
Degree
of
Types
nonlinof
eari ty, nonlineari ty
%
Set
of
kernel
Model
Horizontal
DYHYPer
Monotonic
Synergic
Biphasic
Enhancing
h
h
0'
2
1
namic
asymmetry
saturation
Laminar
DC
de tection
Bipolar
Ba
Bb
On-center
Off -center
Amacrine
Starburst
Depol
Hyper
Depol
HYP-
hor
hl h
1'
6
0
DYnamic
asymmetry
Comparison
DC
subtraction
sustained
DeNa
Nb
Depol
HYper
Monotonic
pressing
h
Laminar?
1
Or:
none
Spaghet ti
sustained
Re-
Spindle
Transien t
C
Depol
h,, h,
45
On-off
gener
slowpotential
Ganglion
On-ten
Off-center
ter
1 -polar
P-polar
3polar
4-polar
Multipolar
Kite
Ya
HYPer
(Concentric)
Spiking
Yb
An tagonis tic
hl’ h2
25
Rectification
Spike
production
Depol
studies (11, 12, 43, 48) there are important
points of variance to be mentioned:
1) Kaneko (11) in the goldfish, Matsumoto and Naka (22) in the frog retinas reported that some of the dye-identified bipolar cells showed monotonic
receptivefield organizations, while in the catfish retina all of the dye-identified bipolar cells in
parts 11 (28) and III (this paper) had bi-
phasic receptive-field organizations. In the
mudpuppy
retina, Nelson (32) reported a
group of neurons referred to as depolarizing
bipolars in which a surround antagonism
was not seen. Three explanations are possible to account for this discrepancy: a) in the
catfish we failed to detect such bipolar
cells, b) as already mentioned by Kaneko
and also by Matsumoto and Naka the stim-
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
External
Intermediate
Internal
WHITE-NOISE
125
longer than the center (or spot) response
only by 20-30 ms. Together with the results in part II (28) we conclude that the
operating modes of bipolar cells in these
two retinas are radically different. EnrothCugell and Pinto (8) obtained evidence to
show that in some of the cat retinal ganglion cells the center-surround
interaction
could be expressed in terms of algebraic sum
of two pure responses, i.e., the responses
elicited by the center or surround signal
alone. If the cat ganglion cell response reflected the bipolar cell activity, their results are what we would expect from the
model we proposed for the catfish bipolar
cell.
Werblin and Dowling (48) were the first
to designate as amacrine cells a class of neurons which gave rise to depolarizing transient potentials with or without spikes superimposed
on them, although
similar
transient
depolarizations
had been previously seen in the frog retina by Naka et al.
(25) and were referred to as type II responses by Tomita et al. (42). Werblin and
Dowling’s identification
was supported by
Kaneko (11) who recorded from and injected Procion dye into goldfish neurons he
classified as amacrine cells because of their
transient depolarizing responses. However,
we note that Kaneko’s amacrine cell (ref 11,
plate 5) had a round soma and thick dendrites, which are characteristic of the catfish
type N neurons. Furthermore,
Matsumoto
and Naka (22) qualified their reference to
the origin of the transient depolarizations
in the frog retina as assumed amacrine cells.
Schwartz (36) was surprised to find, in the
turtle retina, somata of a class of neurons
which gave rise to on-off discharges in the
ganglion cell layer as well as in the INL.
To complicate the issue, Toyoda et al. (44)
claimed to have recorded from the carp
amacrine cells a variety of responses, including those with clear spontaneous spike
discharges, although their criteria for distinguishing
amacrine cells were questionable at best. Curiously, no report is available to indicate the presence of the type N
response in other retinas, although neurons
very similar, structurally, to type N neurons
were seen by Ramon y Cajal in many animals, including lizards (ref 5, plate IV, Fig.
9\
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
ulus parameters were inadequate in their
experiments, or c) those bipolar cells with
monotonic receptive fields are type N neurons in the catfish or vice versa.
2) In the catfish retina, illumination
of
the receptive-field
surround
(by annular
light) gives rise either to depolarizing (type
Bb bipolar cell) or hyperpolarizing
(type
Ba bipolar cell) responses, as reported in
the goldfish by Kaneko (12) and in the frog
by Matsumoto and Naka (22), while in the
mudpuppy retina, illumination
of the surround does not produce any response in the
bipolar cells (45, 46, 48). Simultaneous presentation of two stimuli, a spot and a concentric annulus of light, enhances the dynamic gain of each component (seen in all
bipolar cells identified). Thus the surround
functions, not to produce an inhibitory
influence, as commonly assumed, but to provide an integrating
signal from the horizontal cells to the bipolar cells, as originally
proposed by Naka and Nye (26).
Toyoda (43) in his study on the carp bipolar cells observed an increase in the depolarizing response evoked by a step input
in the presence of an annular illumination;
in the carp retina, however, the reverse was
not true.
In his recent study on the mudpuppy retina, Werblin (45, 46) concluded that the
presence of a background (annular) illumination brought forth a lateral shift of the
bipolar cell V-log I curve toward a lower
sensitivity. In the catfish bipolar cells a
simultaneous
presentation
of the center
(through
a spot of light) and surround
(through an annulus of light) stimuli resulted in a mutual enhancement of the dynamic gain of the two receptive-field components. If the presence of an annular input
brought forth a lateral shift of the V-log I
curve to increase the dynamic gain of the
spot component,
a similar shift for the
annular component must take place by the
presence of a spot input, a situation hard
to visualize. Therefore, the observations we
made in this paper cannot be explained by
a simple shift of V-log I curves. In the
mudpuppy it was further shown that effects
of surround illumination
were seen 250 ms
after the onset of the center response (46),
while in the catfish bipolar cells the latency
of the surround (or annular) response was
ANALYSIS
126
NAKA,
MARMARELIS,
Dynamics
of catfish
retinal
neurons
In the cat as well as in the goldfish retinas it was observed that the ganglion cell
responses (as represented by their spike discharges) changed from a strictly low-pass
filter to a band-pass filter when the intensity of the background
illumination
was
increased (9, 35). Catfish retinal neurons
show exactly the same characteristic when
the average mea .n in tensi ty level is increased; this is reflected by a transformation
of the overdamped hl at low-intensity levels
into the under-damped hl at high-intensity
levels (Fig. 25). In the cat ganglion cells
the low-frequency cutoff (i.e., the band-pass
characteristics) was proved to be due not
to lateral inhibition
from the surround but
to the nonlinear
feedback in which the
controlled transhorizon .tal ccl 1 potential
mission from the receptors to the bipolar
CHAN
cells (9). Schellart and Spekreijse (35) have
also suggested that spatial summation
played an important
role in the shift to
higher values of the cutoff frequencies.
Marmarelis and Naka (20) suggested that
the speedup of the horizontal cell responses
at higher mean intensity levels could be
explained conveniently
if the horizontal
cell potentials (integrating
signal) were assumed to feed negatively back to the receptors. The results of analysis on other
retinal neurons give support to this assump
tion.
Kaneko and Hashimoto (13) were the first
to note that the neurons in the INL had
steeper V-log I curves; their observations
were later confirmed by Werblin (45, 46).
As we have already mentioned in RESULTS,
the static V-log I curve plotted in their experiments serves as a measure of sensitivity
only and only if the system under study
has a constant-gain low-pass characteristic
(i.e., if it is not a function of frequency for
frequencies within the system bandwidth).
The horizontal cell response (generated by
a single class of receptors) is the only neuron, except for the receptors, which partially satisfies such a condition. As we have
shown in RESULTS, the dynamic range of the
catfish retinal neurons (except for the horizontal cell and possibly the receptors) is
much larger than what was predicted by the
static V-log I curves. The fact that such a
large dynamic range is seen first in the
bipolar cells gives further support to our
hypothesis that the cell acts essentially as
a comparator of two signals, each with a
large DC bias, one from the receptor and
the other from the horizontal cells.
CONCLUSIONS
A number of (about 150) white-noise experiments have been performed on the catfish retina; the stimulus consists of light
intensities exciting the center (by a spot of
light) and surround of the receptive field
(by a concentric annulus) and modulated by
independent
white-noise signals. The elici ted response is measured intracellularly
from neurons throughout
the retina. For
each two-input
experiment
a set of five
functions (kernels) is computed from these
stimulus-response data., This set of kernels
describes completely the nonlinear dynamic
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
In the catfish retina we have shown conclusively in parts II (28) and III (this paper)
that the majority
of neurons which fit
Ram&r y Cajal’s description (5) of amacrine
cells produced on 1y the type N response
any
(1inear, sustained response without
high-frequency
component), whi .e depolarizing on-off responses (type C responses)
were recorded from a class of neurons with
the characteristic spindle-shaped cell body.
In part II it was further shown that each of
these type C neurons had a characteristic
process which descended down to the ganglion cell layer.
Here we recall our definition of type C
responses as those which lack well-defined
first-order kernels, necessitating a thirdorder term to describe their responses.
When tested by step inputs, the resulting
responses are transient depolarizations
at
the on- and offset of pulses. Some ganglion
cells (type Y neurons) produce similar transient on-off depolarizations
in response to
step inputs (26, 27), but such responses are
modeled with reasonable accuracy with the
first- and second-order terms (18-20).
Clearly, these transient depolarizations
from amacrine cells published so far are
not well documented
in terms of their
functional
and morphological
characteristics and, therefore, we are not in a position to resolve the di screpancy between the
results in the catfish and other animals.
AND
WHITE-NOISE
127
ulations give rise to responses which are synergistic. The static and dynamic ranges of
the cells are limited, and the system is essentially a low-pass filter which detects mainly
the amplitude
of the input signal. The
functional significance of the cells is twofold; first, they integrate the signal over
the entire retinal area and then transmit it
to the bipolar cells, and second, the same
signal is fed back to the receptors to improve their frequency response (and, consequently, that of the subsequent stages).
2) Bipolar cells. There are two opposing
subtypes, one on-center and the other offcenter bipolar cells, both of which are linear
(within the depth of modulation)
and show
characteristics of a low-pass to band-pass
filter. The functional parameters of bipolar
cells cover a large range, as evidenced by
the loose clusters in the scattergram in the
APPENDIX.
In the bipolar cells, stimulation
by a spot of light produces an overdamped
kernel, which is transformed in the presence of an annular input into an underdamped kernel, accompanied
also by a
shortening of the latency and the peak response time. The annular response has always longer latency and peak response time,
but is faster frequencywise, than the spot
response. In the bipolar cells two receptivefield components, one a local signal activated by a spot input and the other an integrating signal derived from the horizontal
cell, are mutually enhancing. All evidence
obtained so far supports the original contention by Naka and Nye (26) that the bipolar cells act as a comparator of the two
signals.
3) Type N neurons. There are two types,
Na which depolarizes and Nb which hyperpolarizes, to any form of input. The neurons are linear, within the depth of modulation used and show characteristics of a
low-pass to band-pass filter without any highfrequency
components.
Morphologically,
these neurons correspond to most of the amacrine cells described by Ramon y Cajal
(3, including the giant amacrine cells in
lizards.
4) Type C neurons. This neuron is highly
nonlinear, requiring a third-order term to
model the response with a reasonable degree
of accuracy. It does not produce any welldefined first-order kernels but has a charac-
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
behavior of each neuron. In still other experiments a single white-noise stimulus is
used (a field or spot or annulus only) and
a similar set of characterizing
kernels is
measured.
The method allows us to describe separately (in the two-input
experiments) the
contributions
(linear as well as nonlinear)
of each input to the response. Furthermore,
as applied to intracellular
recordings, it
allows us nearly to maximize the amount of
diverse stimulus-response data we can obtain over a limited amount of time and it
greatly alleviates the problems caused by
unwanted noise. Besides these advantages,
the universal nature of the white-noise signals results in a global functional identification of each neuron (i.e., over its entire
operational range) and the characterization
in terms of the kernels is given in a canonical form (i.e., in the same fixed format for
all neurons). These three features of the
method, in turn, allow us to proceed in an
objective and efficient way with the functional classification of the retinal neurons.
We have correlated the morphology
of
the catfish retinal neurons with their (nonlinear) dynamic characteristics as derived
from white-noise stimulation.
From such
combined structural and functional studies
we propose to revise the classical classificcation of the retinal neurons, at least in
the catfish retina, based on the functional
properties of each class of neurons as repres$ted by a small set of kernels.
: In our scheme the neurons are classified
as horizontal, bipolar, and types N, C, and
Y neurons, the latter three types encompassing cells otherwise known as amacrine and
ganglion cells. The objectivity of this categorization system and the degree of separation among the types of neurons (or the
clusters formed by classes of neurons) are
shown in a scatter-gram in the APPENDIX,
in
which morphologically
identified neurons
are grouped according to their common
function al features.
The characteristics of the five types of
catfish neurons are:
1) Horizontal cells. Both the external and
internal (and probably the intermediate,
cells form independent
too) horizontal
monotonic
receptive fields through
the
S space, in which center and surround stim-
ANALYSIS
128
NAKA,
MARMARELIS,
APPENDIX
As discussed,
the white-noise
stimulus
is a type
of universal
probe with which
to test a system.
The
resulting
characterization
of the system
in terms of
a set of kernels
is therefore
a global
picture
of the
system
functional
characteristic
and it is given
in
a canonical
form;
i.e., it describes
the system
behavior
over its entire
operational
range
and it is
always
given
in terms
of a set of kernels
of fixed
format.
It is exactly
these three
features,
i.e., the
stimulus,
the
the
white-noise
universality
of
globality
of the functional
characterization,
and
the canonicity
of the kernels,
together
with
the fact
that for all our experiments
in the retina
a fixed
geometric
configuration
(a spot
and a concentric
annulus)
is employed,
that lead us naturally
to the
following
intriguing
questions:
If the kernels
of all
the retinal
neurons
are grouped
into categories
according
to their
features,
a) Do they form
distinct
groups
(clusters)?
b) If yes, do these clusters
correspond,
on a one-to-one
basis, to clusters
formed
by
grouping
the neurons
according
to morphological
features?
c) Can we associate
each cluster
with
a
particular
class of neurons
(e.g., horizontal
cells, bihow
many
distinct
polar
cells, etc.). 7 d) Finally,
classes of neurons
are there in the vertebrate
retina,
and what
are their
average
functional
and morphological
characteristics?
Relying
on the results
of the two-input
white-
CHAN
noise experiments
of the present
study,
we have
performed
a limited
preliminary
anaiysis
in an
attempt
to answer
these questions.
We consider
all
the
two-input
white-noise
experiments
we have
performed
so far on the vertebrate
retina
for which
experimental
conditions
were
approximately
the
-same, such conditions
as levels of the spot and annulus
mean
lights,
length
of experiment,
equal
bandwidth
white-noise
signals,
and the like. For all
these experiments,
each performed
on a different
unit, we have computed
kernels
(hls,a,
hla,B, h2s,a,
h 2a,s, h,,) as well as their responses
to white-noise,
reductions
in MSE, power
spectra,
etc. (as described
previously).
The number
of these experiments
(or
neural
units) is 147. The set of kernels
for each unit
constitutes
a “signature”
of the neuron.
Imagine,
now, each set of kernels
as a point
in an n-dimensional space, the position
(coordinates)
of this point
depending
on the features
of all these kernels.
Then,
different
(functional)
classes of neurons
will
form
different
clusters
of these
points
in this
space.
Clearly,
then,
such a “PlO t” of the kernels
will
allow
us to give
definite
answer
to questions
a
through
d above.
more complete
functional
signature of the neuron
should
include
also the oneinput
kernels,
hrs, hZs, hIa, and h,,.
In this preliminary
study,
we consider
only two
indirect
features
of the kernels
and plot them in a
two-dimensional
space. Both these features
are estimated from the MSE reduction.
The first one simply
measures
how nonlinear
the neuron
is. The second
one measures
the relative
contribution
to the response
of each receptive-field
component
(spot and
annulus).
Both
these measures
are positive
quantities. One addi tional
piece of information
is plot ted
on this plane,
the direction
of polarization
thYperor de-) for each input
(spot
and annulus).
The
latter
is simply
accomplished
by utilizing
the four
quadrants
of the plane
(if hl,s,a
and hl,,*
both
hyperpolarize,
the point
is plotted
in quadrant
I.
depolarizes
and hla,a
hyperpolarizes,
the
point is plotted
in quadrant
II, etc.). The indexes
of
nonlinearity
and relative
input
contribution
for each
neuron
are estimated
as follows.
Let the MSE of
model
response
LMs,a
be El9 and LMa 8 be E,,.
Let the MSE of the linear
model,
i.e., t h e sum of
responses
LMEI,a and LM,,B,
be E,. Let the MSE
of the total model response
MR (computed
from all
linear
and nonlinear
kernels)
be E,. Then,
we define the index
of nonlinearity
In gr each neuron
to be
100 - E,
In =
100 - E,
That
is, the ratio
of MSE reduction
of the nonlinear representation
divided
bY the MSE reduction
of the linear
representation.
Th us, the larger
In is,
the larger is the nonlineari
linear
tY* For a perfectly
neuron
In = 1. The index
of relative
con tri bu tion
Ire of each input
(spot and annulus)
is defined
as
E 1s
-1
(100 - EJ - (100 - E&
E,
-Ire =
= -Ela
(100 - EJ - (100 - El,)
M
If hlf3/a
--1
El
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
teristic second-order kernel which can be
used as a signature to identify the neuron.
Morphologically,
the type C neuron corresponds to those neurons with a spindleshaped soma, commonly found in the INL.
Type C response may or may not be accompanied by spike discharges.
5) Type Y neurons. This neuron is characterized by a noisy but well-defined first-order
kernel and fits more closely the description
of the classical ganglion cells. The response
is normally
accompanied
by spike discharges, the amplitudes of which vary from
neuron to neuron. This neuron type is
probably composed of a large variety of subtypes. Type Y neurons produce either sustained or transient responses to step inputs.
In conclusion, the results obtained in this
series of papers indicate that the relation of
the function of a type of neurons to the
underlying morphology is not as simple or
homologous as hitherto assumed, and any
such correlation at the present time must be
statistical rather than a discrete one-to-one
correspondence. The application of the intracellular dye-injection
technique to the
neurons in the central nervous system for
identification
purposes must be conducted
with prudence.
AND
WHITE-NOISE
ANALYSIS
of the
Note that (100 - EJ is the MSE reduction
total linear
representation,
(100 - El*) is the reduction of the spot component,
and (100 - El,) is the
reduction
of the annulus
component.
In general,
(100 - EJ
+
(100 - ErJ
+ (100
- Er,)
II
!2
I
z
3
In
The
following
observation
can be made
at first
glance:
1. For neurons
exhibiting
high degrees
of
nonlinearity,
the response
contributions
of the spot
(s) and annulus
(a) are about equal, i.e., Ire is always
near 1. 2) The nonlinear
neurons
are concentrated
in quadrants
I and III. That
is, for the nonlinear
neurons
in the retina,
the spot and annular
inputs
produce
polarizations
of the same direction,
i.e.,
either
both hyperpolarize
(quadrant
I) or both depolarize
(quadrant
III).
In this scattergram
dotted
lines are drawn
to indicate
clusters
of neurons
with
similar
functional
and morphological
characteristics,
as described
in
RESULTS. We note:
1) The type C neurons
form a well-distinguished
cluster
in the III quadrant.
The polarity
of the h,
was arbitrarily
chosen
in the depolarizing
direction
(because
of the noisy
h, from
type C neurons
it
was not possible
to indicate
clearly
its polarity).
The choice of depolarizing
direction
is based on the
fact that the response
of the neuron
is depolarizing
transients.
Type C neurons
are highly
nonlinear.
2) The
type
N neurons
form
one well-distinguished
cluster
for Na in the III quadrant
and one
cluster
for Nb in the I quadrant.
In type N neurons
In is small, while
Ire is large; their linear
responses
are largely
due to annular
inputs.
3) There
are two classes of bipolar
cells (Ba and
Bb) in quadrants
II and IV. However,
their clusters
are not well defined
and are rather
expansive,
indieating a large degree of variation
in their response
parameters.
This
may be due to the various
sizes
I
a:hyp
s:dep
a : hyp
s : hyp
a: dep
s:dep
a : dep
s : hyp
e Ire
Ire*
ID
m
In
0
BIPOLAR
0
--
*
$-,
-
.-&-
-,--L-f
(cx3 3 93
.---
0
Ba _ --.
- -
\>
\
0
larger
annular
[a]
Ya NEURONS=?‘1
=------------
bI
contribution
[a]
/c--N
//I14 o’, \
19 cl ’\
II G ,cl II
I
larger
[SI
annular
e
contribution
C NEURONS
FIG.
27.
Scattergram
for
functional
classification
of the
catfish
retinal
neurons.
For
details
see the
text.
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
of the spot and
If Els = Ela, i.e., the contributions
annular
inputs
are equal,
the Ire = 1. If Ire > 1
then
the contribution
of the annular
signal
is
greater
than
the contribution
of the spot signal,
i.e., in a sense, the annular
stimulus
is more effective
in eliciting
a response
from
the neuron.
The
reverse holds
(spot is more
effective)
if Ire < 1.
For all the neurons
in the catfish
retina
for which
two-input
white-noise
experiments
were performed,
indexes
In and Ire were computed.
There
are 14’7
of them
corresponding
to the number
of experiments.
Each experiment
(neuron)
is plotted
on the
plane according
to its In and Ire indexes
(as coordinates).
Both of these indexes
are positive
numbers.
However,
one of the four quadrants
is chosen
in
each case according
to the direction
of polarization
of the cell potential
for spot and annulus
stimuli:
quadrant
I:
h, ,a hyperpolarizes,
hIa,*
hyperPJ arizes
quadrant
II:
h,,/,
depolarizes,
hla,g
hyperpolarizes
quadrant
III:
hlg,a depolarizes,
h1a,s
depolarizes
quadrant
IV:
hIala
hyperpolarizes,
hla,B depolarizes
The plot of the neuron
labels is shown
in Fig. 27.
129
130
NAKA,
MARMARELIS,
CHAN
belong
either
to one of the other
clusters
or,
properly,
form
a new separate
cluster.
As noted earlier,
this is only a preliminary
analysis of the clustering
of the retinal
neurons
according
to their
functional
traits
(as reflected
by their
sets
of kernels).
Although
it is obviously
limited-since
it considers
only
two parameters
(degree
of nonlinearity
and relative
contribution
of each receptivefield component)
plus the polarizations-the
results
seem to be impressive
in two aspects:
a) this analysis is congruent
to our
subjective
classification
scheme
presented
in RESULTS and b) it could classify
to a satisfactory
degree
75%
of all the neurons
analyzed.
Considering
the difficulties
involved
in
our
experiments,
which
encompassed
white-noise
analysis
and Procion
dye injection,
we think
this
is a very favorable
score.
A more
complete
study
of “clustering”
of these
neurons
according
to their
functional
traits
(kernels) would
have
to take into
account
a host of
other
features
reflecting
the response
dynamics
of
each
neuron
(e.g.,
latency,
peak
response
time,
damping,
forms of nonlinear
kernels,
etc.). It might
alto be possible
to produce
a similar
scattergram
of
these
neurons
according
to their
morphological
traits and correlate
in a more quantitative
(and objective)
fashion
the structure
of a given
class of
neurons
to their functional
traits.
ACKNOWLEDGMENTS
The
Service
research
Grants
was supported
by
NS 10628, EY 00898,
Public
Health
and NB 19234.
REFERENCES
1. BAYLOR, D. A. AND FUORTES, M. G. F. Electrical responses
of single
cones in the retina
of
London
207: 77-92,
1970.
the turtle.
J. Physiol.,
2. BAYLOR, D. A., FUORTES, M. G. F., AND O’BRYAN,
P. M. Receptive
fields of single
cones in the
retina
of the turtle.
J. Physiol.,
London
214:
265-294,
1971.
3. BAYLOR,
D. A. AND HODGKIN,
A. L.
Detection
and resolution
of visual stimuli
by turtle
photo163-198,
receptors.
J. Physiol,
London
234:
1973.
4. BLACKMAN,
R. B. AND TUKEY,
J. W.
The
Measurement
of Power
Spectra.
New
York:
Dover,
1958.
5. CAJAL,
RAM~N
Y, S. The
Structure
of the
Retina,
translated
by S. A. Thorpe
and
M.
Glickstein.
Springfield,
Ill.: Thomas,
1972.
6. DOWLING,
J. E. AND RIPPS, H.
Adaptation
in
skate photoreceptors.
J. Gen. Physiol.
60: 698716, 1972.
7. DOWLINC,
J. E. AND WERBLIN,
F. S. Organization
of retina
of the mudpuppy,
Necturus
macuZosus.
I. Synaptic
structure.
J. Neurophysiol.
32: 315-338,
1969.
8. ENROTH-CUGELL,
C. AND PINTO,
L. H.
Properties
of the surround
response
mechanism
of
cat retinal
ganglion
cells and center-surround
interaction.
J. Physiol.,
London
220: 403439,
1972.
9.
10.
11.
12.
13.
14.
15.
16.
ENROTH-CUGELL,
C.
AND
SHAPLEY,
R.
M.
Adaptation
and dynamics
of cat retinal
ganglion
cells. J. Physiol.,
London
233: 271-309,
1973.
FUORTES, M. G. F., SCHWARTZ,
E. A., AND SIMON,
E. J. Color-dependence
of cone responses
in
the turtle
retina.
J. Physiol.,
London
234: 199216, 1973.
KANEKO,
A. Physiological
and morphological
identification
of horizontal,
bipolar
and amacrine cells in goldfish
retina. J. Physiol.,
London
207: 623-633,
1970.
KANEKO,
A. Receptive
field
organization
of
bipolar
and amacrine
cells in the goldfish
retina.
J. Physiol.,
London
235: 133-153,
1973.
KANEKO,
A. AND HASHIMOTO,
H.
Electrophysiological
study
of single
neurons
in the inner
nuclear
layer
of the carp retina.
Vision
Res.
9: 37-55,
1969.
KANEKO,
A. AND YAMADA,
M.
S-potentials
in
the dark-adapted
retina
of the carp. J. Physiol.,
London
227: 261-273,
1972.
LEE, Y. W. AND SCHETZEN, M.
Measurement
of
the Kernels
of a Nonlinear
System
by CrossCorrelation.
Quarterly
Progress
Report
No. 60.
Cambridge:
Massachusetts
Institute
of Technology
Research
Laboratory
of
Electronics,
1961.
M~ARELIS,
P. Z. AND NAKA,
K.-I.
Whitenoise analysis
of a neuron
chain:
an application
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
of dendritic
expansion
or varying
degree of contributions
from
different
receptors.
4) The
horizontal
cells,
both
internal
and external,
form
very close clusters
indicating
a very
small difference
in the response
parameters
seen in
the cells. As we have
already
mentioned,
this is
probably
due to the fact that they form a syncytium
(or laminar
layer)
in which
responses
from
individual cells are pooled
and averaged
(S space of Naka
and Rushton,
ref SO).
I) The type Y neurons
tend to be rather
diffuse
in this plane, giving credence
to our impression
from
the functional
and
structural
studies
that
these
neurons
encompass
a whole spectrum
of functional
and structural
characteristics.
However,
they
tend
to have intermediate
values
of In (around
1.5-2)
and intermediate
values
of Ire. There
are two subclasses of type Y neurons:
one, type Ya, in the III
quadrant
and the other,
Yb, in the I quadrant.
These
two types of Y neurons
are seen in Fig. 22 in
which
A is for type Yb and B is for type Ya.
111 are classified
into 8
6) Out of 147 neurons,
clusters;
36 neurons
(or 25 %) are left unclassified,
due to a failure
either
by us to identify
them positively
in Procion
preparations
and/or
by them
to
meet
the functional
criteria
of a given
class of
neurons.
Those
unclassified
neurons
between
the
two clusters
Yb and Nb are morphologically
unidentified,
but functionally
they could
possibly
be
included
in either
one of the two nearby
clusters.
Those
in quadrant
I close to the origin
of the axes
do not fit any of the functional
criteria
used to
classify
neurons
in this study;
it is possible
that they
AND
WHITE-NOISE
of Wiener’s
1972.
17.
theory.
Science
175:
1276-1278,
32.
MARMARELIS,
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
131
NELSON,
R. A comparison
of electrical
properties of neurons
in Necturus
retina.
J. Neurophysiol.
34: 519535.
O’BRIEN,
P. M.
Properties
of the depolarizing
synaptic
potential
evoked
by
peripheral
illumination
in cones of the turtle
retina.
J.
Physiol.,
London
235: 207-223,
1973.
SAITO, T., MILLER,
W. H., AND TOMITA,
T.
Cand L-type
horizontal
cells in the turtle
retina.
Vision Res. 14: 119-123,
1974.
SCHELLART,
N. A. M. AND SPEKREIJSE, N.
Dynamic
characteristics
of retinal
ganglion
cell responses
in goldfish.
J. Gen. Physiol.
59: l-21,
1972.
SCHWARTZ,
E. A. Organization
of on-off
cells
in the retina
of the turtle.
J. Physiol.,
London
230: l-14,
1973.
SIMON,
E. J. Two
types
of luminosity
horizontal
cells
in the retina
of the
turtle.
J.
Physiol.,
London
230: 199-211,
1973.
SPEKREI JSE, H.
Rectification
in the goldfish
retina.
Analysis
by sinusoidal
and auxiliary
stimulation.
Vision
Res. 9: 1461-1472,
1969.
STARK, L.
Neurological
Control
Systems.
New
York:
Plenum,
1968.
STELL, W. K.
The morphological
organization
of the vertebrate
retina.
In: The Handbook
of
Sensory
Physiology.
Physiology
of Photoreceptor
Organs,
edited
by M. G. F. Fuortes.
Berlin:
Springer,
1971, vol. VII/Z,
p. 111-213.
TOMITA,
T.
Electrophysiological
study
of the
mechanisms
subserving
color coding
in the fish
retina.
Cold Spring
Harbor
Symp.
@ant.
Biot.
30: 559-566,
1965.
TOMITA,
T.,
MURAKAMI,
M., HASHIMOTO,
Y.,
AND SASAKI, Y. Electrical
activity
of single
neurons
in the frog’s
retina.
In:
The
Visual
System:
Neurophysiology
and
Psychophysics,
edited
by R. Jung
and H. Kornhuber.
Berlin:
Springer,
1961, p. 24-30.
TOYODA,
J. Membrane
resistance
changes
underlying
the bipolar
cell response
in the carp
retina.
Vision
Res. 13: 283-294,
1973.
TOYODA, J., HASHIMOTO,
H., AND OHTSU, K. Bipolar-amacrine
transmission
in the carp retina.
Vision
Res. 13: 295-307,
1973.
WERBLIN,
F. S. Adaptation
in a vertebrate
retina:
intracellular
recording
in Necturus.
J.
Neurophysiol.
34: 228-241,
1971.
WERBLIN,
F. S. Control
of retinal
sensitivity.
II. Lateral
interactions
at the outer
plexiform
layer. J. Gen. Physiot.
63: 62-87, 1974.
WERBLIN,
F. S. AND COPENHAGEN,
D. R.
Control
of retinal
sensitivity.
III.
Lateral
interactions
at the inner
plexiform
layer.
J. Gen.
Physiol.
63: 88-110,
1974.
WERBLIN,
F. S. AND DOWLINC,
J. E. Organization of the retina
of the mudpuppy,
Necturus
macutosus.
II. Intracellular
recording.
J. Neurophysiol.
32: 339-355,
1969
WIENER,
N.
Nonlinear
Problems
in Random
Theory.
New York:
Wiley,
1958.
Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 18, 2017
P. 2. AND NAKA,
K.-I.
Spatial
distribution
of potential
in a flat cell: application
to the
catfish
horizontal
cell
layers.
Biophys.
J. 12: 1515-1532,
1972.
18. MARMARELIS,
P. 2. AND NAKA, K.-I.
Nonlinear
analysis
and
synthesis
of receptive-field
responses in the catfish
retina.
I. Horizontal
cell
to ganglion
cell chain. J. Neurophysiol.
36: 605618, 1973.
19. MARURELIS,
P. Z. AND NAKA, K.-I.
Nonlinear
analysis
and
synthesis
of receptiveifield
responses
in the catfish
retina.
II.
One-input
white-noise
analysis.
J. Neurophysiol.
36: 619633, 1973.
P. Z. AND NAKA, K.-I.
Nonlinear
20. MARMARELIS,
analysis
and
synthesis
of receptive-field
responses
in the catfish
retina.
III.
Two-input
white-noise
analysis.
J. Neurophysiol.
36: 634~
648, 1973.
P. Z. AND NAKA, K.-I.
Identifica21. MARMARELIS,
tion
of multi-input
biological
systems.
IEEE
Trans. Bio-Med.
21: 88-101,
1974.
N. AND NAKA, K.-I.
Identification
22. MATSUMOTO,
of intracellular
responses
in the frog
retina.
Brain
Res. 42: 59-71,
1972.
W. H., HASHIMOTO,
Y., SAITO, T., AND
23. MILLER,
TOMITA,
T.
Physiological
and morphological
identification
of L- and C-types
S-potentials
in
the turtle
retina.
Vision Res. 13: 443-447,
1973.
K.-I.
AND CARRAWAY,
N. R. G. Mor24. NAKA,
phological
and
functional
identifications
of
catfish
retinal
neurons.
I. Classical
morphology.
J. Neurophysiol
38: 53-71,
1975.
INOMA,
S., KOSUCI, Y., AND TONG,
25. NAKA, K.-I.,
C.
Recording
of action
potentials
from
single
cells in the frog retina.
Jupan.
J. Physiol.
10:
436442,
1960.
AND NYE,
P. W.
Receptive-field
26. NAICA, K.-I.
organization
of the catfish
retina:
Are at least
two
lateral
mechanisms
involved?
J. Neurophysiol.
33: 625-642,
1970.
Role of horizon27. NAKA, K.-I. AND NYE, P. W.
tal cells in organization
of the catfish
retinal
receptive
field.
J. Neurophysiol.
34: 785-801,
1971.
K.-I.
AND OHTSUKA,
T.
Morphological
28. NA~A,
and functional
identifications
of catfish
retinal
neurons.
II.
Morphological
identification.
J.
Neurophysiol.
38: 72-91, 1975.
K.-I.
AND
RUSHTON,
W.
A.
H.
S29. NAKA,
potentials
from color units in the retina
of the
fish (Cyprinidae).
J. Physiol.,
London
185: 536
555, 1966.
30. NAKA,
K.-I.
AND RUSHTON,
W. A. H.
The
generation
and spread
of S-potentials
in fish
(Cyprinidae).
J. Physiol.,
London
192: 437-461,
1967.
K.-I.
AND WITKOVSKY,
P. Dogfish
gan31. NAKA,
glion
cell discharges
resulting
from
extrinsic
polarization
of the horizontal
cells. J. Physiol.,
London
223: 449-460,
1972.
ANALIZSB