* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 521 THE CHOLINERGIC LIMBIC SYSTEM: PROJECTIONS TO
Holonomic brain theory wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Subventricular zone wikipedia , lookup
Metastability in the brain wikipedia , lookup
Apical dendrite wikipedia , lookup
Haemodynamic response wikipedia , lookup
Development of the nervous system wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroeconomics wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroplasticity wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
Basal ganglia wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Circumventricular organs wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Synaptic gating wikipedia , lookup
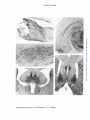
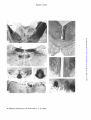
521 BY P. R. LEWIS AND C. C. D. SHUTE (From the Anatomy School, University of Cambridge) INTRODUCTION EVIDENCE has accumulated for a close functional relationship between the brain-stem reticular formation and the hippocampal formation (hippocampus and dentate gyrus) of the fore-brain. On the one hand, it has been shown that stimulation of the mid-brain reticular formation not only causes desynchronization of the neocortical EEG, inhibiting slow rhythms and replacing them with high frequency low amplitude activity, but also produces a synchronized hippocampal EEG, with slow high amplitude waves (e rhythm: Jung and Kommuller, 1938; Green and Arduini, 1954), on which fast activity may be superimposed. The significance, however, of these different patterns of electrical activity is not understood. It has also been suggested that the hippocampus may itself modify the activity of the reticular formation and through it modulate the activity of the cerebral cortex, but whether in such a way as to produce facilitation or suppression is not clear (Green, 1960). Apart from the fact that reticular influences probably reach the hippocampus via the septum, the mechanism by which the reticular formation and the hippocampus are able to interact is quite unknown. Our studies on the distribution of acetylcholinesterase (AChE) in rat brain have shown: (1) that ascending reticular pathways from the brainstem contain AChE along their length and are probably cholinergic, and (2) that AChE-containing neurones, proven to be cholinergic, project on to the hippocampal formation from the septum (for references, see preceding 22 BRAIN—VOL. XC Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 THE CHOLINERGIC LIMBIC SYSTEM: PROJECTIONS TO HIPPOCAMPAL FORMATION, MEDIAL CORTEX, NUCLEI OF THE ASCENDING CHOLINERGIC RETICULAR SYSTEM, AND THE SUBFORNICAL ORGAN AND SUPRA-OPTIC CREST. 522 P. R. LEWIS AND C. C. D. SHUTE paper Shute and Lewis, 1967). We have also been struck by the fact that a number of regions in the mid-brain and diencephalon which are known to receive projections from the hippocampus are rich in AChE, and so may be cholinergic centres. In this paper we describe in detail the routes taken by the cholinergic hippocampal afferent fibres, the locations of AChE-containing nuclei receiving hippocampal efferents, the connexions made by some of these nuclei with the AChE-containing ascending reticular system, and the projections of others on to the areas of medial cortex which constitute the so-called limbic lobe. MATERIALS AND METHODS OBSERVATIONS (i) The septal radiation to the hippocampalformation Hippocampal afferents other than those arising from the septal region, e.g. those derived from the entorhinal cortex (temporo-ammonic fibres), from the dentate gyms terminating on the hippocampus proper (mossy fibres), and from the hippocampus of the opposite side (commissural fibres) contain no cholinesterase and may be presumed to be non-cholinergjc. The fibres which arise from the medial septal nucleus and the nucleus of the diagonal band, on the other hand, have been shown to be cholinergic (Lewis, Shute and Silver, 1964), and supply the hippocampus proper, the dentate gyms and the transitional region known as the subiculum. These fibres, constituting what we have termed the septal radiation (Shute and Lewis, 19636, c), travel to the hippocampal formation via the medial supracallosal stria of Lancisi, the dorsal fornix, the alveus and the fimbria (fig. A). In the fimbria the cholinergic fibres are concentrated on its outer side. Afferent hippocampal-fibres also travel in the inner part of the fimbria, but these are mainly commissural fibres. Some details of the regional distribution of the septal radiation can be made out from normal material. The supra-callosal stria is presumably afferent to the dorsal hippocampal rudiment, which lies at the base of the Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 Stereotaxic lesions were placed so as to interrupt the cholinergic afferent hippocampal pathways in anesthetized rats, which were then allowed to survive long enough to produce accumulation of AChE on the cell body side and loss of AChE on the opposite side of the cut fibres. The rationale of this method has already been discussed (Shute and Lewis, 1967). AChE was rendered visible in serial frozen sections by a modification ofthethiochoUne method, with 10-*M ethopropazine added as an inhibitor of non-specific cholinesterase (ChE). Since a number of nuclei receiving hippocampal projections were found to contain ChE as well as AChE, in many series alternate sections were incubated with butyrylthiocholine instead of acetylthiocholine as substrate. As in our previous study, a propionylthiocholine substrate was occasionally used, combined either with 10~*M ethopropazine to show AChE or with 5 x 10—*M 62 C 47e (an AChE inhibitor) to show ChE. Suppression of AChE staining made the connexions of ChE-containing nuclei much easier to detect. 523 CHOLINERGIC LIMBIC SYSTEM SEPTAL RADIATION SUPRACAUOSAl STtIA (IO CNOU1AJE CTMTlXl OOKSAl FCKINIX ITO DOOM Htrroc MEDIAL SEPTAL N. ICU.US ttltOfUXUl (TO vtNTIM »9fOCAMrU%) Fio. A.—Expanded diagram (natural spacing of transverse sections increased five times) showing the septal radiation of cholinergic fibres arising from the medial septal nucleus (MS) and the nucleus of the diagonal band (DB), and supplying the subfomical organ (SFO), cingulate cortex via the supracallosal stria (SS), dorsal hippocampus (DH) via the dorsal fornis (DF) and alveus (AL), and ventral hippocampus (VH) via the fimbria (FI). The fasciculus retroflexus, with cholinesterase-containing fibres running from the habenular nuclei (H) to the interpeduncular nucleus (IP), is also included. cingulate cortex immediately above the corpus callosum. Some fibres of the stria turn dorsally in layer i of the cingulate cortex to innervate its superior part. Otherfibre'sreach the subiculum by looping down behind the splenium of the corpus callosum. The dorsal fornix innervates the medial part of the dorsal hippocampal formation from its anterior end. Using the nomenclature of Lorente de N6 (1933, 1934), the more medial fibres of the dorsal fornix supply hippocampal area CAi and the adjacent subiculum, also the dentate gyms, especially at the apex of its curvature, and the anterior tip of hippocampal area CA4, which lies in the hilum of the dentate gyms. The more lateral fibres of the dorsal fornix diverge in a lateral direction to supply hippocampal area CAa. The alveus and upper part of the fimbria supply especially area CAa, and also areas CA2 and CA« of the dorsal hippocampus, and the hilum of the dentate gyrus adjacent to CA4. The bulk of the fimbria innervates the ventral hippocampal formation. "The Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 N. DIAGONAL BAND 524 P. R. LEWIS AND C. C. D. SHUTE regional innervation of the dorsal hippocampus and dentate gyms are illustrated in fig. B. The cholinergic hippocampal afferents break up into a preterminal, neuropil Which contains most of the AChE of the hippocampal formation, (Shute and Lewis, 1966). The exizyme is located in definite layers (Shute and Lewis, 1961). In the stratum oriens of the hippocampus proper, AChE is aggregated at the base of the pyramidal cell layer, i.e. in the region of the basal pyramidal dendrites, and extends between the bodies of the pyramidal cells. The staining is far heavier in area CA3 than in other hippocampal areas. Enzyme is also found in the stratum radiatum in the region of the mossy fibres, i.e. in association with the bases of the apical pyramidal dendrites, and in the stratum lacunosum, which is formed by horizontal branches of the apical dendrites. In the dentate gyrus, AChE is found on either side of the granular layer, and the staining is heavier on the side remote from the hilum. Little enzyme can be seen between the granular cells, which are very tightly packed. In addition to the staining due to neuropil, AChE is found in the cell bodies of Golgi type II neurones scattered in the stratum oriens, and more densely aggregated in the hilum of the dentate gyms. Regional differences in the intensity of staining for AChE in different parts of the hippocampal formation seem to be determined by the mode of approach of the cholinergic fibres. Thus, the heavy staining in area CA 3 deep to the pyramidal cells lies opposite the main inflow from the alveus and fimbria, while that of the anterior ends of the subiculum, area CAX and the apex of the dentate above the granular cells, is close to the site of entry of fibres from the dorsal fornix (fig. B). In regions of the hippocampal Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 Fio. B.—Transverse section through the dorsal hippocampal formation showing the route taken by cholinergic afferents from the dorsal fornix (DF), alveus (AL) and flmbria (FI) to the hilum (HI) of the dentate gyrus and to hippocampal areas CAU CA, CA, and CA,. Cholinergic fibres in the ventral part of the fimbria supply the ventral hippocampus (VH). CHOLINERGIC L1MBIC SYSTEM 525 Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 formation more remote from the main inflow, the cholinergic neuropil is more dispersed. The direction of fibre travel in the septal radiation was confirmed by lesions involving its various components. In an experiment with a survival period of four days, AChE accumulated rostral to the lesion in fibres of the medial supracallosal stria and the dorsal fornix. Loss of staining occurred caudal to the lesion in the supracallosal stria and dorsal fornix, and in the dorsal hippocampal formation (area CAX and dentate gyrus). In another four-day experiment, enzyme accumulated in the lateral fibres of the dorsal fornix supplying hippocampal area CA,. Lesions of the alveus and fimbria were produced in animals which were allowed to survive for 4, 5, 6, 7, 25 and 30 days. In the short term experiments accumulation of AChE occurred rostral to the lesion (PI. LXVIII,fig. 1) and in the six-day animal could be traced as far forwards as the medial septal nucleus. Some residual excess of enzyme was still present in the twenty-five-day animal. A comparison of the normal and operated sides in the five-day experiment showed that not all AChE-containing fibres in the fimbria were accumulating enzyme as a result of being divided. Approximately one quarter of the total number of cholinergic fibres visible by light microscopy appeared to respond in this way. In all the animals subjected to interruption of the alveus and fimbria, AChE was found to have disappeared from fibres caudal to the lesion, and especially from the layers of terminal neuropil in the hippocampal formation. The AChE-containing Golgi cells of the hippocampal stratum oriens and those of the hilum of the dentate gyrus were unaffected (PL LXVm, fig. 2). In cases where the fimbria alone was involved, as in the five-day experiment, the loss of hippocampal staining was confined to the ventral hippocampal formation below the flexure (PL LXV1TI, fig. 4, VH). In the seven and twenty-five-day experiments much of the alveus (especially that part adjacent to area CA3) was involved as well as fimbria, and the extent of enzyme loss included the posterior end of the dorsal hippocampal formation, immediately above the flexure. These animals also showed loss of staining in the posterior end of the medial amygdaloid nucleus, which becomes continuous with the ventral hippocampus. In the animals with fimbrial lesions which survived for twenty-five and thirty days, adjacent sections were stained by the thiochohne technique and by a modified Bielschowsky silver method. In each case an attempt was made to compare on the operated side, in sections in front of the lesion, the number of AChE-containing fibres in the fimbria with the number of fimbrial fibres which still stained with silver. Accurate counting was only possible along the lateral margin of the fimbria. Here the number of fibres present in thiocholine and silver stained sections were found to be approximately equal, suggesting that, in this part of the fimbria, the afferent hippocampal fibres are predominantly cholinergic. 526 P. R. LEWIS AND C. C. D. SHUTE Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 (2) Presumed cholinergic nuclei innervated by the hippocampal fornix projection system. The main projection pathway from the hippocampus, which arises from the pyramidal cells, travels in the fornix and ends mainly on the medial mammillary nuclei, does not contain AChE. The same is true of the mammillo-thalamic and mammiUo-tegmental tracts, which arise from the medial mammillary nuclei, and project respectively to the anterior thalamic nuclei and to the dorsal and deep tegmental nuclei, forming second neurones on the efferent pathway. One may conclude, therefore, that the initial outflow from the hippocampus is non-cholinergic. The non-cholinergic neurones, however, impinge upon a number of nuclei whose cells are rich in cholinesterase (often containing ChE as well as AChE), and give rise to cholinesterase-containing fibres. These nuclei, which are presumably cholinergic, either project directly to medial cortical areas or to the olfactory bulb, or connect with relays on the ascending cholinergic reticular system. Other AQiE-containing neurones which are probably innervated by hippocampal efferents supply the subfornical organ. (a) Nuclei supplying medial cortex.—The anterior thalamic nuclei are supplied directly by fornix fibres as well as indirectly through the mammillothalamic tract (Guillery, 1966; Nauta, 1956). The anteroventral nucleus is rich in both AChE and ChE, which is located in the cells and extracellularly in terminal neuropil; in the antero-medial nucleus, on the other hand, the enzymes are entirely extracellular in neuropil (PI. LXEX, fig. 6). The cells of the antero-dorsal nucleus are unusual in that they contain far more ChE than AChE, and there is less extracellular enzyme than in the other anterior thalamic nuclei. We have found a similar preponderance of ChE in the cells of the dorsal motor nucleus of the vagus (Navaratnam, Lewis and Shute, 1964). The anterior thalamic nuclei project on to the cingulate cortex (Rose and Woolsey, 1948). Fibres from the antero-medial nucleus travel farthest anteriorly to the anterior limbic area and area infralimbica, respectively equivalent to Krieg's (1946) areas 32 and 25 of the rat cortex. Those from the antero-ventral nucleus supply the cingular area corresponding to Area 23 (not Area 24 as stated by Krieg), and those from the antero-dorsal nucleus travel farthest posteriorly to the retro-splenial area which is equialent to Krieg's cortical Area 29b. None of the projections from the anterior thalamic nuclei on to medial cortex could be detected in normal material prepared by the thiocholine technique, but it was noticeable that in the retrosplenial area, supplied by the antero-dorsal nucleus, prominent bands of ChE were present in cortical layers i and iii. We were able to prove that these bands of enzyme were produced by terminals of neurones located in the antero-dorsal nucleus, since the normal staining was absent on the operated side (PI. LXIX, fig. 11, CC) twenty-nine days after a lesion which totally destroyed this nucleus CHOLINERGIC LIMBIC SYSTEM 527 Another nucleus closely related to hippocampal projection fibres in the fornix is the nucleus of the anterior commissure, the cells of which likewise contain ChE as well as AChE, although the ChE is present in lesser amounts than in those of the interstitial nucleus of the ventral hippocampal commissure. The cholinesterase-containing cells of this nucleus are located, in the rat, mainly on the dorsal aspect of the anterior commissure, immediately lateral to the descending columns of the fornix. They give rise to fibres which project forwards on to the lateral part of the nucleus accumbens, reaching it by looping over the anterior limb of the anterior commissure. Their terminals contribute to the accumbens neuropil, which is derived mainly from AChE-containing cells of the lateral preoptic area and of the olfactory tubercle. The accumbens nucleus also receives direct projections from the hippocampus via the precommissural fornix (Sprague and Meyer, 1950; Carman, Cowan and Powell, 1963). The medial portion of the accumbens nucleus, which appears to be an extension of the deep or polymorph layer of the olfactory tubercle, contains cells which are also rich in ChE as well as AChE (PL LXVIII, fig. 5, A). Their axons appear to project to olfactory areas. The main cholinesterase-containing, presumed cholinergic, links between the hippocampal projection system and medial cortex are listed in Table I and illustrated in fig. C. Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 (PL LXTX, fig. 6. AD) without involving the other anterior thalamic nuclei. There is, therefore, a correspondence between the type of enzyme present in the nerve terminals and the enzyme contained in the cell body. A similar relationship is found in the vagus, where the terminals in the cardiac ganglia also contain ChE (Navaratnam, Lewis and Shute, 1964). The interstitial nucleus of the ventral hippocampal commissure is also rich in ChE as well as AChE (PL LXVIII, fig. 3, C). The connexions of this large nucleus have not been previously described. From its position, it is probable that afferents, derived from the hippocampus, reach it via the fornix bundles. In material stained specifically for ChE, the axons derived from the interstitial nucleus form a prominent discrete bundle which can be followed forwards through the dorsal part of the septum below the corpus callosum (PL LXVm, fig. 5). Immediately in front of the genu of the corpus callosum, this bundle joins a group of AChE-containing precallosal cells which form a dorsal extension of the AChE-containing cells of the olfactory tubercle (fig. C). From this point, fibres derived from the precallosal cells continue forwards on the medial side of the frontal lobe, and are distributed to the area infralimbica and anterior limbic area (i.e. to cortex supplied by the non-cholinergic anrero-medial thalamic neurones). The ChE-containing bundle derived from the interstitial nucleus of the ventral hippocampal commissure can be traced on forwards below the rhinal fissure into the olfactory bulb. 528 P. R. LEWIS AND C. C. D. SHUTE TABLE I.—ANALYSIS OF THE CHOLINERGIC LIMBIC SYSTEM Group 1 neurones: afferent to the hippocampal formation nucleus of diagonal band T f dentate gyrus medial septal nucleus dorsal and ventral hippocampus J Group 2a neurones: afferent to medial cortex retrosplenial area antero-ventral thalamic nucleus cingular area interstitial nucleus of ventral hippocampal commissure anterior limbic area and area infralimbica precallosal cells olfactory bulb nucleus accumbens olfactory tubercle Group 2b neurones: afferent to the cerebellum latero-dorsal tegmental nucleus (1.d.t.n.) —>- brachium conjunctivim dorsal tegmental nucleus (d.t.n.) nucleus reticularis tegmenti pontis (n.r.t.p.) —*• brachium pontis Group 1c neurones: afferent to the ascending cholinergic reticular system (1) to the dorsal tegmental pathway: d.t.n. —*• 1.d.t.n. —*• nucleus cuneiformis (2) to the ventral tegmental pathway: d.t.n. and deep tegmental nucleus dorsal and median raphe nuclei interpeduncular nucleus habenular nuclei d.t.n. —*• l.d.t.n. —*• nucleus cuneiformis d.t.n. —*• n.r.t.p. -•- —»• substantia nigra ventral tegmental area Group Id neurones: afferent to non-neural structures cells in dorsal fornix "I f subfornical organ cells in septal raphe J (_ supra-optic crest Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 antero-dorsal thalamic nucleus CHOLINERGIC LIMBIC SYSTEM 529 FIG. C.—Diagram showing cholinesterase-containing nuclei of the mid-brain and fore-brain (indicated by stipple) connected with the hippocampus, their projections to the medial cortex, and their connexions with the ascending cholinergic retiailar system. Abbreviations: A, nucleus accumbens; ATH, antero-ventral and antero-dorsal thalamic nuclei; BC, brachium conjunctivum; BP, brachium pontis; C, interstitial nucleus of the ventral hippocampal commissure; CBL, cerebellum; CC, cingulate cortex (cingular and retrosplenial areas); CU, nucleus cuneiformis; DB, diagonal band; DE, deep tegmental nucleus (ventral tegmental nucleus of Gudden); DO, dorsal tegmental nucleus; F, fornix; FC, frontal cortex (area infralimbica and anterior limbic area); FR, fasciculus retroflexus (habenulo-interpeduncular tract); H, habenular nuclei; HF, hippocampal formation; IP, interpeduncular nucleus; LD, laterodorsal tegmental nucleus; LP, lateral preoptic area; M, mammillary body; MS, medial septal nucleus; MT, mammillo-tegmental tract; MTH, mammillo-thalamic tract; OB, olfactory bulb; OT, olfactory tubercle; PC, precallosal cells; R, dorsal and median nuclei of raphe (nucleus central is superior); SFO, subfornical organ; SH, stria habenularis; SR, septal radiation; TP, nucleus reticularis tegmenti pontis (of Bechterew); VT, ventral tegmental area. (b) Nuclei connecting with the cerebellum.—The dorsal tegmental and the deep tegmental nucleus (nucleus of Gudden) are both innervated by mammiJlo-tegmental fibres and so come under the influence of the hippocampal-fornix projection system. The cells of both nuclei are rich in ChE as well as AChE (PI. LXTX, fig. 7, DO, DE). Cholinesterase-containing fibres emanating from the dorsal tegmental nucleus can be seen in normal material to project to the latero-dorsal tegmental nucleus and to the nucleus reticularis tegmenti pontis (fig. C). The two last-named nuclei both send cholinesterase-containing fibres to the cerebellum, the latero-dorsal tegmental nucleus via the brachium conjunctivum and the nucleus reticu- Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 -ToOT 530 P. R. LEWIS AND C. C. D. SHUTE Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 laris tegmenti pontis via the brachium pontis (Shute and Lewis, 1965). These connexions, which are presumably cholinergic, between the hippocampal-tegmental projection and the cerebellum are illustrated in fig. C. (c) Nuclei connecting with the ascending cholinergic reticular system.— The latero-dorsal tegmental nucleus sends cholinesterase-containing fibres not only to the cerebellum but also to the nucleus cuneiformis (fig. C). In this way the hippocampal-tegmental projection is linked with the dorsal tegmental pathway of the ascending cholinergic reticular system (Shute and Lewis, 19636, 1967). Furthermore, a continuous chain of cholinesterasecontaining neurones can be traced from the dorsal and deep tegmental nuclei via the dorsal and median nuclei of the raphe, which also contain AChE and ChE, to the interpeduncular nucleus. This nucleus contains large amounts of AChE, particularly around its periphery (PI. LXIX, fig. 8). It also contains more choline acetylase than has been recorded so far in any other region of the mammalian brain (Lewis, Shute and Silver, 1967). The AChE of the nucleus interpeduncularis is located in nerve cells as well as in terminals. The nucleus contains a little ChE, mainly at the posterior end, at the site of entry of the fibres derived from the dorsal and deep tegmental nuclei. The AChE-containing neurones of the interpeduncular nucleus appear to connect with those of the ventral tegmantal area (fig. Q . Our findings here conflict with the report that the dorsal and deep tegmental nuclei project mainly to the mammillary nuclei via the mammillary peduncle (Cowan, Guillery and Powell, 1964). Unlike the axons of the dorsal and deep tegmental neurones, the fibres which make up the mammillary peduncle do not contain cholinesterase, and so may be regarded as non-cholinergic. We conclude that the projections of the dorsal and deep tegmental nuclei on to the interpeduncular nucleus, and through it on to the AChE-containing neurones of the ventral tegmental area, provide a cholinergic link between the hippocampal fornix projection system and the ventral tegmental pathway. The anterior end of the interpeduncular nucleus is supplied by fibres of the fasciculus retroflexus or habenulo-interpeduncular tract containing ChE as well as AChE. In its dorsal part these fibres form only the central core of the fasciculus retroflexus. More ventrally, i.e. immediately above its entry into the interpeduncular nucleus, they constitute the whole of the bundle. The non-cholinergic fibres of the dorsal part are presumably those which leave the fasciculus retroflexus and project to the mid-brain tegmental nuclei (Nauta, 1958). The direction of the cholinesterase-containing fibres in the fasciculus retroflexus was investigated experimentally. Destruction of the lateral habenular nucleus in an animal surviving for six days (PI. LXIX, fig. 10 H) produced diminished fibre staining in the fasciculus on the operated side (PI. LXIX, fig. 12, FR). The persisting fibres could be seen to emerge from the intact medial habenular nucleus. The fasciculus retroflexus itself was damaged in animals surviving for 3, 4, 7 and 10 days. In all CHOLINERGIC LIMBIC SYSTEM 531 The main chohnesterase-containing links between the hippocampal projection system and the nuclei associated with the dorsal and ventral tegmental pathways of the ascending cholinergic reticular system are listed in Table I and illustrated in fig. C. (d) Innervation of the subfomical organ and supraoptic crest.—The subfornical organ or intercolumnar tubercle is a cellular structure, highly vascularized, which lies beneath the ventral hippocampal commissure. AChE is present in large amounts, particularly around the periphery of the organ (PI. LXIX, fig. 9). In normal material prepared by the thiocholine technique the subfornical organ can be seen to be innervated by AChEcontaining cells, some of which are situated in the dorsal fornix above the hippocampal commissure, while others he close to the mid-line raphe in the upper part of the septum. The axons of these cells reach the subfornical organ by passing through and on either side of the hippocampal commissure (Shute and Lewis, 1963a). Other AChE-containing cells in the ventral part of the septum supply the histologically similar organ known as the supraoptic crest, which lies immediately rostral to the optic chiasma. The axons supplying the supraoptic crest travel on either side of the anterior commissure (fig. D). The subfornical organ was destroyed in an animal which was then allowed to survive for six days. Accumulation of AChE was produced in Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 cases, enzyme accumulated dorsal to the lesion, and staining was diminished ventral to it. In the 7-day experiment the lesion was placed medial to the fasciculus retroflexus. Increase of staining dorsally and diminution ventrally still occurred in response to the lesion, although the fibres were not actually interrupted. Increased staining was much more marked in the animal which survived for 20 days (PI. LXIX, fig. 13) than in the shorter term experiments. It was considered that the late accumulation of enzyme in the fasciculus retroflexus might be associated with the small calibre of its fibres. A comparable response occurred in the fine terminal branches of the cingulate radiation, some weeks after a lesion of the superior cortex (Shute and Lewis, 1967): These experiments proved that the cholinesterase-containing fibres of the fasciculus retroflexus arise from the habenular nuclei. In normal material it can be seen that a proportion of the cells which form these nuclei contain AChE and ChE. Some fornix fibres are known to reach the habenular nuclei via the habenular striae (Votaw, 1960), so it is possible that these neurones too, like the other chohnesterase-containing nuclei mentioned above, are in the sphere of influence of the hippocampus. If the interpeduncular nucleus projects on to cholinergic neurones of the ventral tegmental area, the fasciculus retroflexus can be regarded as providing an additional route by which the hippocampus can act on the ventral tegmental pathway. 532 P. R. LEWIS AND C. C. D. SHUTE DF FIG. D.—Mid-line sagittal section through the anterior wall (lamina tenninalis) of the third ventricle (in V) and through the optic chiasma (CH), showing the course, in relation to the ventral hippocampal commissure (HC) and the anterior commissure (AC), taken by AChE-containing fibres, derived from cells in the dorsal fornix (DF) and septal raphe (RS), which supply the subfornical organ (SFO) and the supraoptic crest (SOC). fibres from the septum arid dorsal fornix passing ventral to and through the hippocampal commissure. In a long-term experiment lasting fourty-four days, a lesion of the dorsal fornix resulted in diminished staining of the subfornical organ at its posterior end. Loss of staining in the supraoptic crest was produced by a septal lesion in an animal which survived for six days. These experiments proved that the massive AChE content of the subfornical organ and supraoptic crest is derived from incoming fibres. The presence within the subfornical organ of unmyelinated axons and nerve endings in a synaptic relationship with the parenchymal cells was confirmed by electron microscopy (Shute and Lewis, 1963a). DISCUSSION We apply the term "cholinergic limbic system" to the groups of AChEcontaining neurones which are intimately related to the hippocampal formation and its projection pathways in the fore-brain and mid-brain. Our results show that the cholinergic limbic system consists of two groups of nuclei (Table I). The nuclei of Group 1 are afferent with respect to the hippocampal formation. Those of Group 2 are innervated by the hippocampal system and project (a) to medial cortex, (6) to the cerebellum, (c) to nuclei of the ascending cholinergic reticular system which probably forms the structural basis of the ascending reticular activating system of Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 ISOC CHOLINERGIC LIMBIC SYSTEM 533 Group 1 neurones (hippocampal offerents). The Group 1 neurones whose cell bodies are located in the nucleus of the diagonal band he on the course of the ventral tegmental pathway of the ascending cholinergic reticular system. It would seem reasonable, therefore, to suppose that these neurones play some part in detenriining the electrical activity of the hippocampus during arousal. In considering what their effects may be, it must be remembered that the cholinergic fibres are not the only afferent projection to the hippocampus travelling via the septum and fornix. In addition there is an extensive innervation by monoamine-containing fibres (the monoamine being mainly noradrenaline) which arise from cells of the mid-brain lying in the mid-line dorsal to the nucleus interpeduncularis (Dahlstrom and Fuxe, 1964; Fuxe, 1965). The monoamine-containing terminals, which are presumably monoaminergic, do not have the same distribution in the hippocampal formation as those of the cholinergic system. Monoaminergic terminals are found mainly in the stratum radiatum and stratum lacunosum of the hippocampus in relation to apical dendrites of pyramidal cells, and in the subgranular layer of the dentate gyrus in relation to basal dendrites of granule cells (Fuxe, 1965). Cholinergic terminals, on the other hand, are located predominantly in the stratum oriens of the hippocampus in relation to basal dendrites of pyramidal cells (although they are also found in the stratum radiatum and lacunosum), and in the supragranular layer or the dentate gyrus in relation to apical dendrites of granule cells (Shute and Lewis, 1961). It is possible that the effects of the cholinergic and monoaminergic systems on hippocampal activity are in some degree opposed to one another. Such a view is favoured by the report that hippocampal neurones are consistently facilitated by acetylcholine whereas those which show sensitivity to either noradrenaline or 5-hydroxytryptamine are depressed (Salmoiraghi and Stefanis, 1966). The firing rate of hippocampal cells is also accelerated by the cholinomimetic drug eserine (Green, Maxwell, Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 neurophysiologists (Shute and Lewis, 1963c, 1967), and (d) to the problematical subfornical organ and supraoptic crest. In the rat, the nuclei of Group 1 and Group Id contain AChE only, whereas those of Groups la, 1b and 1c contain ChE as well as AChE. The neurones of the interpeduncular nucleus contain AChE only. This nucleus may form a link between the cholinergic limbic system and the ventral tegmental pathway of the ascending cholinergic reticular system, which also contains only AChE. The dorsal tegmental pathway of the ascending cholinergic reticular system, which contains ChE as well as AChE, receives connexions from ChEcontaining nuclei of Group 2c. We have suggested that ChE may assist in the hydrolysis of acetylcholine at sites of high activity (Shute and Lewis, 19636). 534 P. R. LEWIS AND C. C. D. SHUTE Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 Schindler and Stumpf, 1960). It is not easy to deduce, however, from the pharmacological responses of unit cells what effect the cholinergic and monoaminergic systems are likely to have on the electrical activity of the hippocampus as a whole, as revealed in the EEG recoid. In favour of the monoaminergic system being concerned in the production of 6 rhythm, the following pieces of evidence can be cited. (1) 6 rhythm is depressed by reserpine, which depletes the brain of monoamines (Killam and Killam, 1957). (2) Slow hippocampal rhythms can be produced by electrical stimulation of the medial preoptic area, medial hypothalamic region, and the central grey matter and the dorsilateral tegmentum of the mid-brain (Torii, 1961). None of these areas, except the last, are sites of cholinergic cells, whereas some at least may coincide with monoaminergic pathways and monoaminergic cell groups (e.g. the mid-line Groups AlO and Al 1 of Dahlstrom and Fuxe (1964): Group A l l occupies the central grey). (3) Although it has been shown that some unit cells in the hippocampus are active during 9 rhythm (Green and Machne, 1955), the firing rate of other hippocampal cells may be depressed (Green, Maxwell, Schindler and Stumpf, 1960), and it is possible that slow rhythms in the hippocampus are associated with a relatively low overall level of activity (Grastyan, Lissak, Madarasc and Donhoffer, 1959), such as might result from an inhibitory innervation from the monoaminergic system. In favour of the cholinergic system also playing a part in 9 rhythm is the fact that slow waves can be induced in the hippocampus by administering eserine, so long as the septum is intact (Green, Maxwell, Schindler and Stumpf, 1960). The effects of eserine may be exerted at the septum, if the hippocampal afferents at that level, cholinergic or otherwise, are cholinoceptive. It has been claimed that neurones located in the medial part of the septum, which we have shown to be a source of cholinergic fibres supplying the hippocampus, act as a pacemaker and produce 9 rhythm through their own slow rhythmical discharge (Petsche, Stumpf and Gogolak, 1962; Stumpf, Petsche and Gogolak, 1962). These neurones may themselves be influenced by the monoaminergic system, since monoaminergic endings are reported in the septum (Fuxe, 1965). Another possible role for the cholinergic Group 1 neurones of the medial septum and diagonal band may be to produce fast low amplitude activity in the hippocampus. Such activity is superimposed on 9 rhythm during moderate degrees of reticular stimulation and after administration of eserine, and replaces the 9 rhythm when reticular stimulation is very strong (Stumpf, 1965). Hippocampal responses would in this way be brought into line with those of the neocortex, where cholinergic projections are probably responsible for the desynchronized fast activity associated with increased unit firing which occurs during arousal. Although the hippocampal record commonly shows 6 rhythm when the neocortical EEG is of the alert type, fast activity only is said to occur in unanaesthetized animals confronted CHOUNERGIC LIMBIC SYSTEM 535 Group 2 neurones of the cholinergic limbic system A major output from the hippocampus passes either directly or through a relay in the mammillary body to elements of the cholinergic limbic system which connect directly with medial cortex (Group 2a neurones) or, through links with the ascending cholinergic reticular system, with the lateral cortex of the cerebral hemispheres (Group 2c neurones). These connexions provide a means by which the hippocampus can influence electrical activity in other regions of fore-brain cortex, as in arousal. The importance of the 2a innervation is emphasized by the finding that stimulation of medial cortex, particularly the anterior limbic area, produces manifestations of arousal in unanaesthetized animals (Kaada, Jansen and Andersen, 1953). Green and Arduini (1954) noted that, during the transition from drowsiness to alertness, hippocampal arousal rhythms usually precede those of the neocortex, while with behavioural changes in the reverse direction the hippocampal 6 rhythm associated with arousal is suppressed before the appearance of neocortical "sleep spindles." These time sequences suggest that the hippocampus may be concerned in initiating the reticular activity associated with the alert state. Experimental ablation of the hippocampal formation, whether carried out surgically (Votaw, 1959) or produced by seizure activity (MacLean, Flanigan, Flynn, Kim and Stevens, 1955-6), tends to result in a state of sluggishness and placidity which may be an expression of reticular hypoactivity. Green (1957) reported that in hippocampectomized cats electrocortical arousal was Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 > with a novel unconditioned stimulus, as part of a "startle response" (Grastyan, Lissak, Madarasz and Donhofler, 1959). Fast, low amplitude activity can in fact be produced experimentally in the hippocampus by stimulating the medial septum (Torii, 1961). The same result is obtained from stimulation of the medio-ventral tegmentum and lateral hypothalamus— areas traversed by cholinergic neurones of our ventral tegmental pathway, which probably connect with the cholinergic neurones of the diagonal band and septum. Fast activity produced in this way is abolished by atropine (Longo, 1956). It has, however, been found that the fast activity resulting from reticular stimulation or administration of eserine can survive septal lesions (Mayer and Stumpf, 1958; Stumpf, 1965). This must mean either that fast hippocampal activity can be produced by cholinoceptive projections on to the hippocampus which do not traverse the septum (e.g. those from entorhinal cortex), or that some of the septal neurones responsible escaped damage. Destruction in the septal region would need to be very extensive to involve all the Group 1 cells. 9 rhythm, unlike fast activity, is always abolished by septal lesions. It is possible that at the level of the septum the monoaminergic supply to the hippocampus, which arises more caudally in the brain-stem, forms a more compact and, therefore, more vulnerable bundle than the cholinergic fibres. 536 P. R. LEWIS AND C. C. D. SHUTE SUMMARY (1) The hippocampal formation (hippocampus and dentate gyrus) receives a cholinergic innervation from the medial septum and diagonal band. Hippocampal efferents travelling by the fornix project, directly or indirectly on to cholinesterase-containing, presumably cholinergic neurones in the hippocampal commissure, anterior thalamus, habenular and Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 difficult to produce by reticular stimulation and, unlike the arousal obtained in normal animals, lasted only for the duration of the stimulus. Hippocampal stimulation does not usually lead to generalized neocortical desynchronization (Green, 1957), but so long as it is not so intense as to cause a seizure discharge, has been found to produce a generalized reaction stimulating behavioural arousal in anaesthetized monkeys (Votaw, 1959) and attentive or searching behaviour in free-running rabbits with implanted electrodes (Cazard and Buser, 1963). In addition to effects on arousal, it is possible that Groups 2a and 2c neurones may be involved in the reputed role of the hippocampus in learning and memory, both in man (Penfield and Milner, 1958) and in experimental animals (Thompson, Duke, Malin and Hawkins, 1961; Thompson, Langer and Rich, 1964; Flexner, Flexner, Roberts and de la Haba 1964; Nielson, Mclver and Boswell, 1965). It is likely that hippocampal effects on memory processes are achieved by influences acting on some part of the brain external to the hippocampus, rather than as an intrinsic property of the hippocampus itself (Green, 1964). Some evidence of the importance of cholinergic mechanisms is provided by the observation that in man memory impairment, coupled with loss of attention, drowsiness and decrease in spontaneous speech, follows the administration of atropine (Ostfield, Machne and Unna, 1960). Little can be said at present of the significance of other Group 2 neurones of the cholinergic limbic system. The Group 2b projection would account for the hitherto unexplained evoked potentials produced in the cerebellum by stimulating the fornix (Green and Morin, 1953). The function of the organs supplied by the Group Id fibres is not known. Some of the dendrites of cells of the supraoptic nucleus are said to reach the equivalent of the subfornical organ in the frog, and on these grounds it has been concluded that it may act as an osmoreceptor responding to changes in the osmotic pressure of the blood and cerebrospinal fluid (Dierickx, 1963). The subfornical organ and supraoptic crest may possibly be concerned in the increased water intake which results in rats from hippocampal stimulation (Fisher and Coury, 1962). Some preliminary observations which we have made by electron microscopy have shown that many of the nerve endings in the subfornical organ contain AChE, and that the synapses on parenchymatous cells of the organ are of motor rather than of sensory type. CHOLINERGIC LIMBIC SYSTEM 537 interpeduncular nuclei, and the mid-brain tegmentum. In the rat, these neurones contain non-specific cholinesterase as well as acetylcholinesterase. They project to medial cortex, to nuclei of the ascending cholinergic reticular system, and to the subfornical organ and supraoptic crest. Together with the cholinergic neurones of the medial septal nucleus and the nucleus of the diagonal band, they constitute the cholinergic limbic system. ACKNOWLEDGMENTS We thank Mrs. Annette Bond for technical assistance, Messrs. J. F . Crane and G. Oakes for photography, and Messrs. May and Baker Ltd. and the Wellcome Research Laboratories for gifts of inhibitors. The work was supported by a grant from the Medical Research Council. REFERENCES BLOOM, F. E., COSTA, E. and SALMOIRAGHI, G. C. (1965) / . Pharmacol. 150, 244. CARMAN, J. B., COWAN, W. M. and POWELL, T. P. S. (1963) Brain, 86, 525. CAZARD, P. and BUSER, P. (1963) Electroenceph. din. NeurophysioL, 15, 413. COWAN, W.M., GUILLERY, R. W. and POWELL, T. P. S. (1964) / . Anat., Lond. 98, 345. DAHLSTROM, A. and FUXE, K. (1964) Actaphysiol. scand. 62, Suppl. 232. DIERICKX, K. (1963) Naturwissenschaften 50, 163. EIDELBERG, E., WHITE, J. C , and BRAZIER, M. A. B. (1959) Exp. Neurol., 1, 483. EMMERS, R. (1961) Arch. ital. Biol., 99, 322. FISHER, A. E., and COURY, J. N . (1962) Science, 138,691. FLEXNER, L. B., FLEXNER, J. B., ROBERTS, R. B., and DE LA HABA, G. (1964) Proc. nat. Acad.Sci., Wash.,S2,U65. FUXE, K. (1965) Actaphysiol. scand., 64, Suppl. 247, p.37. GAULT, F. P., and COUSTAN, D . R. (1965) Electroenceph. din. NeurophysioL, 18, 617. GRASTYAN, E., LISSAK, K., MADARASZ, I., and DONHOFFER, H. (1959) Electroenceph. clin. NeurophysioL, 11,409. GREEN, J. D. (1957 In: "Reticular Formation of the Brain," edited by H. H. Jasper et al. London, p. 607. , 23 (1960) In: American Physiological Society, Handbook of Physiology, Washington, D.C., Section 1. Neurophysiology, Vol. 2, p . 1373. BRAD*—VOL. XC Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 (2) It is suggested that the medial septal and diagonal band portions of the limbic cholinergic system activate the hippocampal formation, and may be responsible for fast activity in the hippocampal EEG. Hippocampal e rhythm, on the other hand, may involve activity of the monoaminergic system. The connexions of the limbic cholinergic system with the ascending cholinergic reticular system and with the medial cortex enable the hippocampus to play a part in arousal and attention, and possibly also in memory and learning. 538 P. R. LEWIS AND C. C. D. SHUTE NAVARATNAM, V., LEWIS, P. R., and SHUTE, C. C. D. (1964) / . Anat., Lond., 98, 287. H. C , MCIVER, A. H., and BOSWELL, R. S. (1965) Exp. Neurol., 11, 147. A. M., MACHNE, X., and UNNA, K. R. (1960) /. Pharmacol., 128, 255. PENFIELD, W., and MILNER, B. (1958) Archs. Neurol. Psychiat., Chicago, 79,475. PETSCHE, H., STUMPF, C , and GOGOLAK, G. (1962) Electroenceph. clin. Neurophysiol., U.Tttl. ROSE, J. E., and WOOLSEY, C. N. (1948) /. comp. Neurol., 89, 279. SALMOIRAOHI, G. C , and STEFANIS, C. N. (1966) Arch. Hal. Biol., 103, 705. NIELSON, OSTFELD, SHUTE, C. C. D., and LEWIS, P. R. (1961) Bibl. anat., 2, 34. , , , , , , (1963a)/. Anat.,Lond., 97,301. (1963ft)/. Anat.,Lond., 97,476. (1963c) Nature., 199,1160. (1965) Nature, Lond., 205,242. (1966) Z.ZeUforsch., 69,334. (1967) .Srafo 90,497. Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 GREEN, J. D. (1964) Pkysiol. Rev. 44, 561. , and ARDUINL. A, A. (1954)/. Neurophysiol. 17. 533. , and MACHNE,X. (1955) Am. J. Physiol. 181,219. , MAXWELL, D. S., SCHINDLER, W. J., and STUMPF, C. (1960) /. Neurophysiol. 23,403. , and MORIN, F. (1953) Am. J. Physiol. 172,175. GUILLERY, R. W. (1956)/. Anat., Lond. 90,350. HERNANDEZ-PEON, R., LAVIN, A., ALCOCER-CUAR6N, C , and MARCELIN, J. P. (1960) Electroenceph. clin. Neurophysiol. 12,41. JUNO, R., and KORNMOLLER, A. E. (1938) Arch. Psychiat. 109,1. KAADA, B. R., JANSEN, J., Jr., and ANDERSEN, P. (1953) Neurology, Minneap., 3, 844. KILLAM, E. K., and KILLAM, K. F. (1957) In: "Brain Mechanisms and Drug Action." Edited by W. S. Fields, Springfield, El. p. 71. KRIEQ, W. J. S. (1946)/. comp. Neurol., 84,221. LEWIS, P. R., SHUTS, C. C. D., and SILVER, A. (1964) / . Physiol. 172,9P. , and (1967)/. Physiol. 191, 215. LONGO, V. G. (1956)/. Pharmacol., 116,198. LORENTE DE N6, R. (1933) /. Psychol. Neurol., Lpz., 45,381. , (1934) /. Psychol Neurol., Lpz., 46,113. MACLEAN, P. D., FLANIGAN, S., FLYNN, J. P., KIM, C , and STEVENS, J. P. (1955-6) Yale, / . Biol. Med., 28,380. MAYER, C. ,and STUMPF, C. (1958) Arch. exp. Path. Pharmak., 234,490. MORELL,F. (1961) In: "Brain Mechanisms and Learning," Edited by J. F. Delafresnaye. Oxford: p. 251. NAUTA, W. J. H. (1956)/. comp. Neurol, 104,247. , (1958) Brain, 81, 319. CHOLINERGIC LIMBIC SYSTEM SFEHLMANN, R. (1963)/. Neurophysiol., 26,127. SPRAGUE, J. M., and MEYER, M. (1950)/. Anat.,Lond., 84,354. STUMPF, C. (1965) EJectroenceph. din. Neurophysiol., 18,477. , 539 PETSCHE, H., and GOGOLAK, G. (1962) Electroenceph. Clitt. Neurophysiol., 14, 212. R., DUKE, R. B., MALIN, C. F., Jr., and physiol. PsychoL, 54,329. THOMPSON, , LANGER, S. HAWKINS, W. F. (1961) /. comp. K., and RICH, I. (1964) Brain, 87,537. TORn, S. (1961) Jap. J. Physiol., 11,147. VOTAW, C. L. (1959)/. comp. Neurol., 112,353. LEGENDS FOR PLATES PLATE LXVm Transverse sections of rat fore-brain showing locations of cholinesterases. AChE appears in sections incubated in a medium containing acetylthiocholine (AThCh) as substrate with 10~'M ethopropazine as a ChE inhibitor. ChE appears in sections incubated in a medium containing either butyrylthiocholine (BuThCh) as substrate or propionylthiocholine (PrThCh) with 5 x 10~6M 62C 47e as an AChE inhibitor. Fio. 1.—Accumulation of enzyme in cholinergic hippocampal afferent fibres following a fimbrial lesion (L). Five days survival. AThCh substrate, ChE inhibitor, x 48. FIG. 2.—Golgi type II cells in the hilum of the ipsilateral dentate gyms following a lesion of the dorsal fornix. Six days survival. AThCh substrate, ChE inhibitor. xlOO. FIG. 3.—Normal brain. Localization of ChE in the interstitial nucleus of the ventral hippocampal commissure (Q. PrThCh substrate, AChE nhibitor. x 18. FIG. 4.—Same animal as Fig. 1. Loss of AChE staining of the ventral hippocampus (VH). Five days after a fimbrial lesion. AThCh substrate, ChE inhibitor, x 12. FIG. 5.—Normal brain. Localization of ChE in the islets of Calleja (I), in the nucleus accumbens (A) and in the projection (marked by arrow) from the interstitial nucleus of the ventral hippocampal commissure to the anterior cingulate area of the frontal lobe. BuThCh substrate, x 18. Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 , (1960)/. comp. Neurol., 114,283. 540 P. R. LEWIS AND C. C. D. SHUTE PLATE LXIX Transverse sections of rat fore-brain and mid-brain showing locations of AChE and ChE. Incubation media as for Plate LXVIII. FIG. 6.—Animal with unilateral destruction of the antero-dorsal thalamic nucleus (AD). The antero-ventral nucleus (heavily stained for ChE) and the antero-medial nucleus (moderately stained) were spared. Twenty-nine days survival. BuThCh substrate, x 24. FIG. 7.—Normal brain. Dorsal (DO), deep (DE) and latero-dorsal (LD) tegmental nuclei. LD contributing to the brachium conjunctivum (BQ. PrThCh substrate, AChE inhibitor, x 251. FIG. 9.—Normal brain. Subfornical organ, heavily stained for AChE. AThCh substrate, ChE inhibitor. X 40. FIG. 10.—Animal with unilateral destruction of the lateral habenular nucleus (H). Six days survival. AThCh substrate, ChE inhibitor. x l 8 . FIG. 11.—Same animal as Fig. 6. Loss of ChE staining in layer iii of the retrosplenial field of the cingulate cortex (CQ, twenty-nine days after destruction of the ipsilateral antero-dorsal thalamic nucleus. BuThCh substrate, x 24. FIG. 12.—Same animal as Fig. 10. Loss of AChE staining in fibres of the fasciculus retroflexus (on the left hand side of the picture) six days after a lesion of the lateral habenular nucleus on the same side. AThCh substrate, ChE inhibitor, x 36. FIG. 13.—Accumulation of AChE in fibres of the fasciculus retroflexus above a unilateral lesion (L). 20 days survival. AThCh substrate, ChE inhibitor, x 30. Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 FIG. 8.—Normal brain. Interpenduncular nucleus, heavily stained for AChE. AThCh substrate, ChE inhibitor, x 10}. PLATE LXVIII ^ 2 ' To illustrate article by P. R. Lewis and C. C. D. Shute. Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 VH PLATE LXIX Downloaded from http://brain.oxfordjournals.org/ by guest on March 5, 2016 To illustrate article by P. R. Lewis and C. C. D. Shute.