* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neurons
Development of the nervous system wikipedia , lookup
Central pattern generator wikipedia , lookup
Signal transduction wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Axon guidance wikipedia , lookup
Patch clamp wikipedia , lookup
Neuroanatomy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neurotransmitter wikipedia , lookup
Membrane potential wikipedia , lookup
Synaptic gating wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Nervous system network models wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Action potential wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Single-unit recording wikipedia , lookup
Node of Ranvier wikipedia , lookup
Electrophysiology wikipedia , lookup
End-plate potential wikipedia , lookup
Resting potential wikipedia , lookup
Synaptogenesis wikipedia , lookup
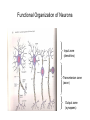
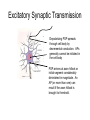
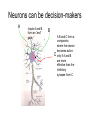
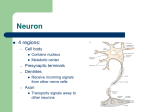
Neurons Functional Organization of Neurons Input zone (dendrites) Transmission zone (axon) Output zone (synapses) The Resting Potential • Almost all cells have a transmembrane electrical charge difference, with the inside roughly 50-100 mV negative relative to the outside. • Voltage = electrical driving force, reflecting the energy required to separate charges – so charge separation is a form of stored or potential energy. Electrical Energy and Chemical Energy Are Interconvertable • At electrochemical equilibrium, the chemical driving force of an ionic gradient is exactly counterbalanced by the gradient’s equilibrium potential. The mathematical statement of this is the Nernst Equation, written here for the K+ gradient: V=RT/zF ln ([K+]out/[K+]in ) R: universal gas constant T: temperature in deg. K Z: ionic charge (+1 here) F: Faraday’s number At mammalian body temperature, each additional order of magnitude of the concentration ratio term of the Nernst Eq. adds an additional 60 mV to the total voltage – i.e. a 1/10 gradient gives -60 mV, a 1/100 gradient gives -120 mV, etc. Concentration Gradients and Permeability Properties Cause the Membrane Potential • The membrane potential arises from the interaction of two factors: • Transmembrane ionic concentration differences • Selective permeability properties of the membrane [K+]=4Eq/l [Na+}=145 mEq/l [K+]=100m Eq/l [Na+]=15 mEq/l You could think of the K+ gradient as “wanting” to drive + charge to the outside of the cell and thus make it insidenegative; the Na+ gradient “wants” to do the opposite. Na+ gradient:, corresponding to an equilibrium potential of about +60 mV K+ gradient: , corresponding to an equilibrium potential of about -90 mV K+ permeability/Na+ permeability = about 20X Which ion wins? Neither K+ nor Na+ wins entirely, but since the K+ gradient and the K+ permeability are both larger, the outcome is an inside-negative potential. Thus, The membrane potential is determined by the relative magnitude of the concentration gradients for Na+ and K+, weighted by their permeabilities. This is expressed in mathematical form by the Goldman Equation + + (PK[K ]out + PNa [Na ]out) V=RT/F ln (PK[K+]in + PNa [Na+]in) Depolarization? Hyperpolarization? • Depolarization =less inside-negative V =action potentials more likely – – – – – – Increase in extracellular [Na+] Increase in extracellular [K+] Decreased K+ conductance Increased Na+ conductance Excitatory synaptic input Anything that causes a net inward flow of + charge • Hyperpolarization: action potentials less likely – Opposites of everything that causes depolarization Action Potentials • rapid, brief stereotypic changes in an excitable cell’s membrane potential • the method of rapid transmission of information over long distances (>1mm). Basic Characteristics of Action Potentials • Initiated by depolarization due to some stimulus • Require a minimum intensity/duration of stimulus to be initiated (“threshold”) • Are followed by a period of decreased responsiveness (“refractory period”) • Are conducted along the axon (or muscle cell) without a decrease in magnitude (“non-decremental conduction”) Factors that determine conduction velocity • Axon diameter: larger = faster: you could think of the surface/volume ratio of the axon determining how much current leaks out of the axon versus how much continues down the axon core. The larger the diameter, the more easily current flows down the core and the farther ahead of itself the AP can reach to depolarize fresh membrane. Myelination = faster conduction than unmyelinated axons of similar diameter Myelination is the result of wrapping of the axon by glial cells (Schwann cells in the peripheral nervous system, oligodendrocytes in the CNS) – the wrapping is interrupted periodically by Nodes of Ranvier. This leads to saltatory conduction. Chemical Communication at Synapses: Steps • AP arrives at synaptic terminal of presynaptic cell • Ca++ entry triggers release of some vesicles of transmitter chemical • Transmitter diffuses across synaptic cleft • Transmitter binds to receptors on surface of postsynaptic cell • Interaction of transmitter and receptor leads to temporary change in postsynaptic cell’s probability of undergoing an action potential – usually this involves a change in the cell’s membrane potential – this change is called a postsynaptic potential (PSP). Excitatory Synaptic Transmission Depolarizing PSP spreads through cell body by decremental conduction. APs generally cannot be initiated in the cell body PSP arrives at axon hillock or initial segment considerably diminished in magnitude. An AP (or more than one) can result if the axon hillock is brought to threshold. Integration of Synaptic Inputs • Most CNS neurons are not “follower cells” – instead, they integrate their synaptic inputs, or add them up over time and space. This is because PSPs summate within the postsynaptic cell’s input segment. The summation is algebraic, because some synaptic inputs are inhibitory. Neurons can be decision-makers A Inputs A and B form an “and” gate B + + - C A B and C form a comparator, where the neuron becomes active only if A and B are more effective than the inhibitory synapse from C In principle, all of the decisions that the NS makes can be made by single neurons integrating their inputs, but in the vertebrate CNS virtually all decisions are made by the interaction of dozens to thousands of neurons. At the whole-system level, we could define “integration” as processing sensory information for an appropriate response. Reflexes – the simplest functional responses of the NS • Reflex: an immediate, brief, predictable response to a stimulus • Reflex arc consists of an afferent (sensory) pathway, an integrating center, and an efferent (motor) pathway that connects to an effector. • Reflexes frequently are involved in protecting or restoring physiological values to their normal ranges. Such stabilizing responses are termed homeostatic. Reflexes and negative feedback Efferent pathway Effectors Set point Error signal Environmental perturbation sensors Afferent pathway Integrating Center To translate this into terms of a real system, core body temperature is closely regulated by a negative feedback system. The sensors of the system are thermosensory neurons scattered about the thorax and abdomen. The integrating center is in the hypothalamus of the brain. The effectors include neuromuscular pathways involved in behavioral responses and shivering thermogenesis, neurovascular pathways that regulate blood flow to the body surface, and pathways that regulate sweating and piloerection. Basic Characteristics of Negative Feedback Systems • System is reactive – it doesn’t respond until after the regulated variable departs from the setpoint. • The gain of the system is defined as departure from the setpoint with no feedback / departure from the setpoint with feedback operating. For example, if a 10o difference between ambient temperature and core body temperature drives a 0.01o decrease in core body temperature, the gain of the body’s thermostat is 10/0.01 =1000. So, the bottom-line statement about negative feedback control is that such systems tolerate a small amount of variation around the setpoint while preventing large departures from it.