* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download MB207_15 - MB207Jan2010
Cellular differentiation wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein moonlighting wikipedia , lookup
Cell nucleus wikipedia , lookup
Extracellular matrix wikipedia , lookup
Kinetochore wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Endomembrane system wikipedia , lookup
Signal transduction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Proteolysis wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
List of types of proteins wikipedia , lookup
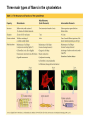
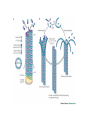
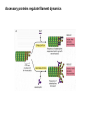
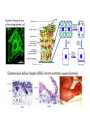
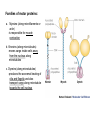
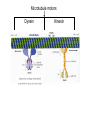
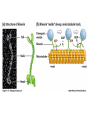
MB 207 Molecular Cell Biology The Cytoskeleton The eukaryotic cytoskeleton The cytoskeleton is a cellular "scaffolding" or "skeleton" contained, as all other organelles, within the cytoplasm. It is a dynamic structure that provide structural support to the cell. Besides that, cytoskeleton also functions in cell motility and regulation. It is a fibrous network made of three families of protein molecules, which assemble to form 3 main types of filaments. Three main types of fibers in the cytoskeleton • Each structural element of the cytoskeleton is formed by the polymerization of different kind of subunit. eg. Microtubules are composed of protein tubulin. Microfilaments are polymers of protein actin. • In addition, each type of cytoskeletal filament has a number of proteins associated with it. eg. Accessory proteins facilitate in the structural and functional diversity of cytoskeletal elements. The cytoskeleton is dynamically assembled and disassembled • • • • • Each type of the cytoskeletal element is constructed from smaller protein subunits. → repetitive assembly of large numbers of the small subunits. These subunits are small that they an diffuse rapidly within cytoplasm whereas assembled filaments cannot. All the three types of cytoskeletal filaments selfassociate using a combination of end-to-end and side-to-side protein contacts. The cytoskeletal ‘polymers’ are held together by weak non-covalent interactions. → Assembly and disassembly can occur rapidly. Cells can undergo structural organization, disassembling filaments at one site and reassembling them at another site far away. Level of organization affects thermal stability Microtubules (MT) • Classified into two general groups, cytoplasmic microtubules and axonemal microtubules. → organization and structural stability. • Cytoplasmic microtubules (loosely organized): i) Animals - required to maintain axons. - maintain polarized shape. ii) Plants - govern the orientation of cellulose microfibrils deposited during the growth of cell walls iii) Others - form the mitotic and meiotic spindles. - facilitate the movement of vesicles and other organelles. • Axonemal microtubules (highly organized) Nucleation - associated with cellular movement (ie. Cilia, flagella and the basal bodies to which these appendages are attached). • MT are polarized structures composed of α- and β- tubulin heterodimer subunits assembled into linear protofilaments. → noncovalent binding. • A single MT is comprised of 10-15 protofilaments (usually 13 in mammalian cells) that associate laterally to form a 24nm wide hollow cylinder. • Different polymerization rates at two ends: → In each protofilament, the heterodimers are oriented with their β-tubulin monomer pointing towards the faster-growing end (plus end) and their α-tubulin monomer exposed at the slower-growing end (minus end). • Each tubulin subunit has a GTP-binding domain at N-terminus, a domain in the middle to which MT poison colchicine can bind and a third domain at the Cterminus that interacts with MT-associated proteins. • MT can form as singlets, doublets or triplets. → Doublets: cilia and flagella Triplets: basal bodies and centrioles Arrangement of protofilaments in singlet, doublet, and triplet microtubules • Singlet composed of a 13 protofilaments tubule (A). • Doublet composed of a singlet (A tubule) and one additional B tubule consisting of 10 protofilaments appended to its side. • Triplet composed of A tubule plus additional B and C tubules of 10 protofilaments. Microtubule assembly • Assemble by polymerization of tubulin dimers. • Addition and loss only at the ends. • Addition of GTP-bound tubulin is favored over GDP-bound. • Assembly and disassembly depend on critical concentration of αβ subunits. Tubulin dimers Oligomers Protofilament Sheets of protofilaments Closing microtubule Elongating microtubule Assemble by polymerization of tubulin dimers • At the start of the nucleation process, several dimers can aggregate into clusters called oligomers. • One an MT has been nucleated, it grows by addition of subunits at either end to form linear chains of tubulin dimers called protofilaments. • The protofilaments can then associate with each other side by side to form sheets. • Sheets containing 13 or more protofilaments can close into a tube, forming a microtubule. • Elongation of the microtubule continues by the addition of tubulin subunits at one or both ends. Addition of tubulin dimers occurs more quickly at the plus ends of microtubules • • • • • One end can inherently grow or shrink much faster than the other. Rapidly growing end of the microtubule is called plus end and the other end is the minus end. Growth rates depending on critical concentrations of free tubulins. → [tubulinsfree] is higher than the [critical] for the plus end but lower at the minus end, assembly will occur at the plus end while disassembly takes place at the minus end. Simultaneous assembly and disassembly → treadmiling Assembly: Relatively smooth ends; some protofilaments are longer (elongate unevenly) Disassembly: Ends are splayed; frayed appearance Drugs that affect the assembly of microtubules • Colchicine → binds to tubulin monomers, strongly inhibiting their assembly into mirotubules and fostering the disassembly of existing ones. • Vinblastine and vincristine → promote tubulin aggregation inside the cell. • Nocodazole → inhibits MT assembly (effects are more readily reversible when the drug is removed) ¤ antimitotic drug – disrupt mitotic spindle of dividing cells, blocking the further progress of mitosis. Taxol → binds tightly to microtubules and stabilizes them, causing much of the free tubulin in the cell to assemble into microtubules and arrests dividing cells in mitosis. GTP cap and its role in dynamic instability of microtubules • Stable when capped with GTP-tubulins and unstable when capped with GDP-tubulins. • Each tubulin heterodimer binds two GTP molecules. → α-tubulin binds one GTP; → β-tubulin binds to the other GTP which can be hydrolyzed to GDP sometime after the heterodimer is added to an MT. Microtubule-organizing enters (MTOC) • serves as a site at which MT assembly is initiated and acts as an anchor for one end of these MTs. • minus ends are anchored in the MTOC and plus ends extend toward the cell membrane. • MTOC in animal cells: centrosome Microtubule-associated proteins (MAPs) • Increase MT stability and affect the density of bundles of MTs. • Stabilizing MAPS → Tau: causes microtubules to form tight bundles in axons. → Map2: causes the formation of looser bundles of MTs. → +-TIP proteins: stabilize plus ends. Microtubule destabilizing MAPs → stathmin/Op18: increases depolymerization by binding tubulin dimers. Microtubule severing MAPs → catastrophins: promoting the peeling of subunits from their ends. Accessory proteins regulate filament dynamics Microfilament (MFs) • smallest cystoskeletal filaments with a diameter of about 7nm. • important in mediating cell movement and maintaining cell shape. → found at sites of attachment of cells to one another and to the extracellular matrix • building block of MFs is actin. → G-actin: individual actin molecules. → F-actin: polymerization of G-actin to form microfilaments • Two major groups of actin: → muscle-specific actins (α-actins) → nonmuscle actins (β- and γ-actins) G-actin monomers polymerize into F-actin microfilaments • F-actin filaments that form are composed of two linear strands of polymerized G-actin wound around each other in a helix, with roughly 13.5 actin monomers per turn. • Subunits assembled in a head-to-tail manner hence protofilaments have polarity. + end: fast growing (barbed) - end: slow growing (pointed) • As G-actin monomers assemble into mirofilament, ATP bound to them is slowly hydrolyzed to ADP. → Ends of growing MF tend to have ATP-F-actin whereas the bulk of the MF is composed of ADP-F-actin. Actin-binding proteins regulate polymerization, length and organization of microfilaments Polymerization • Growth of MFs depend on [ATP-bound-G-actin] • Large amount of free G-actin is not available for assembly into filaments. → bound by protein thymosin β4 • Profilin transfer G-actin monomers from thymosin β4 complex to the end of growing filament only when free filament ends available. Filaments capping • Capping prevents further addition or loss of subunits, thereby stabilizing it. • Example: i) CapZ: binds to the plus ends of actin filaments. II) tropomodulins: binds to the minus ends of actin filaments, preventing loss of subunits from the pointed ends of F-actin. Crosslinking actin filaments • filamin: long molecule consist of two identical polypeptides joined head to head, with an actin-binding site at each tail. → joining two MFs together where they intersect. Severing actin filaments • gelsolin: breaking MFs and capping newly exposed plus ends, thereby preventing further polymerization. → can be regulated by polyphosphoinositides. Promoting actin branching and growth • Arp2/3 complex: helps branches to form by nucleating new branches on the sides of existing filaments. Intermediate filaments (IF) Size is intermediate filaments: 10 nm outer diameter Polypeptides (constituent of polypeptides vary, six major classes) have an alpha-helical core and flanking globular domains Dimers form a coiled-coil, with amino terminal at the same end – Dimers may be homo- or heterodimers Dimers can associate with each other in an antiparallel fashion to form the tetramers (the protofilaments for IF assembly and are antiparallel) Tetramers is the soluble subunit of IF. 8 protofilaments pack together laterally to form the IF assembly (10nm rope like filament, having 16 dimers in cross section) Accessory proteins e.g. filaggrin and plectin help to bundle IF as well as link them to other filaments as well as motor proteins Intermediate filament structure depends on the lateral bundling and twisting of coiled coils IF structure ad assembly • General functions: Provide mechanical strength and resistance to remove stress. Various types of IF in vertebrate cells TYPES OF IF COMPONENT POLYPEPTIDES CELLULAR LOCATION Keratins type I Acidic keratins epithelia Keratins type II Neutral/basic keratins Epithelia Vimentin-like IFs Vimentin During embryogenesis Desmin Muscle Glial fbrllary acidic protein Astrocytos Peripherin Neurons Neurofilaments Internexin Neurons Lamins Lamin Nucleus, almost ubiquitous Nestins Nestin Neuro-epithelial stem cells Others Filensin, phakinin lens Keratin defects in the skin Motor proteins 1. Cellular functions 2. Structure and organization 3. Cytoskeletal motor proteins 3.1 Actin motors 3.1.1 Myosin 3.2 Microtubule motors 3.2.1 Kinesin 3.2.2 Dynein 4. Diseases associated with motor protein Motor Proteins • Motor proteins attach to microtubules or microfilaments and to their cargo (vesicles, organelles, chromosomes, other cytoskeletal filaments). Motor proteins are the driving force behind most active transport of proteins and vesicles in the cytoplasm. • Functions: – Carry membrane organelles or secretory vesicles to their appropriate locations in cell – Cause cytoskeletal filaments to slide against each other , generating force that drives phenomenon such as muscle contraction, ciliary beating and cell division • Structure: Most eukaryotic motor proteins consist of 2 distinct domains: Motor head domain Tail domain • With ATPase function, the proteins carries out the movement by binding to a specific site on the substrate and changing conformation. • Can either form fibers (muscle myosin) or attach to cargo. • Example chromosomes during anaphase of mitosis (kinesin) or vesicles during endocytosis (dynein) • Binds adaptor proteins that allow for stable interactions with the cargo to be moved along the substrate. Families of motor proteins: a. Myosins (along microfilaments or actin) -is responsible for muscle contraction b. Kinesins (along microtubules) -moves cargo inside cells away from the nucleus along microtubules c. Dyneins (along microtubules) -produces the axonemal beating of cilia and flagella and also transport cargo along microtubule towards the cell nucleus Myosins • More than 20 known classes of myosins. • General structure: ¤ have at least one polypeptide called heavy chain with a globular head group at one end attached to a tail of varying length. ¤ Globular head binds to actin and uses energy of ATP hydrolysis to move along an actin filament. ¤ structure of tail region varies among different kinds of myosin, giving myosin molecules the ability to bind to various molecules or cell structures. ¤ tail structure also determines the ability of myosins to bind to other identical myosins to form dimers or large arrays. ¤ small polypeptides, light chain, bound to the globular head group which often play a role in regulating the activity of the myosin ATPase. • Functions: ¤ muscle contraction (muscle myosin II) ¤ cell movement (nonmuscle myosin II) ¤ phagocytosis (myosin VI) ¤ vesicle transport or other membrane-associated events (myosin I, V) Microtubule motors Dynein Kinesin Kinesins • At least 10 families. • General structure: ¤ A globular head region that attaches to microtubules and is involved in hydrolysis of ATP. ¤ A coiled helical region. ¤ A light chain region that is involved in attaching kinesin to other proteins and organelles. • Movement: ¤ One of the two globular heads moves forward to make an attachment to a new βtubulin subunit. ¤ This is followed by detachment of the trailing globular head which can now make an attachment to a a new region of the MT. ¤ This movement is coupled to the hydrolysis of ATP bound at specific sites within the heads which resulted kinesin moves toward the plus end of an MT in an ATP-dependent manner. • Function: ¤ moving and localizing substances within cells. Dyneins • Consist of two types: ¤ Cytoplasmic dyneins → contains two heavy chains that interact with MTs, two intermediate chains, two light intermediate chains and various light chains. → moves toward the minus ends of MTs. → associated with protein complex, dynactin. → involved in shaping endomembrane system and vesicle transport. ¤ Axonemal dynein → basal body consists of nine sets of tubular structures arranged around its circumference, ‘9+2’ pattern (nine outer doublets of tubules and two additional microtubules in the center). → Each outer doublet consists of one complete MT (A tubule) and one incomplete MT (B tubule). → Sidearms that project out from each of the A tubules which is responsible for sliding MTs within the axoneme past one another to bend the axoneme. → Radial spokes project inward from each of the 9 MT doublets for slidingof adjacent doublets into the bending motion that characterizes the beating of these appendages. • Functions: Motility (Cilia and flagella) Diseases associated with motor protein defects a. Kinesin deficiencies have been identified as cause for Charcot-Marie-Tooth disease and some kidney diseases. b. Dynein deficiencies can lead to chronic infections of the respiratory tract as cilia fail to function without dynein. c. Defects in muscular myosin predictably cause myopathies, whereas defects in unconventional myosin are the cause for Usher syndrome and deafness.