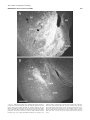
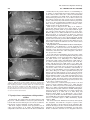
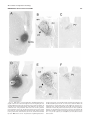
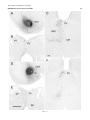
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
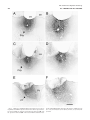
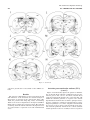
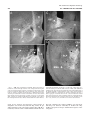
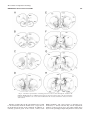
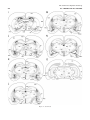
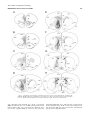
Download Projections of the paraventricular and paratenial nuclei
Environmental enrichment wikipedia , lookup
Executive functions wikipedia , lookup
Human brain wikipedia , lookup
Neuroesthetics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Cortical cooling wikipedia , lookup
Biology of depression wikipedia , lookup
Neuroplasticity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Affective neuroscience wikipedia , lookup
Neuroeconomics wikipedia , lookup
Aging brain wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Emotional lateralization wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Basal ganglia wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
THE JOURNAL OF COMPARATIVE NEUROLOGY 508:212–237 (2008) Projections of the Paraventricular and Paratenial Nuclei of the Dorsal Midline Thalamus in the Rat ROBERT P. VERTES* AND WALTER B. HOOVER Center for Complex Systems and Brain Sciences, Florida Atlantic University, Boca Raton, Florida 33431 ABSTRACT The paraventricular (PV) and paratenial (PT) nuclei are prominent cell groups of the midline thalamus. To our knowledge, only a single early report has examined PV projections and no previous study has comprehensively analyzed PT projections. By using the anterograde anatomical tracer, Phaseolus vulgaris leucoagglutinin, and the retrograde tracer, FluoroGold, we examined the efferent projections of PV and PT. We showed that the output of PV is virtually directed to a discrete set of limbic forebrain structures, including ‘limbic’ regions of the cortex. These include the infralimbic, prelimbic, dorsal agranular insular, and entorhinal cortices, the ventral subiculum of the hippocampus, dorsal tenia tecta, claustrum, lateral septum, dorsal striatum, nucleus accumbens (core and shell), olfactory tubercle, bed nucleus of stria terminalis (BST), medial, central, cortical, and basal nuclei of amygdala, and the suprachiasmatic, arcuate, and dorsomedial nuclei of the hypothalamus. The posterior PV distributes more heavily than the anterior PV to the dorsal striatum and to the central and basal nuclei of amygdala. PT projections significantly overlap with those of PV, with some important differences. PT distributes less heavily than PV to BST and to the amygdala, but much more densely to the medial prefrontal and entorhinal cortices and to the ventral subiculum of hippocampus. As described herein, PV/PT receive a vast array of afferents from the brainstem, hypothalamus, and limbic forebrain, related to arousal and attentive states of the animal, and would appear to channel that information to structures of the limbic forebrain in the selection of appropriate responses to changing environmental conditions. Depending on the specific complement of emotionally associated information reaching PV/PT at any one time, PV/PT would appear positioned, by actions on the limbic forebrain, to direct behavior toward a particular outcome over a range of outcomes. J. Comp. Neurol. 508: 212–237, 2008. © 2008 Wiley-Liss, Inc. Indexing terms: medial prefrontal cortex; subiculum of hippocampus; nucleus accumbens; bed nucleus of stria terminalis; central and basal nuclei of amygdala The paraventricular and paratenial nuclei are prominent cell groups of the midline thalamus (Swanson, 1998; Van der Werf et al., 2002). The paraventricular nucleus (PV) lies dorsally on the midline directly below the third ventricle and extends rostrocaudally virtually throughout the thalamus. The paratenial nucleus (PT) borders PV laterally and overlaps with approximately the rostral onethird of PV. Based on the early demonstration that low-frequency stimulation of the midline and intralaminar nuclei of the thalamus produced slow synchronous activity over widespread regions of the cortex (recruiting responses) (Dempsey and Morrison, 1942), the midline thalamus was viewed as ‘nonspecific’ thalamus, exerting nonspecific or © 2008 WILEY-LISS, INC. global effects on the cortical mantle (Bentivoglio et al., 1991; Groenewegen and Berendse, 1994). The notion, however, of the midline thalamus as ‘nonspecific’ has been revised based on the subsequent anatomical demonstra- Grant sponsor: National Institute of Mental Health; Grant numbers: MH42900, MH63519. *Correspondence to: Dr. Robert P. Vertes, Center for Complex Systems and Brain Sciences, Florida Atlantic University, Boca Raton, FL 33431. E-mail: [email protected] Received 29 August 2007; Revised 20 December 2007; Accepted 10 January 2008 DOI 10.1002/cne.21679 Published online in Wiley InterScience (www.interscience.wiley.com). The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI 213 tion that nuclei of the midline thalamus do not project widely throughout the neocortex, but rather selectively to specific regions of cortex, primarily those of the prefrontal cortex (Berendse and Groenewegen, 1991; Van der Werf et al., 2002; Groenewegen and Witter, 2004; Vertes, 2006). In addition, recent reports have shown that stimulation of individual nuclei of the midline thalamus produce selective effects on their cortical targets—as opposed to widespread actions throughout the cortex (Dolleman-Van der Weel et al., 1997; Bertram and Zhang, 1999; Kung and Shyu, 2002; Zhang and Bertram, 2002; Viana Di Prisco and Vertes, 2006). In a series of reports, Groenewegen and colleagues (Berendse and Groenewegen, 1990, 1991; Berendse et al., 1992; Groenewegen et al., 1999) showed that major targets of midline thalamic nuclei were the prefrontal cortex and ventral striatum (nucleus accumbens, ACC), and further that recipient zones in the medial prefrontal cortex (mPFC) and ACC were themselves directly connected (mPFC to ACC). With respect to the paraventricular nucleus, PV distributes to the prelimbic cortex (of mPFC) and to the medial shell region of ACC, and PL, in turn, projects to the shell of ACC (Berendse and Groenewegen, 1990, 1991; Berendse et al., 1992). Undoubtedly owing to the early emphasis on PV projections to the ventral striatum and to mPFC, subsequent reports largely focused on these target sites. Using dual retrograde labeling techniques, Otake and Nakamura (1998) reported that of the nuclei of the midline thalamus, PV contained the largest percentage of cells with collateral projections to ACC and mPFC. In like manner, Bubser and Deutch (1998) showed that ⬇15% of PV cells distribute via collaterals to the medial shell of ACC and PL, while Pinto et al. (2003) demonstrated that PV fibers terminate in close apposition to dopaminergic (DA) terminals in nucleus accumbens, but not to DA terminals in the mPFC. Abbreviations AA ac AC ACC,c,s AGm AGl AH AI,d,p,v AM AON AV BLA BMA BST CA1,3 cc CEA,c,l,m CL CLA CM COA,a,p CP DBh DMh EC,l ECT EN fa FG FI FP,l,m FS GI GP HF IAM IL IMD IP LA LD LH LHy LO LP LPO LS MA MD MEA Anterior amygdaloid area Anterior commissure Anterior cingulate cortex Nucleus accumbens, core and shell divisions Medial agranular (frontal) cortex Lateral agranular (frontal) cortex Anterior nucleus of hypothalamus Agranular insular cortex, dorsal, posterior, ventral divisions Anteromedial nucleus of thalamus Anterior olfactory nucleus Anteroventral nucleus of thalamus Basolateral nucleus of amygdala Basomedial nucleus of amygdala Bed nucleus of stria terminalis Field CA1 and CA3 of Ammon’s horn Corpus callosum Central nucleus of amygdala, capsular, lateral, and medial divisions Central lateral nucleus of the thalamus Claustrum Central medial nucleus of thalamus Cortical nucleus of amygdala, anterior, posterior divisions Caudate-putamen Nucleus of diagonal band, horizontal limb Dorsomedial nucleus of hypothalamus Entorhinal cortex, lateral division Ectorhinal cortex Endopiriform nucleus Forceps of the corpus callosum Fluorogold Fimbria of hippocampus Frontal polar cortex, lateral, medial divisions Fundus of striatum Granular insular cortex Globus pallidus Hippocampal formation Interanteromedial nucleus of thalamus Infralimbic cortex Intermediodorsal nucleus of thalamus Interpeduncular nucleus Lateral nucleus of amygdala Lateral dorsal nucleus of thalamus Lateral habenula Lateral hypothalamus Lateral orbital cortex Lateral posterior nucleus of thalamus Lateral preoptic area Lateral septum Magnocellular preoptic nucleus Mediodorsal nucleus of thalamus Medial nucleus of the amygdala MFB MG MH MO mPFC MPO MPN MRF MS mt OC OT PAp PC PFC PH PHA-L PIR PL PO PRC PT PV,a,p RE RH RN RSC RT SC SCN SI sm SM SNr SPZ SSI SSII st SUB,v SUM TE TT,d,v V3 VAL VB VL VLO VM VO VTA ZI Medial forebrain bundle Medial geniculate nucleus of thalamus Medial habenula Medial orbital cortex Medial prefrontal cortex Medial preoptic area Medial preoptic nucleus Mesencephalic reticular formation Medial septum Mammillothalamic tract Occipital cortex Olfactory tubercle Posterior parietal cortex Paracentral nucleus of thalamus Prefrontal cortex Posterior nucleus of hypothalamus Phaseolus vulgaris-leucoagglutinin Piriform cortex Prelimbic cortex Posterior nucleus of thalamus Perirhinal cortex Paratenial nucleus of thalamus Paraventricular nucleus of thalamus, anterior and posterior divisions Nucleus reuniens of thalamus Rhomboid nucleus of thalamus Red nucleus Retrosplenial cortex Reticular nucleus of thalamus Superior colliculus Suprachiasmatic nucleus Substantia innominata Stria medullaris Submedial nucleus of thalamus Substantia nigra, pars reticulata Subparaventricular zone of hypothalamus Primary somatosensory cortex Secondary somatosensory cortex Stria terminalis Subiculum, ventral division Supramammillary nucleus Temporal cortex Tenia tecta, dorsal and ventral divisions Third ventricle Ventral anterior nucleus of thalamus Ventral basal nucleus of thalamus Lateral ventricle Ventrolateral orbital cortex Ventral medial nucleus of thalamus Ventral orbital cortex Ventral tegmental area Zona incerta The Journal of Comparative Neurology 214 To our knowledge, only a single report (Moga et al., 1995) has examined the general distribution of PV projections “with special emphasis on the projections to the hypothalamus and amygdala.” Focusing on circadian circuitry, Moga et al. (1995) described PV projections to the suprachiasmatic nucleus (SCN) as well as to other sites involved in circadian rhythms including the dorsomedial nucleus and subparaventricular zone of the hypothalamus. These results, coupled with the demonstration that PV receives input from all major components of the circadian system including SCN, led Moga et al. (1995) to conclude that, “the anterior PV is ideally situated to relay circadian timing information from the SCN to brain areas involved in visceral and motivation aspects of behavior and to provide feedback regulation of the SCN.” Consistent with this, Peng and Bentivoglio (2004) recently demonstrated at the light and electron microscopic (EM) levels that SCN fibers synaptically contact PV cells projecting to the amygdala and concluded that PV serves an important role in the “transfer of circadian timing information to the limbic system.” PV receives a vast array of afferents from monoaminergic and neuropeptide containing systems of the brainstem and hypothalamus—systems known to have activating effects on the forebrain (Chen and Su, 1990; Vertes, 1991; Freedman and Cassell, 1994; Bhatnagar et al., 2000; Krout et al., 2002; Otake, 2005). Accordingly, PV (and other midline thalamic nuclei) are thought to serve a direct role in processes of arousal and attention (Krout et al., 2002; Van der Werf et al., 2002; Vertes, 2002, 2006). Consistent with a role for PV in attention, Kirouac et al. (2005) recently showed that PV receives pronounced input from orexin (hypocretin) containing cells as well as from cocaine- and amphetamine-regulated transcript containing (CART) neurons of the hypothalamus (Kirouac et al., 2006), and that both orexin and CART fibers synapse with PV cells projecting to the shell of ACC (Parsons et al., 2006). They proposed that PV links visceral/arousal systems to limbic forebrain regions involved in behavioral responses (Parsons et al., 2006). Taken as a whole, the foregoing suggests that PV may represent an important relay in the transfer of visceral/ arousal, homeostatic, and circadian information to parts of the limbic system—thereby priming them (state of readiness) for behavioral responding. In this regard, PV neurons show elevated levels of c-fos expression during periods of wakefulness (compared to sleep) (Peng et al., 1995) as well as during stressful conditions (Chastrette et al., 1991; Bubser and Deutch, 1999; Sica et al., 2000; Otake et al., 2002)—which could be seen as heightened states of arousal. In view, then, of its pivotal role in limbic circuitry, we sought to comprehensively examine the efferent projections of PV in the rat. Although the paratenial nucleus of the thalamus also appears to receive a vast array of afferent information (Chen and Su, 1990; Krout et al., 2002) and may selectively target structures of the limbic forebrain (Kelley and Stinus, 1984; Carlsen and Heimer, 1986), little is known regarding the connections of PT. The purpose, then, of the present study was to analyze, compare, and contrast efferent projections of PV and PT nuclei of the midline thalamus. We show that, with some important differences, the output of both PV and PT is mainly directed to the medial prefrontal and entorhinal cortices, the ventral subiculum of the hippocampus, claustrum, the dorsal and R.P. VERTES AND W.B. HOOVER ventral striatum, lateral septum, bed nucleus of stria terminalis, and to most of the amygdala, with a concentration in the central and basal nuclei of the amygdala. Materials and Methods Single injections of Phaseolus vulgaris-leucoagglutinin (PHA-L) were made into either the PV or PT nuclei of the midline thalamus in 31 male Sprague–Dawley rats (Charles River, Wilmington, MA) weighing 275– 400 g. Of the 31 injections, 10 were confined to PV, 8 were confined to PT, 4 overlapped PV and PT, 4 overlapped PV and the mediodorsal nucleus (MD); 3 overlapped PV and the intermediodorsal nucleus (IMD), and 2 were localized to the interanteromedial nucleus (IAM). Another 16 male Sprague–Dawley rats weighing 350 – 450 g received single injections of the retrograde fluorescent tracer FluoroGold (FG) (Fluorochrome, Denver, CO) into some PV and PT targets: the central (CEA) and basolateral (BLA) nuclei of the amygdala and the core and shell of nucleus accumbens. Of the 16 injections, seven were made in CEA or BLA of the amygdala and three were control injections in other nuclei of the amygdala. Of the seven injections in the central and basal nuclei of the amygdala, two were made in CEA, two in the basolateral nucleus (BLA), two in BLA and CEA, and one in BLA and the basomedial nucleus. Of the six injections in nucleus accumbens, three were localized to the core of ACC and three to the shell of ACC. The experiments were approved by the Florida Atlantic University Institutional Animal Care and Use Committee and conform to all federal regulations and National Institutes of Health guidelines for the care and use of laboratory animals. PHA-L procedures Powdered lectin from PHA-L was reconstituted to 4 –5% in 0.05 M sodium phosphate buffer (PB), pH 7.4. The PHA-L solution was iontophoretically deposited in the brains of anesthetized rats (sodium pentobarbital, 75 mg/ kg, ip) by means of a glass micropipette with an outside tip diameter of 40 – 60 m. Positive direct current (5–10 A) was applied through a Grass stimulator (Model 88) coupled with a high voltage stimulator (Frederick Haer, Bowdoinham, ME) at 2 seconds “on” / 2 seconds “off” intervals for 40 –50 minutes. After a survival time of 7–10 days, rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with a heparinized buffered saline wash (100 mL/animal) followed by a fixative (4% paraformaldehyde, 0.2– 0.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4) (300 –500 mL/animal). The brains were removed and stored for 2 days at 4°C in 30% sucrose in 0.1 M PB. On the following day, 50-m frozen sections were collected in phosphate-buffered saline (PBS, 0.9% sodium chloride in 0.01 M sodium phosphate buffer, pH 7.4) using a sliding microtome. Six series of sections were taken. A complete series of sections was treated with 1% sodium borohydride in 0.1 M PB for 30 minutes to remove excess reactive aldehydes. Sections were then rinsed in 0.1 M PB, followed by 0.1 M Tris-buffered saline (TBS), pH 7.6. Following this, sections were incubated for 60 minutes at room temperature (RT) in 0.5% bovine serum albumin (BSA) in TBS to minimize nonspecific labeling. The sections were then incubated overnight at RT in diluent (0.1% BSA in TBS containing 0.25% Triton X-100) and biotinylated goat anti PHA-L (Vector Labs, Burlingame, The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI CA) at a concentration 1:500. Sections were then washed in 0.1 M PB (4 ⫻ 8 minutes) and placed in a 1:500 concentration of biotinylated rabbit antigoat immunoglobulin (IgG) and diluent for 2 hours. Sections were washed and then incubated in a 1:100 concentration of peroxidaseavidin complex from the Elite kit (Vector) and diluent for 1 hour. Following another 0.1 M PB wash the peroxidase reaction product was visualized by incubation in a solution containing 0.022% 3,3⬘ diaminobenzidine (DAB, Aldrich, Milwaukee, WI) and 0.003% H2O2 in TBS for 6 minutes. Sections were then rinsed again in PBS (3 ⫻ 1 minutes) and mounted onto chrome-alum gelatin-coated slides. An adjacent series of sections from each rat was stained with cresyl violet for anatomical reference. Sections were examined using light and darkfield optics. Injection sites, cells, and labeled fibers were plotted on representative schematic coronal sections through the brain using sections adapted from the rat atlas of Swanson (1998). Brightfield and darkfield photomicrographs of injection sites and labeled fibers were taken with a Nikon DXM1200 camera mounted on a Nikon Eclipse E600 microscope. Digital images were captured and reconstructed using Image-Pro Plus 4.5 (Media Cybernetics, Silver Springs, MD), and adjusted for brightness and contrast using Adobe PhotoShop 7.0 (Mountain View, CA). Patterns of labeling were classified as light, moderate, and dense (Table 1), with ‘light’ referring to a few labeled fibers widely dispersed throughout a structure, ‘dense’ as a heavy concentration of labeled fibers generally occupying a significant portion (or most) of a structure, and ‘moderate’ between these two patterns. FluoroGold procedures FluoroGold (FG) (Fluorochrome) was dissolved in a 0.1 M sodium acetate buffer (pH 4.0 to 5) to yield a 4 –5% concentration. The FG solution was iontophoretically deposited in the brains of anesthetized rats by means of a glass micropipette with an outside tip diameter of 25–50 m. Single FG injections were made into one of four structures in separate rats: the central and basolateral nuclei of the amygdala and the core and shell of ACC. The procedures for FG injections were basically the same as described for PHA-L injections, with the following exceptions: 1) the outside tip diameter of the glass micropipettes was 25–50 m, and 2) the length of injections was 2–10 minutes. Following a survival time of 7 days, rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 100 mL of a heparinized saline wash followed by 450 mL of fixative (4% paraformaldehyde in 0.01 M sodium PB, pH 7.4). The brains were then removed and stored for 48 hours in a sucrose solution (30% sucrose in 0.1 M PB) at 4°C. Following this, 50-m coronal sections were taken on a freezing microtome and collected in 0.1 M PB and stored at 4°C. Six series of sections were taken. A complete series of sections was incubated in a sodium borohydride solution (1% sodium borohydride in 0.1 M PB) for 30 minutes, and washed with 0.1M PB four times at 6 minutes each (4 ⫻ 6 min). The sections were then blocked in a Tris-saline solution (0.5% BSA, Sigma Chemicals, St. Louis, MO; 0.25% Triton X-100 in 0.1 M Tris-saline, pH 7.6) for 1 hour. Following the blocking procedure the sections were incubated for 48 hours at RT in a primary antiserum directed against FG (rabbit anti-FluoroGold) (Chemicon, Temecula, CA) at a concentration of 1:1,000 in diluent. 215 TABLE 1. Relative Density of Anterogradely Labeled Fibers Produced by PHA-L Injections into PVa, PVp, and PT of the Midline Thalamus Labeling Structure Telencephalon Cortex Agranular insular, dorsal Agranular insular, posterior Agranular insular, ventral Agranular, lateral (primary motor) Agranular, medial (secondary motor) Anterior cingulate, dorsal Anterior cingulate, ventral Ectorhinal Entorhinal Frontal polar, lateral Frontal polar, medial Granular insular Infralimbic Lateral orbital Medial orbital Parietal Perirhinal Piriform Prelimbic Retrosplenial Subiculum, ventral Somatosensory, secondary Ventral Orbital Ventrolateral Orbital Accumbens n. Core Shell Amygdala Anterior amygdaloid area Basolateral n. Basomedial n. Central n. Cortical n. Lateral n. Medial n. Posterior n. Amygdaloid-piriform area Bed n. of stria terminalis Claustrum Diagonal band of Broca, horizontal Endopiriform n. Globus pallidus Medial preoptic area Olfactory tubrical Septum Medial Lateral Striatum Substantia innominata Tenia tecta Diencephalon Hypothalamus Arcuate n. Dorsomedial n. Lateral hypothalamic area Paraventricular n. Posterior hypothalamus Suprachiasmatic n. Supramammillary n. Thalamus Central medial n. Interanteromedial n. Mediodorsal n. Parafascicular n. Parataenial n. Paraventricular n. Reticular n. Reuniens n. Rhomboid n. PVa PVp PT ⫹⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫹ ⫹ ⫹ ⫹ ⫺ ⫹ ⫺ ⫹ ⫺ ⫹ ⫹ ⫹⫹ ⫺ ⫹⫹ ⫺ ⫹⫹ ⫺ ⫹⫹ ⫹⫹ ⫺ ⫺ ⫺ ⫹ ⫹ ⫹⫹ ⫹⫹ ⫹ ⫹ ⫹⫹ ⫹⫹⫹ ⫺ ⫹ ⫺ ⫹⫹ ⫹⫹⫹ ⫺ ⫹⫹ ⫺ ⫺ ⫺ ⫹⫹⫹ ⫹ ⫺ ⫺ ⫺ ⫹⫹⫹ ⫹⫹ ⫹ ⫹⫹⫹ ⫺ ⫹⫹⫹ ⫺ ⫹⫹⫹ ⫺ ⫹⫹⫹ ⫺ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹ ⫺ ⫹⫹ ⫺ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹ ⫹⫹ ⫹⫹⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫹⫹⫹ ⫹⫹ ⫺ ⫺ ⫹ ⫺ ⫹⫹ ⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫺ ⫺ ⫹⫹⫹ ⫹⫹ ⫺ ⫺ ⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹ ⫺ ⫺ ⫺ ⫹ ⫹⫹⫹ ⫺ ⫹ ⫹⫹ ⫺ ⫹ ⫺ ⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹ ⫺ ⫹⫹⫹ ⫹⫹⫹ ⫺ ⫹⫹ ⫹⫹ ⫹ ⫺ ⫺ ⫺ ⫹⫹ ⫺ ⫺ ⫹⫹ ⫺ ⫺ ⫺ ⫹⫹ ⫺ ⫺ ⫺ ⫹⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫹ ⫺ ⫹, light labeling; ⫹⫹, moderate labeling; ⫹⫹⫹, dense labeling; ⫺, absence of labeling; n, nucleus Following incubation in the primary antiserum, sections were washed (4 ⫻ 6 min) in 0.1 M PB, then incubated in a secondary antiserum (biotinylated goat antirabbit IgG) (Vector) at a concentration of 1:500 in diluent for 2 hours. Sections were then washed again (4 ⫻ 6 minutes) and The Journal of Comparative Neurology 216 Fig. 1. High-power brightfield photomicrographs at two levels of magnification of PHA-L injections sites in the anterior paraventricular nucleus (A,B), the posterior paraventricular nucleus (C,D), and the paratenial nucleus (E,F) of the dorsal midline thalamus. Note R.P. VERTES AND W.B. HOOVER clearly visible PHA-L filled cells in PVa (B), PVp (D), and PT (F). Scale bar ⫽ 375 m for A,E; 200 m for B; 400 m for C; 300 m for D; 225 m for F. The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI 217 Fig. 2. Schematic representation of labeling present in select sections through the forebrain and midbrain (A–N) produced by a PHA-L injection (dots in I,J) in the anterior part of the paraventricular nucleus of the thalamus (case 6). Sections modified from the rat atlas of Swanson (1998). See list for abbreviations. incubated in avidin-biotin complex (Vector) at a 1:100 concentration in diluent for 1 hour. After a final set of 4 ⫻ 6 minute rinses the peroxidase reaction product was visualized by incubation in a solution containing 0.022% of DAB (Aldrich), 0.015% nickel chloride (NiCl), and 0.003% H2O2 in TBS for 6 minutes. Sections were rinsed again in PBS (3 ⫻ 1 minutes) and mounted onto chrome-alum gelatin-coated slides. An adjacent series of representative sections from each rat was stained with cresyl violet for anatomical reference. The resulting material was pro- The Journal of Comparative Neurology 218 R.P. VERTES AND W.B. HOOVER Figure 2 cessed for presentation as described for the PHA-L sections. Results The pattern of distribution of projections from the PV and PT nuclei of the thalamus are described. Figure 1 shows sites of injection in the anterior PV (PVa) (Fig. 1A,B), the posterior PV (PVp) (Fig. 1C,D), and PT (Fig. 1E,F) at two levels of magnification. As depicted, PHA-Lfilled cells are restricted to respective nuclei. The patterns of labeling obtained with the schematically depicted cases are representative of patterns seen with nonillustrated cases. (Continued) Anterior paraventricular nucleus (PVa) (case 6) Figure 2 schematically depicts the pattern of distribution of labeled fibers following a PHA-L injection in the anterior part of PV (case 6). As shown, labeled fibers coursed ventrolaterally from the site of injection (Fig. 2F) within the thalamic peduncle to regions of the lateral hypothalamus and from there took three principal routes. A major contingent continued ventrolaterally in amygdalopetal pathways to reach the amygdala and surrounding regions of cortex, others coursed rostrally to the anterior forebrain primarily bound for the ventral striatum and the prefrontal cortex or caudally en route to regions of The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI the hypothalamus. Some labeled fibers of the ascending bundle joined the stria terminalis and traveled with it to reach the amygdala and adjacent regions of cortex. Overall, labeling was light at the anterior pole of the forebrain (Fig. 2A,B). A small collection of labeled fibers was seen along the medial wall of the prefrontal cortex (PFC), mainly within the anterior prelimbic (PL) cortex. Some diffuse labeling was also observed in the dorsal tenia tecta (TTd) (Fig. 2A,B). Further caudally at the rostral forebrain (Fig. 2C,D), labeling was primarily confined to PL of the mPFC and to rostral aspects of nucleus accumbens (ACC). As depicted in Figure 3A, labeled fibers mainly encircled the outer boundaries of ACC, and were much less concentrated in the core of the rostral ACC. Additional labeled sites were the anterior claustrum (CLA), ventromedial regions of the dorsal striatum bordering ACC (Fig. 2D), and to a lesser degree the dorsal agranular insular cortex (AId) (Fig. 2C). There was a noticeable lack of labeling at this level (Fig. 2C), as well as caudally, over most of the cortical mantle. Labeling was stronger on the left than on the right side of the brain (Fig. 2A–M), reflecting the fact that the PV injection was slightly lateralized to the left side (Fig. 1A,B). At septal levels (Fig. 2D–H), labeling was pronounced and relatively restricted to the core and shell regions of ACC, to ventral aspects of the lateral septum (LS) (Figs. 2F, 3B), and to the bed nucleus of the stria terminalis (BST). The dense labeling within the shell of ACC and to a slightly lesser degree in the core of ACC is depicted in the photomicrographs of Figure 3B, while equally pronounced labeling of BST, above and below the anterior commissure, is shown in Figure 3C. Outside of these sites, CLA, the olfactory tubercle (OT), and ventral regions of the dorsal striatum were moderately labeled, while the ventral globus pallidus (Fig. 2H) was lightly labeled. At mid levels of the forebrain (Fig. 2I–K), labeling was mainly confined to the amygdala and parts of the hypothalamus. The arcuate and suprachiasmatic (SCN) (Fig. 2I) nuclei of the hypothalamus were moderately labeled. Within the (rostral) amygdala, labeling was very heavy within the central nucleus (CEA) (Fig. 2J,K), particularly within the lateral CEA (Fig. 3D), moderately dense in the basomedial (BMA) and basolateral (BLA) nuclei, and relatively light in the medial and cortical nuclei of amygdala. Some labeled fibers were also present on the lateral convexity of cortex within the posterior agranular insular cortex, rostrally (Fig. 2I,J) and in the perirhinal (PRC), rostral entorhinal (EC) and piriform cortices, caudally (Fig. 2K). At further caudal levels of the forebrain (Fig. 2L,M) and the rostral midbrain (Fig. 2N), the bulk of labeled fibers was localized to the amygdala and surrounding regions including caudal parts of the dorsal striatum (Fig. 2L,M), deep layers of the perirhinal, entorhinal and piriform cortices (Fig. 2L–N), and the ventral subiculum of the hippocampus (Fig. 2N). Within the amygdala, labeling was predominantly restricted to the basal nuclei— dense within BMA and moderate within the medial part of BLA. Some labeled fibers were also present in the posterior PV (PVp) and in the dorsomedial nucleus of the hypothalamus (Fig. 2L,M). 219 Posterior paraventricular nucleus (PVp) (case 32) Anterior and posterior PV injections largely gave rise to similar patterns of labeling but, as described below, overall density of labeling was stronger with PVp than with PVa injections. Labeled fibers from PVp mainly coursed rostrally through the dorsal thalamus (Fig. 4K–N) and approximately at the level of the rostral pole of the hippocampus (Fig. 4I,J) turned ventrolaterally to exit the thalamus. From there, they either continued on the same trajectory to reach the amygdala and surrounding regions of the dorsal striatum and cortex or ascended or descended through the medial forebrain bundle (MFB) en route to the basal forebrain and prefrontal cortices, rostrally, or to parts of the hypothalamus, caudally. Similar to PVa, labeling at the anterior pole of the forebrain (Fig. 4A,B) was generally moderate and mainly present in inner layers of the anterior PL and anterior cingulate (AC) cortices and to considerably lesser degrees in medial frontal polar (FPm), medial orbital (MO), and dorsal agranular insular (AId) cortices. Moderate numbers of labeled axons were also visible in TTd. Further caudally at the rostral forebrain (Fig. 4C), labeled fibers spread widely over ventral aspects of the brain mainly localized to the ventral mPFC, claustrum, dorsal agranular insular cortex (AId), rostral ACC, and the olfactory tubercle (OT). As depicted (Fig. 4C), labeling was quite dense in the inner layers of the infralimbic (IL) and prelimbic cortices and somewhat less pronounced in AId, CLA, rostral ACC, and OT. A few labeled fibers were also seen in AC. The main targets of labeled fibers further caudally in the forebrain were the dorsal and ventral striatum (Fig. 4D–F). As depicted schematically (Fig. 4D,E) and in the micrograph of Figure 5A, the shell of ACC (ACCs) was intensely labeled. With the exception of the region surrounding the anterior commissure, which was heavily labeled, the core of ACC (ACCc) was moderately labeled. Unlike PVa, significant numbers of labeled axons were also present in ventral aspects of the dorsal striatum (CP), progressively thinning from the ventrolateral to dorsomedial CP. Other moderately to heavily labeled sites at these levels were ventral LS, OT, CLA, and AId (Fig. 4D–F). Immediately caudal to ACC (Fig. 4F–H), labeled axons spread densely throughout the extent of the bed nucleus of the stria terminalis (BST) and were also present in sizeable numbers in medial aspects of CP, CLA, OT, AId, and the suprachiasmatic nucleus (SCN). Figure 5B shows a discrete group of labeled axons bilaterally within SCN. Additional light to moderately labeled sites included the posterior agranular insular cortex (AIp) (with some extension dorsally into the granular insular cortex), substantia innominata (SI), medial preoptic area (MPO) and the globus pallidus (GP) (Fig. 4F–H). At mid to caudal levels of the forebrain (Fig. 4I–N), labeled fibers were mainly confined to the dorsal striatum and to the amygdala, spreading massively throughout the amygdala. As shown (Figs. 4I–N, 6A–D), labeled fibers virtually blanketed the amygdala, with the densest concentration in the central (CEA) (Fig. 6A,B) and basomedial (BMA) (Fig. 6A–D) nuclei of amygdala. The medial (MEA) and basolateral nuclei of amygdala were also fairly heavily labeled, whereas the lateral and The Journal of Comparative Neurology 220 R.P. VERTES AND W.B. HOOVER Fig. 3. A–D: Low-magnification darkfield photomicrographs of transverse sections through anterior (A–C) and posterior (D) regions of the forebrain depicting patterns of labeling in rostral and caudal nucleus accumbens (ACC) (A,B), the bed nucleus of the stria terminalis (C), and the amygdala (D) produced by a PHA-L injection into anterior paraventricular nucleus of thalamus. A: Note that labeled fibers mainly encircle but largely avoid the central core of the rostral ACC, and also note significant labeling in the prelimbic cortex (PL) of the medial prefrontal cortex. B: By contrast with the rostral ACC (A), labeled fibers distribute massively to caudal part of ACC. Note pronounced labeling in both the shell and core of ACC, with additional labeling in the adjacent lateral septum (LS) and ventromedial parts of the dorsal striatum (caudate-putamen) (CP). C: Note strong terminal labeling in BST above and below the anterior commissure. D: Note a dense aggregate of labeled fibers in the central medial and medial part of the medial part of the basomedial nucleus and prominent but less dense labeling in the basolateral nucleus of the amygdala. Scale bar ⫽ 550 m for A; 500 m for B–D. See list for abbreviations. parts of the anterior and posterior cortical nuclei of amygdala were moderately labeled (Fig. 4I–N). At pre and beginning levels of the hippocampus (Fig. 4H–K), a relatively narrow band of labeled fibers within the me- dial CP, abutting the globus pallidus, was observed. Labeling was densest ventrally in medial CP (Fig. 4I–M) at its point of merger with medial aspects of the amygdala. The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI 221 Fig. 4. Schematic representation of labeling present in select sections through the forebrain and midbrain (A–O) produced by a PHA-L injection (dots in N) in the posterior part of the paraventricular nucleus of the thalamus (case 32). Sections modified from the rat atlas of Swanson (1998). See list for abbreviations. Further caudally (Fig. 4L–N), labeled fibers were found to extend laterally from the medial CP to occupy most of the mediolateral expanse of the caudal CP. In addition, as rostrally, posterior parts of the lateral, central, basal (BMA and BLA), and cortical nuclei of amygdala were moderately to densely labeled (Fig. 4L–N). Significant numbers of labeled axons were also visible within inner layers of the parahippocampal and piriform cortices; that The Journal of Comparative Neurology 222 R.P. VERTES AND W.B. HOOVER Figure 4 (Continued) The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI is, a continuous column in layers 5 and 6 stretching from the ectorhinal, perirhinal, and entorhinal cortices to the piriform cortex (Fig. 4L–N). Although most regions of the diencephalon were devoid of labeled fibers (Fig. 4L–N), 223 moderate numbers were present in the dorsomedial nucleus of the hypothalamus (DMH) (Fig. 5C) and a few within the midline thalamus–reuniens (RE) and rhomboid (RH) nuclei. At the level of the midbrain (Fig. 4O) moderate numbers of labeled fibers were present in the caudal perirhinal cortex, lateral EC, and the ventral subiculum of the ventral hippocampus. Although labeling progressively thinned caudally, labeled axons continued to be present in lateral EC and the ventral subiculum throughout caudal reaches of the brain (not shown). With a few exceptions, there was a general absence of labeling at the rostral midbrain (Fig. 4O) and throughout the brainstem. Paratenial nucleus (PT) (case 27) As depicted (Fig 1E,F), the injection in the paratenial nucleus (PT) was confined to left PT, and accordingly labeling was virtually restricted to the left side of the brain. Labeling was minimal contralateral to the injection. Similar to PV, labeled fibers from PT exited ventrolaterally through the thalamus and then either continued on the same course to the amygdala or ascended through the MFB to the anterior forebrain or descended in the MFB to parts of the caudal diencephalon. At anterior pole of the forebrain (Fig. 7A,B), virtually the entire medial wall of the mPFC was densely labeled. This includes the medial frontal polar, prelimbic (PL), and medial orbital (MO) cortices, rostrally (Fig. 7A) and the anterior cingulate, PL, and MO cortices, further caudally (Fig. 7B). This is shown in the photomicrographs of Figure 8A,B. As depicted, labeled fibers spread to all layers of respective cortices, but were most heavily concentrated in layers 2/3 of PL and the ventrally adjacent MO. In addition, moderate numbers of labeled fibers were present in the ventral orbital cortex (VO) and TTd, but considerably fewer in AId. Further caudally in the anterior forebrain (Fig. 7C), labeling remained pronounced along the medial wall of the ventral mPFC, particularly pronounced in layers 1/3 of the infralimbic (IL) and prelimbic cortices (Figs. 7C, 8C, 9). Equally dense labeling was observed within the rostral ACC and parts of OT. Additional lightly to moderately labeled sites included AId, AC, and the CLA. This pattern of labeling is depicted in the brightfield photomicrograph of Figure 9. At early septal levels (Fig. 7D–F), labeled axons were primarily localized to the ventral mPFC, CP, ventral striatum, CLA, and AId. Labeling was pronounced (or massive) in IL and PL of the mPFC, ventromedial sectors of CP, the core and shell of ACC, and parts of OT (Fig. 7D–F). Some regions of AC, AId, and LS were also heavily labeled (Fig. 7E,F). As depicted schematically (Fig. 7D–F) and in Fig. 5. A–C: Low-magnification darkfield photomicrographs of transverse sections through the forebrain depicting patterns of labeling produced by a PHA-L injection into posterior paraventricular nucleus. A: Note the massive labeling throughout the shell division of the nucleus accumbens (ACCs) and intense but lesser labeling in the core of ACC (ACCc) surrounding the anterior commissure. B: Note the strong labeling bilaterally in the suprachiasmatic nucleus of the hypothalamus (SCN) above the optic chiasm. C: Note the pronounced labeling bilaterally in the dorsomedial nucleus (DMh) of the hypothalamus lateral to the third ventricle. Scale bar ⫽ 500 m for A; 250 m for B; 300 m for C. See list for abbreviations. The Journal of Comparative Neurology 224 R.P. VERTES AND W.B. HOOVER Fig. 6. A–D: Series of low-magnification darkfield photomicrographs of transverse sections rostrocaudally through the forebrain (A–D) depicting patterns of labeling within the amygdala produced by a PHA-L injection into posterior paraventricular nucleus. A,B: Note very intense dense labeling in the central (CEA), basomedial (BMA) and basolateral nuclei of amygdala, and prominent but less dense labeling in the parts of the medial (MEA), lateral (LA), and anterior cortical nuclei of amygdala. C,D: Note labeling at caudal levels of the amygdala mainly confined to BMA and BLA. Scale bar ⫽ 750 m. See list for abbreviations. the photomicrograph of Figure 10A, labeling was uneven mediolaterally across ACC and within bordering regions of CP; that is, dense in the internal (medial) shell of ACC, considerably lighter in the lateral shell (with some relatively clear pockets), very strong in the (dorsal) core of ACC, extending into the ventromedial CP, and moderate in the lateral CP. At caudal levels of the septum (Fig. 7G,H) significant numbers of labeled axons were present in rostral BST, on the lateral and ventral border of the anterior commissure, and medially within CP abutting lateral wall of the lateral ventricle, dorsal to BST. Light to moderate labeling was also observed in AC, LS, ventromedial CP, olfactory tubercle, and CLA. There was marked decline in labeling further caudally in the forebrain (Fig. 7I–K) with labeled fibers mainly observed in the rostral amygdala and adjoining regions of the piriform cortex. As shown (Fig. 7I–K), labeled fibers spread widely (and moderately) throughout the amygdala to the anterior amygdaloid area (AA), CEA, MEA, BLA, BMA, and the anterior cortical nucleus (COAa). There was a noticeable absence of labeling in the core of CEA (Fig 7J,K). Other lightly to moderately labeled sites were AC, dorsomedial CP, CLA, and anterior and lateral nuclei of the hypothalamus. At mid-levels of the forebrain (Fig. 7L–N), labeled fibers were essentially confined to the ventrolateral sector of the brain; that is, to CP, to the amygdala, and to the perirhinal, entorhinal, and piriform cortices. An intensely labeled band of tissue stretching diagonally through the amygdala was observed (Fig. 7L–N, 10B) that included medial aspects of the lateral and basolat- The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI 225 Fig. 7. Schematic representation of labeling present in select sections through the forebrain and midbrain (A–O) produced by a PHA-L injection (dots in I,J) in the paratenial nucleus of the thalamus (case 27). Sections modified from the rat atlas of Swanson (1998). See list for abbreviations. eral amygdala and virtually the extent of posterior BMA. As rostrally, the core of CEA was largely devoid of labeled fibers (Fig. 7L), but moderate numbers were seen in the capsular CEA as well as in the posterior, amygdaloid-piriform area, and posterior cortical nuclei of the amygdala (Fig. 7L–N). A few labeled fibers were also present in RE, the zona incerta (ZI), and throughout the lateral hypothalamus. The Journal of Comparative Neurology 226 R.P. VERTES AND W.B. HOOVER Figure 7 (Continued) The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI 227 Fig. 8. A–C: Series of rostrocaudally (A–C) aligned lowmagnification darkfield photomicrographs of transverse sections through the anterior forebrain depicting patterns of labeling within the medial prefrontal cortex (mPFC) produced by a PHA-L injection into the paratenial nucleus of the thalamus. Note the presence of intense labeling along the ventral medial wall of the mPFC mainly confined to the prelimbic (PL) (A–C), medial orbital (MO) (A,B), and infralimbic (C) (IL) cortices. As depicted, labeling was particularly dense in layers 1 and 3 of these prefrontal fields. Scale bar ⫽ 750 m. At the rostral midbrain (Fig. 7O), labeled fibers continued to mainly occupy ventrolateral regions of the brain localized to perirhinal cortex, lateral EC, and the ventral subiculum of the hippocampus. This pattern of labeling is depicted in the micrographs of Figure 11A–C showing prominent labeling in these areas— particularly dense rostrocaudally throughout the lateral EC. The few labeled fibers present dorsally in the retrosplenial cortex (Fig. 7O) mainly appeared bound for the dorsal subiculum which was lightly labeled (Fig. 7O). ACCs injections; and 3) labeling was slightly stronger in PVp with ACCc than ACCs injections. This supports anterograde results showing pronounced terminal labeling in the shell and core of ACC with PVa, PVp, and PT injections. Figure 13 shows FG injections in the rostral BLA (Fig. 13A) and rostral CEA (Fig. 13D), together with patterns of cell labeling in PVa and PT (Fig. 13B,E) and PVp (Fig. 13C,F) produced by these injections. As depicted, there is a small number of labeled neurons in PT (Fig. 13B) with the BLA injection and fewer still in PT (Fig. 13E) with the CEA injection. This is consistent with anterograde findings showing light terminal labeling in rostral BLA (Fig. 7I,J) and general lack of labeling in rostral CEA (Fig. 7I,J) with PT injections. FG injections in BLA gave rise to pronounced cell labeling in PVp (Fig. 13C), but light labeling in PVa (Fig. 13B), while those in CEA produced significant labeling in both PVa (Fig. 13E) and PVp (Fig. 13F). This is consistent with anterograde results demonstrating strong terminal labeling in rostral CEA with PVa (Fig. 2I,J) and with PVp injections (Figs. 4I,J, 6A,B), as well as weak labeling in BLA with PVa injections (Fig. 2I,J) and dense labeling in BLA (Figs. 4I,J, 6A) with PVp injections. As depicted, labeled cells are also present in RE (Fig. 13B,E), the intermediodorsal (IMD), and the central medial (CM) nuclei of the thalamus (Fig. 13C,F) with BLA and CEA injections. Retrograde tracing experiments Two major destinations of labeled fibers of PVa, PVp, and PT were the nucleus accumbens (shell and core) and the amygdala—mainly CEA and basal nuclei (see above). To confirm anterograde findings and provide further information on the distribution of PV and PT fibers to these sites, retrograde injections (FG) were made in ACC and the amygdala and patterns of labeling in PV and PT determined. Figure 12 shows FG injections in the shell (Fig. 12A) and the core (Fig. 12D) of ACC together with patterns of cell labeling in PVa and PT (Fig. 12B,E) and PVp (Fig. 12C,F) obtained with these injections. As depicted: 1) labeled cells were present in PVa, PVp, and densely in PT with injections in the shell (ACCs) and core (ACCc) of ACC; 2) labeling was heavier in PVa with ACCc than The Journal of Comparative Neurology 228 R.P. VERTES AND W.B. HOOVER (LS), the core and shell of ACC, OT, BST, several nuclei of the amygdala, including the lateral, medial, central, basal (BLA and BMA), and the anterior and posterior cortical nuclei, and the suprachiasmatic (SCN), arcuate, and dorsomedial nuclei of the hypothalamus. Secondary targets were the anterior cingulate and ectorhinal cortices, dorsal tenia tecta (TTd), the medial preoptic area, reuniens (RE), and rhomboid nuclei of the thalamus and the lateral hypothalamus. There is a significant overlap in projections from the anterior (PVa) and posterior (PVp) PV (Figs. 2, 4; Table 1). With some exceptions, PVp is the source of stronger projections to most commonly innervated sites. Perhaps the most significant difference between PVa and PVp projections is that PVa distributes minimally to the dorsal striatum (caudate-putamen), whereas PVp projects quite massively to CP, mainly to medial/ventromedial regions of CP. In addition, while PVa and PVp project commonly to the amygdala, PVp distributes more widely and heavily throughout the amygdala than PVa, particularly to the basal nuclei of amygdala. On the other hand, PVa is the source stronger projections to the ventral subiculum of the hippocampus. Overview of PT projections and comparisons with PV projections Fig. 9. Low-magnification brightfield photomicrograph of a transverse section through the anterior forebrain depicting patterns of labeling within the anterior cingulate (AC), prelimbic (PL), infralimbic (IL), and dorsal agranular insular cortices, the olfactory tubercle (OT), and the rostral pole of the nucleus accumbens (ACC) produced by a PHA-L injection in the paratenial nucleus of thalamus. Scale bar ⫽ 500 m. See list for abbreviations. Discussion We examined, compared, and contrasted the efferent projections of the PV and PT nuclei of the dorsal midline thalamus in the rat. The main (or virtually sole) targets of PV and PT were ‘limbic/limbic related’ structures of the forebrain. With the possible exception of the piriform cortex, there was essentially lack of PV/PT projections to ‘nonlimbic’ regions of the cortex including sensorimotor, special sensory, or associational cortices as well as few projections to most of the thalamus and hypothalamus. As developed below, based on widespread afferents from the brainstem and hypothalamus coupled with output to select structures of the limbic forebrain, PV/PT appear critical for routing visceral/emotional information to structures of the limbic forebrain, including the limbic cortex, in the control of goal-directed behaviors. Overview of PV projections and comparisons between PVa and PVp projections The main targets of PV (anterior and posterior parts) were the prelimbic (PL), dorsal agranular insular, perirhinal (PRC) and entorhinal cortices, the ventral subiculum of the hippocampus, the claustrum, the lateral septum The main targets of PT were the medial frontal polar (FPm), anterior cingulate, prelimbic, infralimbic, medial orbital, dorsal agranular insular, piriform and entorhinal cortices, the ventral subiculum of hippocampus, the claustrum, the core and shell of nucleus accumbens, the medial striatum (CP), BST, and caudal parts of the central and basal nuclei of amygdala. PT also distributes to the ventral orbital and perirhinal cortices, the dorsal subiculum of hippocampus, lateral septum, olfactory tubercle, medial and cortical nuclei of amygdala, RE of thalamus, and the lateral hypothalamus. Although there is considerable overlap in PT and PV projections, there are several important differences between the two sets of projections. PT sends considerably stronger projections than PV to the mPFC, to the lateral entorhinal cortex, to the ventral subiculum, and to anterior regions of the dorsal and ventral striatum. Differences are particularly notable with respect to the mPFC. PT strongly targets the mPFC, distributing throughout the ventral mPFC to the medial frontal polar, medial orbital (MO), anterior cingulate, prelimbic and infralimbic cortices, and particularly heavily in outer layers (1 and 3) of MO, PL, and IL (Fig. 8A–C). By contrast, the projections of PV (PVa and PVp) to the mPFC are modest and mainly confined to IL and PL. With respect to nucleus accumbens (ACC), PT distributes more heavily to the rostral pole (Fig. 9) and core of ACC (Figs. 10A, 12E), but less densely to the shell of ACC (Figs. 3B, 5A) than does PV. Unlike PVa, but similar to PVp, PT strongly targets the dorsal striatum. PT fibers terminate densely (and selectively) in the rostromedial CP, dorsal to the core of ACC (Fig. 10A), while PVp distributes rostrocaudally throughout CP and heavily to the caudal CP—a region basically devoid of fibers from PT. Finally, in contrast to robust PV projections to virtually the entire amygdala, PT distributes significantly to caudal, but at best modestly, to rostral parts of the amygdala (see Fig. 13B,E). The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI Fig. 10. A,B: Low-magnification darkfield photomicrographs of transverse sections through the forebrain depicting patterns of labeling the dorsal and ventral striatum (A) and the amygdala (B) produced by a PHA-L injection into the paratenial nucleus of the thalamus. A: Note the dense labeling, but uneven labeling (sparsely labeled pockets) in the shell of nucleus accumbens (ACC) and the massive labeling in the core of ACC (dorsal/dorsomedial to the anterior com- 229 missure) with a continuation of equally dense labeling into ventromedial parts of the dorsal striatum (caudate putamen, CP). B: Note heavy labeling in parts of the lateral and basolateral nuclei, somewhat less pronounced labeling in the basomedial nucleus and an absence of labeling in the lateral part of the central nucleus of amygdala. Scale bar ⫽ 550 m for A; 500 m for B. See list for abbreviations. The Journal of Comparative Neurology 230 Fig. 11. A–C: Series of rostrocaudally aligned low magnification darkfield photomicrographs of transverse sections through the forebrain depicting patterns of labeling within the ventral subiculum (SUBv) and lateral entorhinal cortex (ECl) produced by a PHA-L injection into the paratenial nucleus of the thalamus. Note strong labeling in ECl as well as in the molecular layer of SUBv. Scale bar ⫽ 500 m. See list for abbreviations. PV projections: comparisons with previous studies As discussed, the output of PV is restricted; that is, PV projects to a limited number of sites, but quite massively to them. The foremost PV targets are nucleus accumbens, bed nucleus of stria terminalis, and the amygdala. The most complete analysis of PV projections was an early report by Moga et al. (1995). Our findings were comparable to theirs for the anterior PV (PVa) but differed R.P. VERTES AND W.B. HOOVER considerably for the posterior PV. In accord with Moga et al. (1995), we demonstrated pronounced PVa projections to the shell of ACC and to the central and basomedial nuclei of amygdala, but unlike them, also described substantial PVa projections to the core of ACC as well as to the medial, basolateral, and cortical nuclei of amygdala. On the other hand, they demonstrated denser projections to nuclei of the hypothalamus including the retrochiasmatic nucleus, subparaventricular zone, and the ventromedial nucleus of the hypothalamus. With respect to PVp, however, Moga et al. (1995) reported that PVp projections were much lighter overall than PVa projections, while we generally found the opposite: stronger PVp than PVa projections to most sites. Further, Moga et al. (1995) described an essential lack of PVp projections to several sites in which we observed them including the infralimbic, piriform, perirhinal and agranular insular cortices, the ventral subiculum, medial regions of the striatum, posterior BLA and BMA, and most of the hypothalamus. The reasons for these differences are unclear but could involve differences in size and locations of the PVp injections. PV projections to emotional/visceral associated forebrain areas. PV distributes to several forebrain sites associated with emotional behavior including the infralimbic cortex, the lateral septum, bed nucleus of stria terminalis, and almost the entire amygdala—with massive projections to CEA. In accord with present findings, previous reports, using various tracers, have described significant PV projections to the infralimbic cortex in rats (Berendse and Groenewegen, 1991; Conde et al., 1995; Moga et al., 1995; Bubser and Deutch, 1998; Otake and Nakamura, 1998; Pinto et al., 2003), mainly targeting inner layers (5/6) of IL (Berendse and Groenewegen, 1991; Pinto et al., 2003). We recently identified labeled cells rostrocaudally throughout PV following retrograde tracer injections in IL (Hoover and Vertes, 2007). PV also distributes substantially to area 25 (or the infralimbic cortex) in primates (Hsu and Price, 2007). Moga et al. (1995) reported that PVa projects densely, whereas PVp sparsely (or not at all) to the lateral septum (LS). We showed that both PVa and PVp project to LS, but similar to Moga et al. (1995) found that the major output was from PVa. Consistent with this, Risold and Swanson (1997) described labeled cells throughout PV following FG injections in LS, but progressively fewer cells at successive caudal levels of PV. Few reports have examined PV projections to the bed nucleus of stria terminalis (Moga et al., 1995; Van der Werf et al., 2002). In general accord with present findings, Moga et al. (1995) reported that PV distributes heavily to rostral and lateral parts (subnuclei) of BST. Although the efferent projections of BST have been fairly extensively examined (Dong et al., 2001; Gu et al., 2003; Dong and Swanson, 2004, 2006), to our knowledge, only a single early report by Weller and Smith (1982) examined afferents to BST. They showed PV and PT are virtually the sole sources of thalamic input to BST, distributing significantly to BST. We showed that PV distributes massively throughout the amygdala, and with the exception of parts of the caudal amygdala, to most subnuclei of the amygdala. The foremost PV targets are the central and basal nuclei of the amygdala. In accord with present findings, an early examination of thalamic afferents to the amygdala (Ottersen The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI Fig. 12. A–C: Series of low-magnification brightfield photomicrographs of transverse sections through the forebrain depicting the site of a FluoroGold injection in the shell of nucleus accumbens (ACCs) (A) and patterns of retrogradely labeled cells within the anterior paraventricular (PV) and paratenial (PT) nuclei (B) and the posterior paraventricular nucleus (C) produced by this injection. Note significant numbers of retrogradely labeled neurons in PT, moderate numbers in posterior PV, and relatively few in the anterior PV with this injection. D–F: Series of low- magnification brightfield photomicro- 231 graphs of transverse sections through the forebrain depicting the site of a FluoroGold injection in the core of nucleus accumbens (ACCs) (D) and patterns of retrogradely labeled cells within the anterior paraventricular (PV) and paratenial (PT) nuclei (E) and posterior paraventricular nucleus (F) produced by this injection. Note significant number of retrogradely labeled neurons in anterior PV and PT and moderately number in posterior PV produced by this injection. Scale bar ⫽ 500 m for A,D; 350 m for B,E; 400 m for C,F. See list for abbreviations. The Journal of Comparative Neurology 232 and Ben-Ari, 1979) described widespread PV (and PT) projections to the amygdala, stating that “the paraventricular and paratenial nuclei of the thalamus were found to project throughout the amygdaloid complex.” Several subsequent studies have confirmed pronounced PV projections to CEA, BMA, and BLA (Berendse and Groenewegen, 1990; Su and Bentivoglio, 1990; Turner and Herkenham, 1991; Moga et al., 1995; Peng and Bentivoglio, 2004). PV projections to ‘cognitive-associated’ forebrain areas. Groenewegen and colleagues (Room et al., 1985; Groenewegen et al., 1990) initially defined a system of connections (or loop) from the prelimbic cortex ⬎ ventral striatum ⬎ ventral pallidum ⬎ MD of thalamus ⬎ PL which they termed the ‘PL circuit.’ The ‘PL circuit’ has subsequently been expanded to include several additional structures; principal among them are the dorsal agranular insular cortex (AId), hippocampus/parahippocampus, the basolateral amygdala, parts of midline thalamus and the ventral tegmental area (VTA) (Vertes, 2006). PL and its interconnected circuitry serve a recognized role in cognitive functions (Laroche et al., 2000; Groenewegen and Uylings, 2000; Vertes, 2006). PV distributes to several structures of prelimbic circuit: PL, AId, ACC, EC, the ventral subiculum, BLA, and VTA. We showed that PV (PVa and PVp) distributes: 1) selectively to IL and PL of the ventral mPFC; 2) more heavily to PL than to IL; and 3) rostrocaudally throughout PL, terminating most densely in inner layers of PL. These findings are consistent with previous descriptions of significant PV projections to PL (Berendse and Groenewegen, 1991; Conde et al., 1995; Moga et al., 1995; Bubser and Deutch, 1998; Otake and Nakamura, 1998; Pinto et al., 2003; Hoover and Vertes, 2007). We found that PVa and PVp distribute massively throughout the shell and core of ACC. Although early reports showed that PV (and PT) strongly target ACC (Groenewegen et al., 1980; Newman and Winans, 1980; Beckstead, 1984; Jayaraman, 1985; Phillipson and Griffiths, 1985), Groenewegen and colleagues (Berendse et al., 1988; Berendse and Groenewegen, 1990) were the first to show that each of the midline nuclei distribute to select, and only partially overlapping, territories of the ventral striatum. Regarding PV, they reported that of the midline nuclei of thalamus, PV was the predominant source of afferents to the shell of ACC, and while also pronounced to the core, were shared by other midline thalamic groups to the core (Berendse and Groenewegen, 1990). Several subsequent studies have confirmed ‘massive’ PV projections to ACC, and further showed that a fairly significant percentage of PV fibers to ACC collateralize to other sites (Meredith and Wouterlood, 1990; Su and Bentivoglio, 1990; Brog et al., 1993; Freedman and Cassell, 1994; Moga et al., 1995; Bubser and Deutch, 1998; Otake and Nakamura, 1998; Erro et al., 2002; Pinto et al., 2003; Parsons et al., 2006, 2007), mainly to the mPFC (Bubser and Deutch, 1998; Otake and Nakamura, 1998) and to the amygdala (Su and Bentivoglio, 1990). Finally, in accord with previous reports (Berendse et al., 1988; Berendse and Groenewegen, 1990), we found that PV fibers distribute in a nonhomogeneous (or ‘patch/ matrix’) manner to the nucleus accumbens; that is, regions of dense innervation interspersed with relatively fiber free zones (Figs. 2, 3B, 4, 5A). Berendse et al. (1988) reported that densely PV-innervated regions of the rostral ACC and sparsely innervated areas of the caudomedial R.P. VERTES AND W.B. HOOVER ACC overlap with zones of strong and weak enkephalin immunoreactivity, respectively. We did not immunostain for enkephalin and, hence, cannot confirm these findings. We showed that PV distributes moderately to the entorhinal cortex and to the ventral subiculum of the hippocampus—mainly to the rostral EC/subiculum and to ventral aspects of the subiculum, adjoining EC. Previous reports (Berendse and Groenewegen, 1991; Moga et al., 1995) have similarly described PV projections to EC and to the ventral subiculum and, like here, stronger projections from the anterior than posterior PV. Retrograde tracer injections in the hippocampus, involving the ventral subiculum, give rise to labeled cells in PV (mainly PVa) (Wyss et al., 1979; Riley and Moore, 1981; Su and Bentivoglio, 1990), and the hippocampus is the source of significant return projections to PV, originating from the ventral subiculum (Witter, 2006). As described, the amygdala is a major PV target, with projections heaviest to the central (CEA) and basomedial (BMA) nuclei of amygdala. While PV projections to BLA are less dense than to CEA and BMA, they are nonetheless pronounced, mainly targeting the posterior BLA (BLAp), bordering BMA. Earlier reports have similarly described marked PV projections to BLA (Ottersen and Ben-Ari, 1979; Berendse and Groenewegen, 1990, 1991; Su and Bentivolglio, 1990; Turner and Herkenham, 1991; Moga et al., 1995). BLA is an integral part of the “prelimbic circuit.” BLA has strong links with PL (Sesack et al., 1989; McDonald, 1987, 1991; McDonald et al., 1996; Conde et al., 1995; Vertes, 2004; Gabbott et al., 2006; Hoover and Vertes, 2007), as well as with other parts of the circuit including the hippocampus, ACC, claustrum, and the insular cortex (McDonald, 1987; Brog et al., 1993; Petrovich et al., 1996; Pikkarainen et al., 1999; Majak et al., 2002). PV as an interface in the flow of information between the suprachiasmatic nucleus (SCN) and other regions of the brain. In accord with previous reports (Moga et al., 1995; Moga and Moore, 1997; Krout et al., 2002), we showed that PV projects moderately densely to the suprachiasmatic nucleus of the hypothalamus. SCN, in turn, is the source of significant projections to PV (Watts et al., 1987; Novak et al., 2000; Peng and Bentivoglio, 2004; Zhang et al., 2006). Accordingly, PV appears to represent an important relay in the transfer of information to and from the SCN—the circadian pacemaker (Mistlberger, 2005; Morin and Allen, 2006). While afferents to SCN generally serve to entrain SCN activity to light/dark conditions, PV lesions do not disrupt circadian timing or entrainment to light (Ebling et al., 1992). This suggests a nonphotic modulatory influence of PV on SCN. Moga et al. (1995) proposed that PV conveys information on basal levels of activation to the SCN—functions associated with PV/midline thalamus (Van der Werf et al., 2002; Vertes, 2006). Regarding SCN-PV projections, the SCN has few direct outputs to the systems it affects (Deurveilher and Semba, 2005; Morin and Allen, 2006), indicating indirect routes to them, possibly through PV. At the light and EM levels, Peng and Bentivoglio (2004) showed that SCN strongly targets PV, and further that SCN fibers synapse with PV cells projecting to the amygdala. On this basis they concluded that PV “plays a role in the transfer of circadian timing information to the limbic system.” The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI 233 Figure 13 The Journal of Comparative Neurology 234 PT projections: comparisons with previous studies As discussed, there is significant overlap in PV and PT projections. Although a few reports have described PT projections to specific targets, to our knowledge no previous study has examined the totality of PT projections. We showed that PT distributes densely throughout the ventral PFC to AC, PL, IL, and medial orbital (MO) cortices, with projections heaviest to PL. In an early examination of midline and intralaminar thalamic connections with the cortex, Berendse and Groenewegen (1991) similarly described PT projections to the mPFC, but unlike here, projections were modest and largely confined to ventral aspects of the mPFC, mainly to MO and IL. Their PT injection, however, was small and restricted to the medial aspect of the anterior PT (see their fig. 1A, p. 75; Berendse and Groenewegen, 1991). Supporting present findings, retrograde tracer injections in AC, PL, and IL have been shown to give rise to significant numbers of labeled cells in PT (Conde et al., 1995; Hoover and Vertes, 2007). PT also strongly targets the ventral mPFC in primates, mainly IL (area 25) and PL (area 32) (Hsu and Price, 2007). Similar to PV, ACC is a major destination of PT fibers. In general accord with present results, two early studies (Kelley and Stinus, 1984; Carlsen and Heimer, 1986) described robust PT projections to ACC, terminating heavily in the medial two-thirds of ACC (shell region) with some extension dorsally into dorsomedial aspects of CP. Berendse and Groenewegen (1990) confirmed marked PT projections to the shell of ACC, particularly dense to the ventromedial shell of ACC. Although they also reported that PT distributes to the core of ACC and to the dorsomedial CP, projections were considerably less pronounced than the presently described massive distribution of PT fibers to the core of ACC and to the dorsomedial CP (see Figs. 7D–F, 10A). Consistent with our results, Brog et al. (1993) described significant numbers of labeled cells in PT following retrograde tracer injections in the core or shell of ACC. As demonstrated, PT fibers mainly target caudal regions of the amygdala, predominantly the lateral and basal nuclei of amygdala. In contrast to the dense PV innervation of CEA, PT fibers largely avoid the core of Fig. 13. A–C: Series of low-magnification brightfield photomicrographs of transverse sections through the forebrain depicting the site of a FluoroGold injection in the basolateral nucleus (BLA) of the amygdala (A) and patterns of retrogradely labeled cells within the anterior paraventricular (PV) and paratenial (PT) nuclei (B) and the posterior paraventricular nucleus (C) produced by this injection. Note significant numbers of retrogradely labeled neurons in the posterior PV but relatively few numbers in the anterior PV and PT with this injection. D–F: Series of low-magnification brightfield photomicrographs of transverse sections through the forebrain depicting the site of a FluoroGold injection in the central nucleus (CEA) of the amygdala (D) and patterns of retrogradely labeled cells within the anterior paraventricular (PV) and paratenial (PT) nuclei (E) and the posterior paraventricular nucleus (F) produced by this injection. Note moderate numbers of retrogradely labeled cells in anterior and posterior PV, and relatively few in PT. Finally, note the presence of labeled neurons in other nuclei of the midline thalamus (C,F) produced with BLA and CEA injections; namely, in the intermediodorsal and central medial nuclei (C) and the rhomboid and reuniens nuclei (F). Scale bar ⫽ 750 m for A; 300 m for B; 500 m for C; 700 m for D; 400 m for E; 450 m for F. See list for abbreviations. R.P. VERTES AND W.B. HOOVER CEA, projecting instead to the fringes of CEA. Ottersen and Ben-Ari (1979) identified few labeled cells in PT with retrograde injections in CEA, the cortical nuclei, or anterior regions of the basal nuclei, but described significant numbers of reacted cells in PT following large amygdalar injections spanning the rostral and caudal BMA/BLA. Subsequent reports using retrograde (Su and Bentivoglio, 1990) or anterograde tracers (Turner and Herkenham, 1991) similarly demonstrated PT projections to the lateral, basomedial, and basolateral nuclei of amygdala. We showed that PT distributes throughout entorhinal cortex and the ventral subiculum, terminating within fairly restricted zones of both sites: mainly inner layers (3– 6) of the lateral EC and the molecular layer of the ventral subiculum. Berendse and Groenewegen (1991) demonstrated a similar distribution of PT fibers to EC and to the ventral subiculum, but in contrast to the present findings described stronger PV than PT projections to these sites—we found the opposite. Differences could involve relative size and placements of injections in PT and PV. Several reports have identified labeled cells in PT following retrograde tracer injections in the hippocampus (Wyss et al., 1979; Riley and Moore, 1981; Su and Bentivoglio, 1990), or entorhinal cortex (Beckstead, 1978; Wyss et al., 1979; Insausti et al., 1987). Functional considerations Although the projections of PV and PT significantly overlap, suggesting comparable functions, considerably greater attention has been given to the functional characteristics of PV. An accumulating body of evidence indicates that PV receives inputs from several sites of the brainstem and hypothalamus that are known to exert activating and/or ‘wakefulness-promoting’ effects on the forebrain. This includes afferents from monoaminergic, cholinergic, and peptide-containing systems of the brainstem and diencephalon, prominently including orexin/ hypocretin cells of the lateral hypothalamus (Chen and Su, 1990; Vertes, 1991; Freedman and Cassell, 1994; Otake and Ruggiero, 1995; Peyron et al., 1998; Cutler et al., 1999; Vertes et al., 1999; Bhatnagar et al., 2000; Krout et al., 2002; Kirouac et al., 2005; Otake, 2005; Parsons et al., 2006). Accordingly, PV/PT (and other nuclei of the midline thalamus) are thought to serve an essential role in arousal and attention (Van der Werf et al., 2002; Vertes, 2006; Vertes et al., 2006). In line with the foregoing, PV cells show elevated levels of c-fos expression during wakefulness (Peng et al., 1995; Novak et al., 2000) as well as during stressful conditions, elicited by various stressors (Chastrette et al., 1991; Bubser and Deutch, 1999; Sica et al., 2000; Otake et al., 2002). PV appears to be critically involved in adaptive responses to stress (Bhatnagar and Dallman, 1998; Bhatnagar et al., 2000, 2002; Otake et al., 2002) through direct (Sawchenko and Swanson, 1983; present results), or predominantly indirect projections to paraventricular nucleus of the hypothalamus (Sawchenko and Swanson, 1983; Bhatnagar and Dallman, 1998; Dong et al., 2001; Otake et al., 2002; Dong and Swanson, 2006). In addition to its role in promoting arousal/wakefulness, the orexin system participates in feeding behavior (for review, see Willie et al., 2001). Intraventricular injections of orexin (orexin A) stimulates food consumption in satiated rats (Sakurai et al., 1998; Edwards et al., 1999; Haynes et al., 1999), anti-orexin antibodies or receptor The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI antagonists suppress feeding (Haynes et al., 2000), and orexin knockout mice are hypophagic (Hara et al., 2001). These effects may in part be mediated the actions of orexin on PV. Specifically, Nakahara et al. (2004) described elevated c-fos expression in PV in anticipation of feeding (food anticipatory activity) in deprived rats, and further reported that PV lesions attenuated anticipatory locomotor activity associated with feeding. Angeles-Castellanos et al. (2007) similarly reported elevated c-fos expression in PV with anticipated feeding and, interestingly, also described enhanced c-fos expression in several other limbic forebrain structures before and immediately after the delivery of food to deprived rats, including the central and basal nuclei of the amygdala, BST, the lateral septum, ACC (core and shell) and the infralimbic/prelimbic cortices. As demonstrated here, these are the main forebrain targets of PV (and PT), suggesting a PV/PT influence on them in complex behaviors associated with feeding. Related to this, Kelley et al. (2005) recently put forth a model suggestive of a role for PV in motivated behaviors as exemplified by feeding. Although not necessarily limited to feeding, the model addressed mechanisms responsible for the ingestion of palatable foods under satiated conditions. According to Kelley et al. (2005), PV receives diverse inputs which, among other things, code the incentive value of foods and when incentives are high (desirable foods), feeding ensues, even in a satiated state, mainly through the actions of PV on the ventral striatum. They stated: “PVT may act as an interface between signals related to arousal, energy balance, circadian or diurnal rhythms, and reward, and major striatal motor output systems.” In summary, PV (and PT) receive a vast array of afferents from the brainstem, hypothalamus, and limbic forebrain and appear to serve as a critical gateway for the transfer of multimodal information to structures of the limbic system in the selection of appropriate responses to changing environmental conditions. In effect, depending on the relative dominance of sets of inputs to PV/PT, coupled with their output to specific structures of the limbic forebrain, PV/PT would guide behavior toward a particular outcome among various potential outcomes. LITERATURE CITED Angeles-Castellanos M, Mendoza J, Escobar C. 2007. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 144:344 – 355. Beckstead RM. 1978. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res 152:249 –264. Beckstead RM. 1984. The thalamostriatal projection in the cat. J Comp Neurol 223:313–346. Bentivoglio M, Balercia G, Kruger L. 1991. The specificity of the nonspecific thalamus: the midline nuclei. Prog Brain Res 87:53– 80. Berendse HW, Groenewegen HJ. 1990. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299:187–228. Berendse HW, Groenewegen HJ. 1991. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42:73–102. Berendse HW, Voorn P, te Kortschot A, Groenewegen HJ. 1988. Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J Chem Neuroanat 1:3–10. Berendse HW, Galis-de Graaf Y, Groenewegen HJ. 1992. Topographical organization and relationship with ventral striatal compartments of 235 prefrontal corticostriatal projections in the rat. J Comp Neurol 316: 314 – 447. Bertram EH, Zhang DX. 1999. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience 92:15–26. Bhatnagar S, Dallman M. 1998. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039. Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. 2000. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamicpituitary-adrenal function. J Neurosci 20:5564 –5573. Bhatnagar S, Huber R, Nowak N, Trotter P. 2002. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitaryadrenal responses to repeated restraint. J Neuroendocrinol 14:403– 410. Brog JS, Salyapongse A, Deutch AY, Zahm DS. 1993. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338:255–278. Bubser M, Deutch AY. 1998. Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res 787:304 –310. Bubser M, Deutch AY. 1999. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse 32:13–22. Carlsen J, Heimer L. 1986. The projection from the parataenial thalamic nucleus, as demonstrated by the Phaseolus vulgaris-leucoagglutinin (PHA-L) method, identifies a subterritorial organization of the ventral striatum. Brain Res 374:375–379. Chastrette N, Pfaff DW, Gibbs RB. 1991. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res 563:339 –344. Chen S, Su H-S. 1990. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat—a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res 522:1– 6. Conde F, Maire-Lepoivre E, Audinat E, Crepel F. 1995. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol 352:567–593. Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, Arch JR, Wilson S, Buckingham RE, Evans ML, Leslie RA, Williams G. 1999. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides 20:1455–1470. Dempsey EW, Morison RS. 1942. The production of rhythmically recurrent cortical potentials after localized thalamic stimulation. Am J Physiol 135:293–300. Deurveilher S, Semba K. 2005. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience 130: 165–183. Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. 1997. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci 17:5640 –5650. Dong HW, Swanson LW. 2004. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471:396 – 433. Dong HW, Swanson LW. 2006. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494:142–178. Dong HW, Petrovich GD, Watts AG, Swanson LW. 2001. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436:430 – 455. Ebling FJ, Maywood ES, Humby T, Hastings MH. 1992. Circadian and photoperiodic time measurement in male Syrian hamsters following lesions of the melatonin-binding sites of the paraventricular thalamus. J Biol Rhythms 7:241–254. Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. 1999. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol 160:R7–12. Erro ME, Lanciego JL, Gimenez-Amaya JM. 2002. Re-examination of the The Journal of Comparative Neurology 236 thalamostriatal projections in the rat with retrograde tracers. Neurosci Res 42:45–55. Freedman LJ, Cassell MD. 1994. Relationship of thalamic basal forebrain projection neurons to the peptidergic innervation of the midline thalamus. J Comp Neurol 348:321–342. Gabbott PL, Warner TA, Busby SJ. 2006. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139:1039 –1048. Groenewegen HJ, Berendse HW. 1994. The specificity of the “non-specific” midline and intralaminar thalamic nuclei. Trends Neurosci 17:52–57. Groenewegen HJ, Uylings HB. 2000. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res 126:3–28. Groenewegen HJ, Witter MP. 2004. Thalamus. In: Paxinos G, editor. The rat nervous system, 3rd ed. New York: Academic Press. p 408 – 453. Groenewegen HJ, Becker NE, Lohman AH. 1980. Subcortical afferents of the nucleus accumbens septi in the cat, studied with retrograde axonal transport of horseradish peroxidase and bisbenzimid. Neuroscience 5:1903–1916. Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. 1990. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res 85:95–116. Groenewegen HJ, Galis-de Graaf Y, Smeets WJAJ. 1999. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat 16:167–185. Gu G, Cornea A, Simerly RB. 2003. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol 460:542–562. Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. 2001. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30:345–354. Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JR. 1999. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20:1099 – 1105. Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. 2000. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96:45–51. Hoover WB, Vertes RP. 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struc Funct 212:149 –179. Hsu DT, Price JL. 2007. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 504:89 –111. Insausti R, Amaral DG, Cowan WM. 1987. The entorhinal cortex of the monkey: III. Subcortical afferents. J Comp Neurol 264:396 – 408. Jayaraman A. 1985. Organization of thalamic projections in the nucleus accumbens and the caudate nucleus in cats and its relation with hippocampal and other subcortical afferents. J Comp Neurol 231:396 – 420. Kelley AE, Stinus L. 1984. The distribution of the projection from the parataenial nucleus of the thalamus to the nucleus accumbens in the rat: an autoradiographic study. Exp Brain Res 54:499 –512. Kelley AE, Baldo BA, Pratt WE. 2005. A proposed hypothalamic-thalamicstriatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493:72– 85. Kirouac GJ, Parsons MP, Li S. 2005. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059:179 –188. Kirouac GJ, Parsons MP, Li S. 2006. Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol 497:155–165. Krout KE, Belzer RE, Loewy AD. 2002. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 448:53– 101. Kung JC, Shyu BC. 2002. Potentiation of local field potentials in the anterior cingulate cortex evoked by the stimulation of the medial thalamic nuclei in rats. Brain Res 953:37– 44. Laroche S, Davis S, Jay TM. 2000. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus 10:438 – 446. Majak K, Pikkarainen M, Kemppainen S, Jolkkonen E, Pitkanen A. 2002. Projections from the amygdaloid complex to the claustrum and the endopiriform nucleus: a Phaseolus vulgaris leucoagglutinin study in the rat. J Comp Neurol 451:236 –249. R.P. VERTES AND W.B. HOOVER McDonald AJ. 1987. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J Comp Neurol 262:46 –58. McDonald AJ. 1991. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44:1–14. McDonald AJ, Mascagni F, Guo L. 1996. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71:55–75. Meredith GE, Wouterlood FG. 1990. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferaseimmunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol 296:204 –221. Mistlberger RE. 2005. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 49:429 – 454. Moga MM, Moore RY. 1997. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol 389:508 –534. Moga MM, Weis RP, Moore RY. 1995. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359:221–238. Morin LP, Allen CN. 2006. The circadian visual system. Brain Res Rev 51:1– 60. Nakahara K, Fukui K, Murakami N. 2004. Involvement of thalamic paraventricular nucleus in the anticipatory reaction under food restriction in the rat. J Vet Med Sci 66:1297–1300. Newman R, Winans SS. 1980. An experimental study of the ventral striatum of the golden hamster. I. Neuronal connections of the nucleus accumbens. J Comp Neurol 191:167–192. Novak CM, Harris JA, Smale L, Nunez AA. 2000. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus). Brain Res 874:147–157. Otake K. 2005. Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci Res 51:383–394. Otake K, Nakamura Y. 1998. Single midline thalamic neurons projecting to both the ventral striatum and the prefrontal cortex in the rat. Neuroscience 86:635– 649. Otake K, Ruggiero DA. 1995. Monoamines and nitric oxide are employed by afferents engaged in midline thalamic regulation. J Neurosci 15:1891– 1911. Otake K, Kin K, Nakamura Y. 2002. Fos expression in afferents to the rat midline thalamus following immobilization stress. Neurosci Res 43: 269 –282. Ottersen OP, Ben-Ari Y. 1979. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol 187:401– 424. Parsons MP, Li S, Kirouac GJ. 2006. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse 59:480 – 490. Parsons MP, Li S, Kirouac GJ. 2007. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol 500:1050 –1063. Peng ZC, Bentivoglio M. 2004. The thalamic paraventricular nucleus relays information from the suprachiasmatic nucleus to the amygdala: a combined anterograde and retrograde tracing study in the rat at the light and electron microscopic levels. J Neurocytol 33:101–116. Peng ZC, Grassi-Zucconi G, Bentivoglio M. 1995. Fos-related protein expression in the midline paraventricular nucleus of the rat thalamus: basal oscillation and relationship with limbic efferents. Exp Brain Res 104:21–29. Petrovich GD, Risold PY, Swanson LW. 1996. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 374:387– 420. Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996 –10015. Phillipson OT, Griffiths AC. 1985. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16:275–296. Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. 1999. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol 403: 229 –260. Pinto A, Jankowski M, Sesack SR. 2003. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol 459:142–155. The Journal of Comparative Neurology EFFERENTS OF PV AND PT NUCLEI Riley JN, Moore RY. 1981. Diencephalic and brainstem afferents to the hippocampal formation of the rat. Brain Res Bull 6:437– 444. Risold PY, Swanson LW. 1997. Connections of the rat lateral septal complex. Brain Res Rev 24:115–195. Room P, Russchen FT, Groenewegen HJ, Lohman AHM. 1985. Efferent connections of the paralimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. J Comp Neurol 242:40 –55. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573– 585. Sawchenko PE, Swanson LW. 1983. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog Brain Res 60:19 –29. Sesack SR, Deutch AY, Roth RH, Bunney BS. 1989. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242. Sica AL, Greenberg HE, Scharf SM, Ruggiero DA. 2000. Chronicintermittent hypoxia induces immediate early gene expression in the midline thalamus and epithalamus. Brain Res. 883:224 –228. Su HS, Bentivoglio M. 1990. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol 297:582–593. Swanson LW. 1998. Brain maps: structure of the rat brain. New York: Elsevier. Turner BH, Herkenham M. 1991. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J Comp Neurol 313:295–325. Van der Werf YD, Witter MP, Groenewegen HJ. 2002. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39:107–140. Vertes RP. 1991. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313:643– 668. 237 Vertes RP. 2002. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol 442:163–187. Vertes RP. 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. Vertes RP. 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142:1–20. Vertes RP, Fortin WJ, Crane AM. 1999. Projections of the median raphe nucleus in the rat. J Comp Neurol 407:555–582. Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. 2006. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 499:768 –796. Viana Di Prisco G, Vertes RP. 2006. Excitatory actions of the ventral midline thalamus (rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse 60:45–55. Watts AG, Swanson LW, Sanchez-Watts G. 1987. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol 258:204 – 229. Weller KL, Smith DA. 1982. Afferent connections to the bed nucleus of the stria terminalis. Brain Res 232:255–270. Willie JT, Chemelli RM, Sinton CM, Yangisawa M. 2001. To eat or sleep: orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24:429 – 458. Witter MP. 2006. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res 174: 251–264. Wyss JM, Swanson LW, Cowan WM. 1979. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience 4:463– 476. Zhang DX, Bertram EH. 2002. Midline thalamic region: widespread excitatory input to the entorhinal cortex and amygdala. J Neurosci 22: 3277–3284. Zhang L, Kolaj M, Renaud LP. 2006. Suprachiasmatic nucleus communicates with anterior thalamic paraventricular nucleus neurons via rapid glutamatergic and GABAergic neurotransmission: state-dependent response patterns observed in vitro. Neuroscience 141:2059 –2066.