* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download RFC_Cp_C_Wyart_def_EUK-v
Neuropsychology wikipedia , lookup
Neural coding wikipedia , lookup
History of neuroimaging wikipedia , lookup
Multielectrode array wikipedia , lookup
Embodied language processing wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Sensory substitution wikipedia , lookup
Neuroeconomics wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Proprioception wikipedia , lookup
Neuroplasticity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural oscillation wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Neural engineering wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Circumventricular organs wikipedia , lookup
Synaptic gating wikipedia , lookup
Nervous system network models wikipedia , lookup
Evoked potential wikipedia , lookup
Metastability in the brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
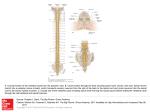
Paris, 24 November 2015 Press release A new neural circuit involved in the control of movement The team led by Claire Wyart, an Inserm researcher at the Brain and Spine Institute, has just demonstrated the ability of sensory neurons located in the spinal cord to modulate movement. In the zebrafish, the researchers have shown that activation of these neurons triggers locomotion when the animal is at rest, and inhibits it when the animal is moving. These results offer hope that it will one day be possible to specifically stimulate these circuits in order to generate movement in patients with spinal cord injuries. This work is published in Current Biology. Spinal cord injuries lead to serious paralysis for which, to date, there is no treatment. When communication between the brain and spinal cord is interrupted, the brain can no longer control movements voluntarily. However, the spinal cord contains autonomous circuits that generate movement, and ensure that locomotion proceeds once the decision to move has been taken at brain level. This capacity for sustaining movement comes from the ability of the spinal locomotor network to generate electrical oscillations. In order to understand the functioning and modulation of the spinal locomotor network, Claire Wyart’s team studies motor activity in the zebrafish. This transparent vertebrate species is particularly suited to optogenetics, an innovative technology that allows stimulation of target neurons using light. In this method, the stimulated neurons light up and are visible in the transparent animal. The researchers exploited this technology to identify and understand the functioning of a new neural circuit involved in the control of movement. By activating the circuit at different times (animal at rest or moving), the researchers demonstrated connections that can generate the oscillations that allow the fish to move. The originality of this circuit is that it depends on the activity of sensory neurons, which, through a cascade effect, ultimately activate motor neurons. Surprisingly, the researchers find that modulation of movement depends on the animal’s initial state. In fact, stimulation triggers movement when the animal is in a resting state, whereas it inhibits it when the animal is already swimming. “This modulation is complex, and will depend on the context,” explains Claire Wyart, the main author of this work. In 2014, this same team had shown that this circuit is conserved among the different vertebrate species, particularly in primates. This original work in the zebrafish thus opens many avenues of research for understanding the modulation of the locomotor circuit in humans. For the first time, a class of sensory neurons that can modulate the spinal locomotor network has been identified. Although several points remain to be clarified, stimulation of the sensory pathways to activate the locomotor network that generates walking in humans represents hope in cases of spinal cord injury. Brain stem Spinal cord Rostral spinal cord Caudal spinal cord Dorsal view of the spinal cord in the zebrafish at 4 days of development. Illustration of the movement-modulating spinal circuit according to the animal’s locomotor context. In red: interneurons known as “excitatory” involved in the control of movement In green: GABAergic sensory neurons that are “inhibitory” on contact with the cerebrospinal fluid. Source State-dependent modulation of locomotion by GABAergic spinal sensory neurons Kevin Fidelin1,2,3,4, Lydia Djenoune1,2,3,4,5, Caleb Stokes1,2,3,4, Andrew Prendergast1,2,3,4, Johanna Gomez1,2,3,4, Audrey Baradel1,2,3,4, Filippo Del Bene4,6 and Claire Wyart1,2,3,4,* 1Brain and Spine Institute (ICM), F-75013, Paris, France 2INSERM UMRS 1127 3French National Centre for Scientific Research (CNRS) UMR 7225 2UPMC – University of Paris 6, F-75005, Paris, France. 5National Museum of Natural History, F-75005, Paris, France 6Institut Curie, CNRS UMR 3215, INSERM U934, F-75005, Paris, France Current Biology. Investigator contact Claire Wyart Inserm Unit 1127, “Brain and Spine Institute” Tel. +33 (0)1 57 27 43 10 [email protected] Press contact [email protected] Access the Inserm press room