* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download In This Issue - The Journal of Cell Biology
Survey
Document related concepts
Cell nucleus wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Signal transduction wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Extracellular matrix wikipedia , lookup
Protein structure prediction wikipedia , lookup
Cell membrane wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Endomembrane system wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
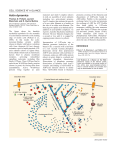
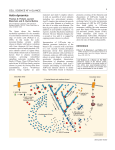
1566iti Page 938 Thursday, March 7, 2002 2:30 PM Published March 18, 2002 In This Issue Finding the dark side of tau APP transport (top) is inhibited by excess tau protein (bottom). view is that tau stabilizes microtubules, and disease results when the protein detaches from microtubules and aggregates into paired helical filaments. According to the authors, the opposite situation—having too much tau attached to microtubules— may be just as bad. Overexpressing tau in neuroblastoma cell lines, primary hippocampal neurons, or retinal ganglion cells leaves microtubules intact. Rather than forming filaments, the overexpressed tau binds to microtubules and appears to lay the groundwork for neurodegeneration. Excess tau causes the depletion of mitochondria and peroxisomes from the cells’ processes, retarding growth and increasing the cells’ sensitivity to oxidative stress. The transport of Golgi-derived vesicles into axons is inhibited, and neurofilament proteins and vesicles carrying the amyloid precursor protein (APP) accumulate in the cell body. These changes are likely to increase the production of toxic amyloid A peptides, a hallmark of Alzheimer’s disease. Thus, whereas low levels of tau are necessary for microtubule stability, higher levels interfere with transport. Detachment of tau from microtubules and aggregation of tau into filaments might be a later consequence of the trafficking problems caused by excess tau attaching to microtubules. The results also suggest a novel regulatory system for microtubule-based motors, in which tau and other proteins on microtubules might act as roadblocks that determine the rate of vesicle trafficking. Tubulin but not microtubule T he pathogens that cause malaria, composed of tubulin protofilaments, cryptosporidiosis, and toxoplasmosis supporting the microtubule hypothesis. share a baffling common feature: a But careful ultrastructural analysis cone-shaped apical structure called the shows that the tubulin in the conoid conoid. Previous research has suggested assembles in a manner never before that the conoid is composed of observed, creating a structure with microtubules and has simultaneously a comma-shaped cross section rather provided evidence that it is not. Now, than a microtubule. Previously on page 1039, Hu et al. resolve this described tubulin structures are longstanding dilemma by demonstrating generally circular or semicircular in that the conoid is made of tubulin, but cross section. not in the form of classical microtubules. Hu et al. also found that the conoid In addition to describing a previously fibers are assembled rapidly during the unknown form of cytoskeletal structure, early phases of cell division. The flattened the results could help in the development cross section explains how tubulin can Conoids consist of tubulin in an of new treatments for some of the conform to the tight bends found in the unconventional arrangement. world’s most devastating diseases. conoid—a ribbon is easier to bend than The conoid is part of the apical a straw—but more work will be needed complex, the defining feature of the phylum Apicomplexa and to understand how the cell directs the assembly of this novel a structure thought to be involved in host cell invasion by structure, and how the increased pitch of the fibers and the these parasites. Although tubulin was considered the most translation of the entire structure contribute to the spring-like likely building block of the conoid, the bends in the structure action of the conoid. The conoid’s unique architecture makes appeared too tight to be accommodated by microtubules. it an attractive drug target, especially since parasite and host Combining transgenic Toxoplasma gondii and an array of microtubules are known to differ in their sensitivity to various imaging techniques, the authors confirmed that the conoid is compounds. 938 The Journal of Cell Biology | Volume 156, Number 6, 2002 Downloaded from on June 17, 2017 n page 1051, Stamer et al. have uncovered what may be a critical early step in the pathogenesis of Alzheimer’s disease and a previously unappreciated regulatory system for microtubulebased motors. The work focuses on the microtubuleassociated protein tau, which is thought to cause the pathological changes seen in some neurodegenerative diseases. The prevailing O 1566iti Page 939 Thursday, March 7, 2002 2:30 PM Published March 18, 2002 TEXT BY ALAN W. DOVE [email protected] Only one way to skin a mouse T he actin cytoskeleton is a highly adaptable framework. In some structures actin polymerizes and depolymerizes rapidly to facilitate quick movement, whereas in others actin monomers turn over quite slowly to maintain structural integrity. On page 1065, Ono and Ono show that competition between two actin-binding proteins, ADF/cofilin and tropomyosin, determines the stability of actin thin filaments in C. elegans muscle. An acid invasion I ntracellular parasites must walk a pH. By amino acid substitution, the fine line, invading and exploiting authors determined that changing a a host cell without killing it too single residue largely relieves this quickly. On page 1029, Glomski et pH dependence, but bacteria al. show how a single amino acid expressing the pH-independent determines not only the pH form of the enzyme are 100-fold less optimum of a bacterial virulent in mice. The hemolysin, but also the virulence defect is not virulence of an important in cellular entry. The pathogen. The data mutants escape from help explain how acidified phagosomes, Listeria monocytogenes grow in the cytosol, distinguishes between and spread from cell the membranes of to cell. But bacteria acidic vesicles, which expressing the mutant the bacterium must LLO permeabilize the pierce to enter the host cell membrane cytosol, and the plasma prematurely. The membrane, which authors propose that A single residue determust remain intact the acidic pH optimum mines acid sensitivity in while the parasite of LLO is an adaptation a bacterial hemolysin. reproduces. to the parasitic lifestyle, The activity of the L. monocytogenes allowing Listeria to penetrate acidic pore-forming hemolysin listeriolysin vesicles while leaving the plasma O (LLO) is strongly induced by low membrane intact. Actin (green) and ADF/cofilin (red) are disorganized in the absence of tropomyosin (bottom). Previously, the authors found that worms with mutations in a muscle-specific ADF/cofilin isoform could not assemble normal myofibrils. ADF/cofilin appears to increase actin turnover, but myofibrils are highly stable structures, suggesting that some additional factor must inhibit ADF/cofilin in order to stabilize the myofibrils. The new study demonstrates that purified tropomyosin and ADF/cofilin compete for binding to purified F-actin, and that ADF/cofilin cannot bind to isolated myofibrils unless the attached tropomyosin is removed from the myofibrils first. RNAi suppression of tropomyosin disrupts myofibril organization in wild-type worms, but not in ADF/cofilin mutant worms. The results suggest that in vivo tropomyosin preserves myofilaments by blocking the destabilizing effects of ADF/cofilin. In This Issue 939 Downloaded from on June 17, 2017 t isn’t often that textbooks have to be revised because of a single research paper, but that appears to be the case for the work described by Furuse et al. on page 1099. The standard view of epithelial structure is that continuous tight junctions (TJs) are required only in simple epithelia like those that separate internal organs, but are not found Tight junctions have turned up in the epidermis. in stratified epithelia like the epidermis. The authors’ analysis of mice lacking the TJ component claudin-1, however, shows that TJs are indeed found in the skin and are at least as necessary there as in simple epithelia. Claudin-1 belongs to a multigene family that contributes to the backbone of TJ strands, so its deletion should affect simple epithelia. Surprisingly, the major defect in claudin-1–deficient mice is lethally leaky skin: the mice are born normally but die within a day, apparently from dehydration. Permeability assays of the claudin-1– deficient skin show excessive water loss. In contrast with previous reports, Furuse et al. found continuous TJs in the stratum granulosum, a subset of the epidermis, of both wild-type and mutant mice. The results suggest that functional TJs are required in both types of epithelia and that loss of claudin-1 increases the permeability of the epidermal TJs without disrupting the organization of the keratinocytes. I Tropomyosin keeps actin tough