* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Category-specific Conceptual Processing of
Broca's area wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Optogenetics wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neurophilosophy wikipedia , lookup
Synaptic gating wikipedia , lookup
History of neuroimaging wikipedia , lookup
Executive functions wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Human brain wikipedia , lookup
Neurolinguistics wikipedia , lookup
Vocabulary development wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Aging brain wikipedia , lookup
Affective neuroscience wikipedia , lookup
Misattribution of memory wikipedia , lookup
Stroop effect wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brodmann area 45 wikipedia , lookup
Time perception wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Emotional lateralization wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neuroesthetics wikipedia , lookup
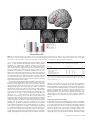
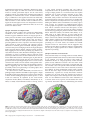
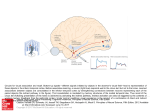
Cerebral Cortex August 2006;16:1193--1201 doi:10.1093/cercor/bhj060 Advance Access publication October 26, 2005 Category-specific Conceptual Processing of Color and Form in Left Fronto-temporal Cortex To investigate the cortical basis of color and form concepts, we examined event-related functional magnetic resonance imaging (fMRI) responses to matched words related to abstract color and form information. Silent word reading elicited activity in left temporal and frontal cortex, where category-specific activity differences were also observed. Whereas color words preferentially activated anterior parahippocampal gyrus, form words evoked category-specific activity in fusiform and middle temporal gyrus as well as premotor and dorsolateral prefrontal areas in inferior and middle frontal gyri. These results demonstrate that word meanings and concepts are not processed by a unique cortical area, but by different sets of areas, each of which may contribute differentially to conceptual semantic processing. We hypothesize that the anterior parahippocampal activation to color words indexes computation of the visual feature conjunctions and disjunctions necessary for classifying visual stimuli under a color concept. The predominant premotor and prefrontal activation to form words suggests action-related information processing and may reflect the involvement of neuronal elements responding in an either-or fashion to mirror neurons related to adumbrating shapes. Keywords: abstract concepts, fMRI, language, prefrontal cortex, reading Introduction A great challenge in cognitive neuroscience is to define the cortical areas engaged in processing semantic meaning of words and concepts. Many researchers agree that temporal cortex provides a unique substrate for semantic binding. Definitions of relevant areas within the temporal lobe range from temporal pole (Hodges, 2001; Rogers et al., 2004) to anterior superior temporal gyrus (Scott and Johnsrude, 2003), posterior inferior temporal areas (Price et al., 2001) and posterior superior temporal sulcus (Hickok and Poeppel, 2000). Activation in temporal cortex, including posterior fusiform and parahippocampal gyri, was found to be specific to categories of knowledge (Chao et al., 1999; O’Craven and Kanwisher, 2000), suggesting that areas of temporal cortex may contribute differentially to semantic category processing. A different line of research emphasizes the relevance of frontal cortex, especially premotor and inferior frontal cortex, for meaning processing (Posner and Pavese, 1998; Rizzolatti et al., 2001). Mirror neurons that bind information about actions and their corresponding perceptual patterns have been discovered in inferior premotor cortex of monkeys and apparently contribute to action-related cognitive processes, including observation or imagination of actions in humans (Jeannerod and Frak, 1999; Buccino et al., 2001). Processing action verbs and tool words with semantic relationship to actions was found to elicit stronger fronto-central cortical activation than object The Author 2005. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: [email protected] Friedemann Pulvermüller and Olaf Hauk Medical Research Council, Cognition and Brain Sciences Unit, Cambridge CB2 2EF, UK words (Preissl et al., 1995; Martin et al., 1996; Pulvermüller et al., 1999). Among the action words, those related to movements of the face, arm or leg activated fronto-central cortex in a somatotopic fashion (Hauk et al., 2004; Shtyrov et al., 2004), consistent with the claim that sensorimotor cortex processes actionrelated aspects of word meaning (Pulvermüller, 2001, 2005). These results can be explained by the so-called sensorimotor theory of conceptual and semantic processing: sensory and action-related semantic features are attached to a symbol by correlation, typically when a child perceives words in the context of specific objects and actions (Wernicke, 1874; Freud, 1891; Warrington and Shallice, 1984; Shallice, 1988; Humphreys and Forde, 2001; Kiefer and Spitzer, 2001). Because perceptions through different senses and actions are processed by different areas of cortex, this explains a dissociation into categories of knowledge, for example visually related and motor categories (Warrington and Shallice, 1984), and even fine-grained semantic categories, such as face-, arm- and leg-action words (Pulvermüller, 2001, 2005). Although the sensorimotor theory explains a range of neuropsychological dissociations in patients with disease of the brain (Humphreys and Forde, 2001) and a multitude of facts revealed by neuroimaging experiments (Pulvermüller, 1999; Martin and Chao, 2001), it has systematic difficulty explaining abstract knowledge (Grossman et al., 2002; Jackendoff, 2002). Words such as ‘rhomb’, ‘hope’ and ‘nevertheless’ do not refer to objects in the world but have abstract meaning, and the brain areas where such abstract knowledge is stored are still largely unknown. Neuroimaging work comparing symbols with abstract and concrete meaning has shed light on the issue, but could not determine an area, or set of areas, consistently activated by abstract meaning processing. Some results suggested differential laterality: highly abstract grammatical function words such as ‘it’ and ‘the’ led to pronounced left-lateralized neurophysiological activity, which contrasted with the more bilateral responses to concrete nouns and verbs (Neville et al., 1992; Pulvermüller et al., 1995). Also, more strongly left-lateralized brain responses to abstract nouns compared with concrete nouns were revealed by event-related potential (ERP) experiments (Kounios and Holcomb, 1994). However, a general difference in laterality related to abstractness could not be confirmed (Friederici et al., 2000). Recent imaging work using functional magnetic resonance imaging (fMRI) indicated lefthemispheric correlates of abstract semantics in Broca’s area (Fiebach and Friederici, 2004), angular gyrus (Skosnik et al., 2002), superior (Wise et al., 2000) and posterior temporal cortex (Grossman et al., 2002), or a widespread set of areas including temporal pole along with inferior temporal and inferior frontal cortex (Noppeney and Price, 2004). In contrast with previous findings of a predominantly left-hemispheric locus of abstract semantics, a few studies also reported stronger activation for abstract words relative to concrete words in different areas of the right non-dominant hemisphere (Kiehl et al., 1999; Perani et al., 1999; Grossman et al., 2002). However, consistent with the differential laterality hypothesis, a recent study by Binder and colleagues reported that concrete word identification led to activation of distributed sources in both hemispheres, whereas abstract words activated a left frontal region including areas in the inferior and middle frontal gyri and lateral premotor cortex (Binder et al., 2005). This variability of results may be due, in part, to the fact that in all earlier studies, widely defined categories of abstract and concrete words were compared with each other. Since the brain basis of concrete action and object meaning can vary substantially, it is reasonable to assume similar local specificity for abstract concepts as well. Crucially, the cognitive abstraction processes necessary for understanding grammatical function words, such as ‘it’ or ‘nevertheless’, are fundamentally different from those related to emotionally related words, such as ‘hope’, which are, in turn, different from those triggered by words referring to visual shapes, such as ‘rhomb’. Thus, the cognitive processes of abstraction differ between subtypes of abstract words, and so may the loci of their cortical processing. To elucidate processes of abstraction, it may therefore be useful to focus on subtypes of words with specific abstract semantic features. Here, we investigated items that still have links to action and object features, but abstract away from concrete entities by referring to form or color features many objects have in common. We looked at words semantically related to color and form, whose meaning is at an intermediate level on the continuum between highly concrete, imageable, object-related words and highly abstract words. According to the MRC Psycholinguistic Database (Coltheart, 1981; Wilson, 1988), concreteness ratings for highly concrete words are >600 (e.g. ‘mouse’, 624; ‘eye’, 634), whereas highly abstract words are rated as <400 (‘love’, 311; ‘plan’, 357). Our stimuli were in the intermediate range (between 400 and 600: ‘blonde’, 502; ‘curve’, 447). As our stimuli were carefully matched for important properties, including physical features, visual imageability, familiarity, abstractness and a range of psycholinguistic variables, a unitary semantic system approach would predict congruent activation of this system for both word categories. A version of the sensorimotor theory, however, may predict differential activation of frontal and temporal circuits as a function of the action- and object-relatedness of the semantics of the stimulus words. Whereas a concrete word, e.g. ‘door’ directly relates to its reference object, a form or color word, e.g. ‘rectangle’, refers to a feature of most of its reference objects that needs to be separated and extracted from other object features. This visual feature extraction process can be computed by neurons in the vicinity of the areas engaged in object processing. Elementary color features of visual stimuli are processed already in primary visual cortex (Hubel, 1995) and temporo-occipital areas have been found to preferentially respond to color processing (McKeefry and Zeki, 1997). To compute a color concept — which covers a range of possible colors, with different levels of brightness and saturation — several alternative feature conjunctions must be classified together, possibly by neural units at higher levels located at gradually more anterior cortical sites. Neural correlates of color concept processing may therefore be 1194 Category-specific Conceptual Processing of Color and Form d Pulvermüller and Hauk present in the temporal lobe, anterior to the temporo-occipital and fusiform areas preferentially activated during color perception (Martin et al., 1995). In animal research, neurons in anterior peri- and entorhinal areas have been proposed to compute increasingly complex conjunctions and disjunctions of features represented by neurons in inferior temporal lobe and closer to the visual input (Bussey and Saksida, 2002; Bussey et al., 2002; Tyler et al., 2004). In the same way, concrete objects can be classified as exhibiting the same shape (e.g. rhomb shape). Establishing a form/shape concept necessitates computation of disjunctions over large sets of concrete objects and elementary visual feature. Again, the neural substrate of progressively abstract computations may be in primary visual (elementary visual features: Hubel, 1995), lateral occipital (object shapes: Kourtzi and Kanwisher, 2001) and inferior and medial temporal cortex. However, in contrast to color concepts, shape concepts always have a motor correspondence, since shapes, but not colors, can be outlined or adumbrated by specific sequences of body movements. Also, when looking at a shape, eye movements follow the shape contour. Because the form of objects can be outlined with different body parts, the brain basis of abstract form concepts may comprise sensorimotor, especially premotor cortex, where action representations are still somatotopic (Rizzolatti et al., 2001), but also neurons in prefrontal cortex related to motor planning (Fuster et al., 2000). Prefrontal cortex just anterior to premotor cortex would be ideally placed for computing conjunctions and disjunctions over sets of specific action programs and could therefore provide a neural substrate for action aspects of abstract form. Here, we set out to investigate the brain basis of words related to abstract color and form concepts (e.g. ‘brown’, ‘blonde’ and ‘bronze’ versus ‘rhomb’, ‘square’, ‘arc’). Word groups matched for physical features, visual imageability, familiarity, abstractness and a range of psycholinguistic variables were presented in a passive reading task while event-related blood-flow changes were measured. We hypothesized that the color- and formrelated meaning of these words is reflected by category-specific activation in temporal lobe, and that form-related words specifically activate premotor and prefrontal cortex. Materials and Methods Imaging Methods Fourteen monolingual, right-handed, healthy native English speakers participated in the study. Their mean age was 25 years (SD 5). Subjects were scanned in a 3 T Bruker MR system using a head coil. Echo planar imaging (EPI) sequence parameters were TR = 3.02 s, TE = 115 ms, flip angle = 90. The functional images consisted of 21 slices covering the whole brain (slice thickness 4 mm, inter-slice distance 1 mm, in-plane resolution 1.6 3 1.6 mm, FOV 20 cm, matrix size 21 3 128 3 128). Imaging data were processed using SPM99 software (Wellcome Department of Cognitive Neurology, London). Images were corrected for slice timing, and then realigned to the first image using sinc interpolation. Phase-maps were used to correct for inaccuracies resulting from inhomogeneities in the magnetic field (Jezzard and Balaban, 1995; Cusack et al., 2003). Any non-brain parts were removed from the T1-weighted structural images using a surface model approach (‘skull-stripping’) (Smith, 2002). The EPI images were co-registered to these skull-stripped structural T1-images using a mutual information co-registration procedure (Maes et al., 1997). The structural MRI was normalized to the 152 subject T1 template of the Montreal Neurological Institute (MNI). The resulting transformation parameters were applied to the co-registered EPI images. During the spatial normalization process, images were resampled with a spatial resolution Figure 1. Cortical activation during passive reading of concrete words semantically related to visual information (P = 0.001, uncorrected). A lateral view is shown on the upper left and frontal slices of an average brain are displayed from top left to bottom right. The diagram at the bottom left gives semantic ratings (and their standard errors) for color and form words; ratings of semantic relatedness to general visual information, form information and color information are given (see Materials and Methods). of 2 3 2 3 2 mm3. Finally, all normalized images were spatially smoothed with a 12 mm full-width half-maximum Gaussian kernel, globally normalized, and single-subject statistical contrasts were computed using the general linear model (Friston et al., 1998). Low-frequency noise was removed with a high-pass filter with time constant 60 s. Group data were analyzed with a random-effects analysis. A brain locus was considered to be activated in a particular condition if 20 or more adjacent voxels all passed the threshold of P = 0.001 (uncorrected). In some cases, correction for multiple comparisons in the left hemisphere was administered using the False Discovery Rate correction approach (P < 0.05) (Genovese et al., 2002). Stereotaxic coordinates for voxels with maximal z-values within activation clusters are reported in Talairach space (Talairach and Tournoux, 1988). Clusters in the left language-dominant hemisphere, which were found to be active in the random-effects analysis of the contrast words versus matched hash mark stings (Fig. 1), were used to define three ‘canonical’ regions of interest (ROIs), in inferior frontal (IF), fusiform (FUS) and parahippocampal gyrus (PH) respectively (see Table 1). Additional ‘supplementary’ ROIs were defined on the basis of earlier findings. Since previous research found an area in the middle temporal gyrus whose activation related to semantic and lexical processing (Chao et al., 1999; Devlin et al., 2004), a middle temporal gyrus (MT) ROI was selected (3 3 3 3 3 voxels centered at –60/–40/0). Because a large proportion of precentral gyrus was activated by action words in an earlier study (Hauk et al., 2004) and recent work indicated involvement of premotor cortex in abstract word processing (Binder et al., 2005), possible involvement of premotor cortex dorsal to the inferior frontal language area was probed (–45/0/35). As abstract action concept processing was predicted in dorsolateral prefrontal cortex and earlier work confirmed involvement of the middle frontal gyrus in abstract concept processing (Binder et al., 2005), an additional ROI was placed in a dorsolateral prefrontal area and anterior to premotor cortex (–50/ 25/25). Previous research has documented activation of this region in a range of tasks tapping into typical prefrontal functions, as for example, abstract mappings between stimuli and responses (Duncan and Owen, Table 1 Areas activated by words relative to strings of hash marks of the same length Brain region Talairach x, y, z All words Fusiform gyrus, BA 20 Fusiform gyrus Inferior frontal gyrus, BA 45 Parahippocampal gyrus LH LH LH LH ÿ36 ÿ38 ÿ18 ÿ42 ÿ47 ÿ13 ÿ46 28 10 ÿ28 ÿ12 ÿ15 T(13) 5.54 4.64 4.91 4.69 Talairach coordinates along with t-values are given for the maximally activated voxel in each local cluster. All clusters that passed the significance threshold at P \ 0.001 are listed. The FDR correction cutoff was at T 5 3.8. 2000). For each subject and each of the six ROIs, average parameter estimates over voxels were calculated. These values were subjected to ANOVAs including the factors Region of Interest and Word Category (color and form). An additional ANOVA was carried out separately on data from canonical ROIs only. Paired two-tailed t-tests were used as planned comparison tests for between condition differences in individual ROIs. The mean values scaled to HRF-peak percentage signal change relative to the mean over all voxels and scans within the corresponding ROI and standard errors over subjects are shown in Figure 2. Stimuli and Experimental Design Words semantically related to visual information, 50 color- and 50 formrelated items, were selected using established procedures (Pulvermüller et al., 1999). Stimulus groups were matched for word length counted in letters and syllables (4.3 versus 4.1 letters, all monosyllabic), standardized lexical frequency (27.9 versus 30.9 occurrences/million words: Francis and Kucera, 1982) (also matched according to Baayen et al., 1993), imageability and concreteness/abstractness [mean concreteness ratings (SE): 533 (9.2) versus 522 (8.6): Coltheart, 1981; Wilson, 1988]. Cerebral Cortex August 2006, V 16 N 8 1195 Figure 2. (Top right) Cortical activation during passive reading of color (in green) and form-related words (red). (Center) Frontal slices of the MNI average brain showing regions where specific activation was seen during reading of color (green/blue/purple) or form (red/orange/yellow) words. (Bottom left) Effect sizes for color and form words in the six ROIs in the left hemisphere (rescaled to HRF peak percentage signal change relative to the mean over all voxels and scans within the corresponding ROI): IF, inferior frontal gyrus; PF, dorsolateral prefrontal cortex; PM, dorsolateral premotor cortex; PH, parahippocampal gyrus; MT, middle temporal gyrus; FUS, fusiform gyrus. The little brain at the bottom right gives the approximate locations of the six ROIs. Stimulus word groups were also matched for their semantic relationship to objects that can be visually perceived, but from which they differed in terms of their semantic relatedness to shape and color, as assessed in a rating study (Fig. 1, bottom). Subjects were asked whether they considered the word as being semantically related to (i) an object that can be perceived visually, (ii) a visual shape or form and (iii) a color. Ratings were given on a scale from 1 to 7, following procedures described in an earlier publication (Hauk and Pulvermüller, 2004). One hundred and fifty filler words and 50 pseudo-words were added in order to avoid focussing the subjects’ attention on specific aspects of the stimuli. The filler words included the face- and arm-related words used in the study by Hauk and colleagues (Hauk, 2004). Stimuli employed during 150 baseline trials consisted of strings of meaningless hash marks varying in length and matched to the word stimuli in length. In addition, 50 null events were included during which a fixation cross remained was displayed. Stimuli were presented for 100 ms, and the stimulus onset asynchrony (SOA) between two stimuli was 2.5 s, so that stimulus presentation and scanner trigger were out of phase by ~500 ms. Two pseudo-randomized stimulus sequences were alternated between subjects. For statistical analysis, the SPM99 canonical haemodynamic response function (HRF) was used to model the activation time course. Results Words activated an area in the left fusiform gyrus in the inferior temporal lobe (activation peaks at –36/–38/–24 and –42/–47/ –13) close to and overlapping with the site labeled the visual word form area (VWFA, –42/–57/–15: McCandliss et al., 2003). In addition, an area of inferior frontal cortex comprising the posterior part of Broca’s area, Brodmann’s area 44, and its anterior section, Brodmann area 45, became active (activation 1196 Category-specific Conceptual Processing of Color and Form d Pulvermüller and Hauk Table 2 Areas activated by words associated with color and form information Brain region Talairach x, y, z T(13) Color words Parahippocampal gyrus Cerebellum Fusiform gyrus, BA 20 LH ÿ30 ÿ14 ÿ20 LH ÿ36 ÿ40 ÿ24 LH ÿ44 ÿ48 ÿ20 5.60 4.38 3.98 Form words Fusiform gyrus, BA 20 Middle temporal gyrus, BA 19/39 Middle temporal gyrus, BA 21 Inferior frontal gyrus, BA 47 Precentral gyrus, BA 6 Middle frontal gyrus, BA 46 Middle and inferior frontal gyrus, BA 9 Precentral gyrus, BA 4 Lentiform Nucleus, Putamen LH LH LH LH LH LH LH RH LH ÿ38 ÿ38 ÿ57 ÿ34 ÿ49 ÿ46 ÿ53 36 ÿ20 ÿ36 ÿ18 ÿ63 18 ÿ43 4 17 ÿ2 0 35 26 19 13 29 ÿ26 55 10 7 6.21 4.88 4.28 7.03 6.29 5.85 4.91 5.38 4.73 Talairach coordinates along with t-values are given for the maximally activated voxel in each local cluster. All clusters that passed the significance threshold at P \ 0.001 are listed. The FDR correction cutoff was at T 5 4.3. peaks at –52/10/20 and –46/28/12). The activation of the VWFA together with inferior frontal areas during passive reading of single words is consistent with earlier findings (e.g. Dehaene et al., 2002; Hauk et al., 2004). There was an additional activation focus in anterior parahippocampal gyrus (–28/–12/ –18; see Fig. 1, Table 1). No significant activation was seen in the right hemisphere. Comparison of color and form words showed that both word categories activated the inferior temporal VWFA. This activation was more pronounced for form than for color words. Closer examination indicated that posterior parts of the activated fusiform area was most responsive to color words, whereas anterior fusiform activation was strongest for form words (–44/–48/–20 versus –38/–36/–18; see Table 2 and Fig. 2). The parahippocampal area was significantly activated by color words, but not by form words. Additional areas in middle temporal gyrus were activated specifically by form words. The most robust and widespread activation elicited by form words was found in left frontal cortex. Significant activation peaks were present in premotor cortex (BA 6, –50/–2/38), inferior prefrontal cortex (BA 45 and 47, –34/18/–2) and dorsolateral prefrontal cortex (BA 9 and 46, –54/12/32 and –46/26/19). To demonstrate that stimulus types activate specific brain regions to different degrees, it is necessary to directly compare the activity patterns they elicit in predefined ROIs. A repeated measures two-way ANOVA was therefore performed to assess significance of word-category-specific activation in the three canonical ROIs defined on the basis of the words versus hash marks contrast. This revealed a significant interaction of the factors Word Category and Region of Interest [F (2,26) = 9.11, P < 0.001]. Analysis of all six ROIs (fusiform, parahippocampal, middle-temporal, premotor, inferior-frontal and dorsolateral prefrontal areas) confirmed the critical interaction of the factor Word Category with Region of Interest [F (5,65) = 3.16, P < 0.01]. There was also a significant main effect of the factor Word Category [F (1,13) = 6.22, P < 0.02], suggesting stronger activation to form words than to color words. For the three canonical ROIs, planned comparison tests revealed stronger activation to form than color words in inferior frontal and fusiform gyri and the reverse, stronger activation to color than form words, in parahippocampal gyrus (F-values > 4.5, P-values < 0.05). Planned comparison tests performed for the three ‘supplementary’ ROIs revealed significant between-category differences in middle-temporal and prefrontal cortex (F > 4.5, P < 0.05), but not in the premotor region (P = 0.15, NS). In summary, these results show that five of the six ROIs were activated differentially by the word categories under investigation. Discussion Using event-related fMRI while subjects performed a passive reading task, we observed inferior temporal and inferior frontal activation of the left dominant hemisphere to words semantically related to form and color information. Critically, color and form words elicited category-specific cortical activation differences in focal areas of frontal and temporal cortex. Parahippocampal areas were more strongly activated by color words than by form words, and stronger activity to form words than to color words was seen in fusiform gyrus, middle temporal gyrus and prefrontal cortex in the inferior and middle frontal gyri. These results provide further evidence that words from different semantic categories —color and form words in the present work— can activate cortical areas to different degrees, possibly revealing the cortical topographies of their categoryspecific distributed semantic memory networks or cell assemblies. These findings are in line with previous neuropsychological evidence suggesting that dissociable brain systems contribute to conceptual processing of color and form information (Zeki et al., 1999; Miceli et al., 2001). General Word-related Activation Passive reading of all word stimuli grouped together led to enhanced blood flow in the left temporal lobe, in fusiform cortex, anterior to an area previously related to the processing of prelexical word-related or letter string information (Cohen et al., 2000; Dehaene et al., 2002). Because the stimulus words under study were highly imageable and semantically related to objects known through the visual channel, the present data are also open to the interpretation that word-evoked fusiform activation indicates semantic processing (Price, 2000; Price and Devlin, 2003). In this context, it is noteworthy that the fusiform activation maximum elicited by color words was posterior to that elicited by form words, and that fusiform activation was generally stronger for form words than for color words. This argues in favor of a semantic origin of the present fusiform activation, i.e. a specialization of subareas of fusiform gyrus in the conceptual processing of color and/or form information. Apart from temporal cortex, left-inferior frontal cortex, including Broca’s area, was also generally activated by all word forms. This confirms earlier reports on a general role of this brain region in word processing (Price et al., 1996; Binder et al., 1997; Pulvermüller et al., 2003; Wilson et al., 2004). The inferior-frontal neurons included in word-related neuronal ensembles would thus be activated not only during speech but during language comprehension as well (Pulvermüller and Preissl, 1991). The role of these inferior frontal neurons may be analogous to that of mirror neurons with a role in action control but also responding to perceptual aspects of actions (Gallese et al., 1996; Iacoboni et al., 1999; Rizzolatti et al., 2001; Kohler et al., 2002), in this case acoustic signals uniquely identifying lexical elements (Fadiga et al., 2002). However, we cannot decide on the basis of the present neuroimaging data whether the inferior frontal activation is due to mirror neurons or to canonical multimodal neurons that do not respond specifically to actions, but rather to objects involved in actions (Rizzolatti and Craighero, 2004). The left-hemispheric parahippocampal activation not generally seen for words could tentatively be attributed to the abstract meaning aspects of the stimulus words (but see Discussion below). Category-specific Semantic Activation Category-specific activation was reported in a variety of earlier studies (e.g. Dehaene, 1995; Martin et al., 1995, 1996). However, as argued previously (for review, see Pulvermüller, 1999), much earlier work did not control for important physical, psychological and psycholinguistic stimulus features, therefore making it difficult to draw inferences on a semantic origin of the observed effects. Although category-specific brain activation was not always observed when these factors were empirically assessed and controlled for (Devlin et al., 2002), a number of studies confirmed differences between the brain activation patterns elicited by words from different semantic categories. Differences were found between widely defined and increasingly specific categories, ranging from content and function words, nouns and verbs, animal and tool names, to action word subtypes with different meaning (e.g. Brown and Lehmann, 1979; Preissl et al., 1995; Pulvermüller et al., 1995, 2005; Cappa et al., 1998; Koenig et al., 1998; Chao et al., 1999; Kiefer, 2001; Hauk et al., 2004; Devlin et al., 2005). The present study provided particularly extensive stimulus control and matching of word categories for variables including word length, Cerebral Cortex August 2006, V 16 N 8 1197 standardized lexical frequency, familiarity, abstractness, imageability and the degree to which the words were semantically related to visually perceivable objects. On the other hand, empirical evidence was gathered that stimulus groups differed significantly on semantic scales of color- and form-relatedness (Fig. 1), a difference at the cognitive level that was reflected by differential activations of a range of cortical areas. The differential activation to color and form words in different sets of regions of interest argues in favor of an interpretation in terms of semantic and conceptual information linked to the word stimuli at an abstract level. Specific Activation in Temporal Lobe Our results provide evidence that cortical areas differentially contribute to the semantics of color and form words and therefore support established theories of category-specific semantic processes in human cortex (Warrington and McCarthy, 1983; Shallice, 1988). Information about color and form is mainly extracted from the visual input and therefore cortical processing differences were expected in the inferior-temporal ventral stream of visual processing. The observed category differences in fusiform gyrus and middle temporal gyrus are consistent with previous imaging work and neuropsychological studies revealing distinct inferior temporal systems preferentially engaging in color and form processing (e.g. Martin et al., 1995; Martin and Chao, 2001; McClelland and Rogers, 2003). An explanation in terms of different inferior temporal neural systems specialized for color and form information can account for the differential activation of fusiform gyrus, in particular the 12 mm distance between form and color word maxima. The specific activation of parahippocampal cortex to color words is consistent with results of recent studies of fine-grained neuropsychological deficits in processing color-knowledge related to objects, suggesting that ‘the mesial temporal stuctures are specifically involved in representing or accessing object color knowledge’ (Miceli et al., 2001). As color and form words differentially activated areas in parahippocampal, fusiform and middle temporal gyrus, it might be possible to relate these differences to aspects of the meaning of these stimuli. Parahippocampal activation specific to color words can tentatively be explained by the visual feature conjunction model (Bussey and Saksida, 2002; Bussey et al., 2002): to classify visual information under a concept, such as a color, logical operations including ‘and’ and ‘either-or’ computations need to be performed by neural elements over a range of input patterns. It is well known that this requires additional sets of neural elements operating on the input (Kleene, 1956; Minsky and Papert, 1969; McClelland and Rumelhart, 1985), which in the inferior visual stream must be located progressively anterior to visual cortex. It is possible that feature conjunctions and disjunctions for color concepts, e.g. binding the different tones and luminances covered by a given color concept, are computed by parahippocampal neurons receiving input from posterior color-related inferior temporal areas. This is consistent with the idea that aspects of color concepts linked to words are processed by anterior temporal sites, thereby supporting the posterior--anterior model of visual feature abstraction (Bussey and Saksida, 2002; Bussey et al., 2002; Tyler et al., 2004). However, as form words did not activate parahippocampal cortex although their meaning, similarly to color words, is abstract and independent of any specific object (feature conjunctions and disjunctions), it appears that other areas in temporal cortex can contribute to conjunctive processing too. For form words, the fusiform and middle temporal cortices are relevant here and the suggestion may therefore be that there are different streams in temporal lobe that can play a category-specific role in feature conjunction or disjunction processing. Specific Activation in Frontal Lobe All words under study were strongly related to visually defined perceptual information, but lacked any obvious action meaning, as, for example, tool names and action verbs usually do. Established theories of category-specificity would therefore predict activity differences between color and form words related to visual information processing in temporal lobe, but not in frontal cortex. The specific inferior frontal and middle frontal activation to form words can be tentatively accounted for as follows. An action relationship of form words is evident from the fact that a shape or form can always be outlined or adumbrated by a specific set of body movements. Premotor activation seen for form words may therefore be related to implicit action aspects of the meaning of form words. However, there is a clear difference between the action relatedness of a concrete action word, e.g. ‘kick’, and an abstract form word, e.g. ‘rhomb’. The former relates to a defined body part whereas Figure 3. Lateral view of the brain with activation maps for abstract form/shape words (in red) contrasted with the areas activated by concrete action words related to specific body parts (face in blue, arm in green; diagram on the left) and actions performed with these body parts (diagram on the right) (cutoffs: P < 0.001, uncorrected, for words, P < 0.05, FDR-corrected, for actions). Note that form words elicited prefrontal activation in the first and second frontal gyrus not evoked by concrete action words or actions (yellow circle). All word activations overlapped in the left inferior frontal cortex (white area, diagram on the left) but actions and abstract word processing did not elicit overlapping activation patterns (right diagram). 1198 Category-specific Conceptual Processing of Color and Form d Pulvermüller and Hauk the latter refers to a shape that can be adumbrated using the hand or finger, but equally well by a head movement or a series of saccades. The form-related action concept should therefore include information that abstracts away the body part information but leaves open the alternatives of using different body parts for perceptually related actions, which we could expect in prefrontal cortex adjacent to premotor areas. To directly test this prediction, an experiment on action words related to different body parts (face-, arm- and leg-related action words, such as ‘lick’, ‘pick’ and ‘kick’, see Hauk et al., 2004) was done with the participants in the color-/form-word study (see Materials and Methods). Consistent with the earlier findings, face- and arm-words produced somatotopic activation in motor and premotor cortex, which partly overlapped with activation during movements of corresponding body parts. The left inferior-frontal activation to form words overlapped with action word activation (Fig. 3, diagram on the left), but no clear overlap was seen between word activation and the precentral activation observed during simple body movements (diagram on the right). Crucially, frontal activation elicited by form words was not restricted to premotor areas (as for concrete action words), but extended into prefrontal cortex in both inferior and middle frontal gyri including Brodmann areas 9 and 46. These areas are anterior to premotor cortex and therefore ideally located for controlling premotor activity. We suggest that the dorso-lateral prefrontal activation specific to form words relates to the activation of neural networks computing conjunctions and disjunctions on concrete action patterns therefore specifying action concepts at an abstract level. The activation of a left inferior frontal area, including middle frontal gyrus and premotor cortex, is consistent with earlier findings about abstract words in general (Binder et al., 2005). As the premotor activation, which reached significance for form words, did not reliably differentiate this word category from color words, the dorsolateral prefrontal focus seen more active for form than color words might be of particular functional relevance for computing abstract form-related action concepts (yellow circles in Fig. 3). Conclusion On the basis of these data, the sensorimotor theory of conceptual and semantic processing (Warrington and Shallice, 1984; Humphreys and Forde, 2001) can be extended to incorporate a model of concepts that may be considered as partially abstract. Neuron populations storing abstract concepts would accordingly be housed in areas adjacent to, and connected with, areas where more elementary and concrete concepts are laid down. Modality-specific systems may be located in inferior temporal, middle temporal and parahippocampal gyri that compute feature conjunctions and dysjunctions on visual form and color features. The mirror and/or canonical neuron system in premotor cortex as a site where actions and perceptions are linked together offers a unique basis for action-related abstraction, which could be carried out by neurons in the adjacent prefrontal cortex performing eitheror operations on motor patterns stored in premotor cortex. This view, which covers partially abstract concepts still grounded in action or visual concepts, suggests similar mechanisms of abstraction in different cortical lobes for specific abstract categories. Although future research is necessary to decide whether this view can be generalized to the various types of abstract words and concepts, corroborating evidence from studies of highly abstract lexical items suggests that the prefrontal circuits play a general role in the computation of abstract semantic information. In conclusion, we report evidence that two subtypes of partially abstract concepts have their respective brain basis in specific temporal and frontal areas. Color/form word-evoked category-specific activation in fusiform, middle temporal and inferior frontal gyri may be shared in part with other semantic categories. Specific activation in dorsolateral prefrontal and parahippocampal gyri may be most characteristic for abstraction processes necessary for classifying sensory and motor patterns into color and form concepts. Notes We thank Rik Henson, Ingrid Johnsrude, Ferath Kherif, Bettina Mohr, Yury Shytov and two anonymous referees for their help at different stages of this work. This work was supported by the Medical Research Council (UK) and by the European Community under the ‘Information Society Technologies Programme’ (IST-2001-35282). Address correspondence to Friedemann Pulvermüller, MRC Cognition and Brain Sciences Unit, 15 Chaucer Road, Cambridge CB2 2EF, UK. Email: [email protected]. References Baayan H, Piepenbrock R, van Rijn H (1993) The CELEX lexical database (CD-ROM). University of Pennsylvania, PA: Linguistic Data Consortium. Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T (1997) Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17:353--362. Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA (2005) Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci 17:905--917. Brown WS, Lehmann D (1979) Verb and noun meaning of homophone words activate different cortical generators: a topographic study of evoked potential fields. Exp Brain Res 2:159--168. Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ (2001) Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13:400--404. Bussey TJ, Saksida LM (2002) The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. Eur J Neurosci 15:355--364. Bussey TJ, Saksida LM, Murray EA (2002) Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci 15:365--374. Cappa SF, Perani D, Schnur T, Tettamanti M, Fazio F (1998) The effects of semantic category and knowledge type on lexical-semantic access: a PET study. Neuroimage 8:350--359. Chao LL, Haxby JV, Martin A (1999) Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci 2:913--919. Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F (2000) The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 123 (Pt 2): 291--307. Coltheart M (1981) The MRC Psycholinguistic Database. Q J Exp Physiol 33A:497--505. Cusack R, Brett M, Osswald K (2003) An evaluation of the use of magnetic field maps to undistort echo-planar images. Neuroimage 18:127--142. Dehaene S (1995) Electrophysiological evidence for category-specific word processing in the normal human brain. Neuroreport 6: 2153--2157. Cerebral Cortex August 2006, V 16 N 8 1199 Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L (2002) The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport 13:321--325. Devlin JT, Jamison HL, Matthews PM, Gonnerman LM (2004) Morphology and the internal structure of words. Proc Natl Acad Sci USA 101: 14984--14988. Devlin JT, Rushworth MF, Matthews PM (2005) Category-related activation for written words in the posterior fusiform is task specific. Neuropsychologia 43:69--74. Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, Tyler LK (2002) Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia 40:54--75. Duncan J, Owen AM (2000) Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475--483. Fadiga L, Craighero L, Buccino G, Rizzolatti G (2002) Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci 15:399--402. Fiebach CJ, Friederici AD (2004) Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia 42:62--70. Francis WN, Kucera H (1982) Computational analysis of english usage: lexicon and grammar. Boston, MA: Houghton Mifflin. Freud S (1891) Zur Auffassung der Aphasien. Leipzig, Wien: Franz Deuticke. Friederici AD, Opitz B, von Cramon DY (2000) Segregating semantic and syntactic aspects of processing in the human brain: an fMRI investigation of different word types. Cereb Cortex 10:698--705. Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998) Event-related fMRI: characterizing differential responses. Neuroimage 7:30--40. Fuster JM, Bodner M, Kroger JK (2000) Cross-modal and cross-temporal association in neurons of frontal cortex. Nature 405:347--351. Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996) Action recognition in the premotor cortex. Brain 119:593--609. Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870--878. Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J (2002) The neural basis for category-specific knowledge: an fMRI study. Neuroimage 15:936--948. Hauk O, Johnsrude I, Pulvermüller F (2004) Somatotopic representation of action words in the motor and premotor cortex. Neuron 41: 301--307. Hauk O, Pulvermüller F (2004) Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Mapp 21:191--201. Hickok G, Poeppel D (2000) Towards a functional neuroanatomy of speech perception. Trends Cogn Sci 4:131--138. Hodges JR (2001) Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology 56:S6--10. Hubel D (1995) Eye, brain, and vision. New York: Scientific American Library. Humphreys GW, Forde EM (2001) Hierarchies, similarity, and interactivity in object recognition: ‘category-specific’ neuropsychological deficits. Behav Brain Sci 24:453--509. Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999) Cortical mechanisms of human imitation. Science 286:2526--2528. Jackendoff R (2002) Foundations of language: brain, meaning, grammar, evolution. Oxford, UK: Oxford University Press. Jeannerod M, Frak V (1999) Mental imaging of motor activity in humans. Curr Opin Neurobiol 9:735--739. Jezzard P, Balaban RS (1995) Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 34:65--73. Kiefer M (2001) Perceptual and semantic sources of category-specific effects: event-related potentials during picture and word categorization. Mem Cogn 29:100--116. Kiefer M, Spitzer M (2001) The limits of a distributed account of conceptual knowledge. Trends Cogn Sci 5:469--471. 1200 Category-specific Conceptual Processing of Color and Form d Pulvermüller and Hauk Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD (1999) Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapp 7:225--233. Kleene SC (1956) Representation of events in nerve nets and finite automata. In: Automata studies (Shannon CE, McCarthy J, eds), pp. 3-41. Princeton, NJ: Princeton University Press. Koenig T, Kochi K, Lehmann D (1998) Event-related electric microstates of the brain differ between words with visual and abstract meaning. Electroencephalogr Clin Neurophysiol 106:535--546. Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G (2002) Hearing sounds, understanding actions: action representation in mirror neurons. Science 297:846--848. Kounios J, Holcomb PJ (1994) Concreteness effects in semantic priming: ERP evidence supporting dual-coding theory. J Exp Psychol Learn Mem Cogn 20:804--823. Kourtzi Z, Kanwisher N (2001) Representation of perceived object shape by the human lateral occipital complex. Science 293: 1506--1509. Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P (1997) Multimodality image registration by maximization of mutual information. IEEE Trans Med Imag 16:187--198. Martin A, Chao LL (2001) Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 11:194--201. Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (1995) Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270:102--105. Martin A, Wiggs CL, Ungerleider LG, Haxby JV (1996) Neural correlates of category-specific knowledge. Nature 379:649--652. McCandliss BD, Cohen L, Dehaene S (2003) The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci 7: 293--299. McClelland JL, Rogers TT (2003) The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci 4:310--322. McClelland JL, Rumelhart DE (1985) Distributed memory and the representation of general and specific information. J Exp Psychol Gen 114:159--188. McKeefry DJ, Zeki S (1997) The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain 120 (Pt 12):2229--2242. Miceli G, Fouch E, Capasso R, Shelton JR, Tomaiuolo F, Caramazza A (2001) The dissociation of color from form and function knowledge. Nat Neurosci 4:662--667. Minsky M, Papert S (1969) Perceptrons. Cambridge, MA: MIT Press. Neville HJ, Mills DL, Lawson DS (1992) Fractionating language: different neural subsystems with different sensitive periods. Cereb Cortex 2:244--258. Noppeney U, Price CJ (2004) Retrieval of abstract semantics. Neuroimage 22:164--170. O’Craven KM, Kanwisher N (2000) Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci 12:1013--1023. Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F (1999) The neural correlates of verb and noun processing. A PET study. Brain 122:2337--2344. Posner MI, Pavese A (1998) Anatomy of word and sentence meaning. Proc Natl Acad Sci USA 95:899--905. Preissl H, Pulvermüller F, Lutzenberger W, Birbaumer N (1995) Evoked potentials distinguish nouns from verbs. Neurosci Lett 197:81--83. Price CJ (2000) The anatomy of language: contributions from functional neuroimaging. J Anat 197(Pt 3):335--359. Price CJ, Devlin JT (2003) The myth of the visual word form area. Neuroimage 19:473--481. Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ (2001) Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci 13:419--429. Price CJ, Wise RJS, Frackowiak RSJ (1996) Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex 6:62--70. Pulvermüller F (1999) Words in the brain’s language. Behav Brain Sci 22:253--336. Pulvermüller F (2001) Brain reflections of words and their meaning. Trends Cogn Sci 5:517--524. Pulvermüller F (2005) Brain mechanisms linking language and action. Nat Rev Neurosci 6:576--582. Pulvermüller F, Preissl H (1991) A cell assembly model of language. Network Comput Neural Sys 2:455--468. Pulvermüller F, Lutzenberger W, Birbaumer N (1995) Electrocortical distinction of vocabulary types. Electroencephalogr Clin Neurophysiol 94:357--370. Pulvermüller F, Lutzenberger W, Preissl H (1999) Nouns and verbs in the intact brain: evidence from event-related potentials and highfrequency cortical responses. Cereb Cortex 9:498--508. Pulvermüller F, Shtyrov Y, Ilmoniemi RJ (2003) Spatio-temporal patterns of neural language processing: an MEG study using minimum-norm current estimates. Neuroimage 20:1020--1025. Pulvermüller F, Shtyrov Y, Ilmoniemi RJ (2005) Brain signatures of meaning access in action word recognition. J Cogn Neurosci 17: 884--892. Rizzolatti G, Craighero L (2004) The mirror-neuron system. Annu Rev Neurosci 27:169--192. Rizzolatti G, Fogassi L, Gallese V (2001) Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2:661--670. Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K (2004) Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev 111:205--235. Scott SK, Johnsrude IS (2003) The neuroanatomical and functional organization of speech perception. Trends Neurosci 26:100--107. Shallice T (1988) From neuropsychology to mental structure. New York: Cambridge University Press. Shtyrov Y, Hauk O, Pulvermüller F (2004) Distributed neuronal networks for encoding category-specific semantic information: the mismatch negativity to action words. Eur J Neurosci 19: 1083--1092. Skosnik PD, Mirza F, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ (2002) Neural correlates of artificial grammar learning. Neuroimage 17:1306--1314. Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143--155. Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. New York: Thieme. Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE (2004) Processing objects at different levels of specificity. J Cogn Neurosci 16:351--362. Warrington EK, McCarthy RA (1983) Category specific access dysphasia. Brain 106:859--878. Warrington EK, Shallice T (1984) Category specific semantic impairments. Brain 107:829--854. Wernicke C (1874) Der aphasische Symptomencomplex. Eine psychologische Studie auf anatomischer Basis. Breslau: Kohn und Weigert. Wilson MD (1988) The MRC Psycholinguistic Database: machine readable dictionary, version 2. Behav Res Methods Instrum Comput 20:6--11. Wilson SM, Saygin AP, Sereno MI, Iacoboni M (2004) Listening to speech activates motor areas involved in speech production. Nat Neurosci 7:701--702. Wise RJ, Howard D, Mummery CJ, Fletcher P, Leff A, Buchel C, Scott SK (2000) Noun imageability and the temporal lobes. Neuropsychologia 38:985--994. Zeki S, Aglioti S, McKeefry D, Berlucchi G (1999) The neurological basis of conscious color perception in a blind patient. Proc Natl Acad Sci USA 96:14124--14129. Cerebral Cortex August 2006, V 16 N 8 1201