* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Emo7onal decision‐making systems and their role in addic7on
Vesicular monoamine transporter wikipedia , lookup
Nervous system network models wikipedia , lookup
Human brain wikipedia , lookup
Environmental enrichment wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuromarketing wikipedia , lookup
Neural engineering wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Affective neuroscience wikipedia , lookup
Neuroinformatics wikipedia , lookup
Emotional lateralization wikipedia , lookup
Neuroethology wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuropsychology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Executive functions wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neurotransmitter wikipedia , lookup
Basal ganglia wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Biology of depression wikipedia , lookup
Metastability in the brain wikipedia , lookup
Aging brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Time perception wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
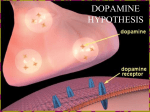
Emo$onal decision‐making systems and their role in addic$on Antoine Bechara Objec&ves: 1. Habit (Impulsive) system: Dopamine mediated (striatal). 2. Inhibitory control (Reflec$ve) system: Prefrontal cortex dependent— a. Decision‐making b. Simple inhibitory control. 3. Insular cortex system: responsive to homeosta$c imbalance (depriva$on states), which ac$vity: a. Exacerbates the ac$vity of the habit/impulsive system. b. Disable (or “hijack”) ac$vity of the prefrontal (control/ reflec$ve) system. 1. The habit (impulsive) system—reward seeking A. The Nature of reward: The term “reward” describes the positive value an individual ascribes to an object, behavioral act or an internal physical state. There are two kinds of rewards that can establish addictive behaviors: (1) Natural rewards are those that reach the brain circuitry of motivation via the Bive senses. The rewards of food for a hungry person, water for a thirsty person, or copulation for sexually receptive individual reach the brain via peripheral sensory pathways (e.g., vision, taste, olfaction, sensation, and audition). (2) “Unsensed” rewards denote those with the capacity to activate the brain substrates of motivation directly, bypassing the peripheral sensory pathways. Drugs taken orally, inhaled, or injected, do not have a particular taste, or smell, that makes them rewarding. They act directly on the brain to produce reward. (3) “Money” is a reward too: Through learning, money becomes strongly associated with natural rewards, like food, water, sex, and shelter. Because learning about money is so extensive and spans our entire life, these learning associations with natural rewards become powerful, automatic, and non‐conscious. Although there are obvious differences between how natural (e.g., sex) and unsensed (e.g., drugs), or money rewards reach the reward systems within the brain, a large volume of studies, dating back to the 1980’s, have implicated the “mesolimbic” dopamine system, and several of its afferents (inputs) and efferents (outputs), in mediating the motivational functions of both types of reward. Both animal and human studies have linked natural rewards to the same mesolimbic dopamine system. Thus drugs are rewarding because they have the capacity to activate directly in the brain the endogenous reward mechanisms subserving natural or biological rewards, which existed before the invention of drugs. B. The dopamine system and reward: There are several systems of dopaminergic neurons or systems (5 in total). However, the three most important of these originate in the midbrain, speciBically: (1) The substantia nigra, which projects to the dorsal striatum or neostriatum= mesostriatal (or nigrostriatal) dopamine system‐‐‐Parkinson Disease. Medications: predominantly D2 receptors—Parkinson DA agonist therapies target these receptors. (2) The ventral tegmental area, which projects to the nucleus accumbens= mesolimbic dopamine system‐‐‐‐reward. Medications: predominantly D3 receptors—Parkinson medications that are D3 agonists (e.g., Mirapex) have high likelihood of inducing addictive behaviors (e.g., pathological gambling) in individuals whom otherwise never gambled before. (3) Another one from the ventral tegmental area, which projects to the prefrontal cortex= mesocortical dopamine system—decision‐making and inhibitory control. C. The history of research on dopamine and reward. Research on the difference between the nigrostriatal and mesolimbic systems in terms of their contribution to movement versus reward is old and dates back to at least the early 1980’s (it started in the late 70’s): 1. The Birst proposal of a unique relationship between dopamine and reward was provided by R. Wise: Wise, R.A., Catecholamine Theories of Reward CriticalReview. Brain Research, 1978. 152(2): p. 215247. Wise, R.A. and M.A. Bozarth, A psychomotor stimulant theory of addiction. Psychological Review, 1987. 94: p. 469492. The literature from that era (the 80’s) clearly demonstrates that the dopaminergic projection to the nucleus accumbens (the mesolimbic dopamine system), but not the dorsal striatum (the dorsal part of the neostriatum, which excludes the ventral striatum, where the nucleus accumbens is located), is the one that plays the most signiBicant role in the reward derived from drugs of abuse (e.g., psychostimulants). 1.a. It was also recognized in the late 1980’s and early 1990’s that the abuse potential of drugs of abuse (e.g., opiates, alcohol, nicotine, caffeine, barbiturates, benzodiazepines, cannabis, and phencyclidine) all are linked, one way or another, to this mesolimbic dopamine system. While these different drugs may act initially on different receptor sites in the brain, ultimately they all act (directly or indirectly) on the mesolimbic dopamine system to exert reward: Wise, R.A., The neurobiology of craving: Implications for the understanding and treatment of addiction. Journal of Abnormal Psychology, 1988. 97: p. 118132. 1.b. It was also well accepted in the late 1980’s and early 1990’s that manipulations of the same mesolimbic dopamine system (with pharmacological blockade of dopamine or with selective lesions of the dopamine neurons using 6‐hydroxydopamine or 6‐OHDA) exert an impact on natural rewards, such as food, sweet drinks, and sex. Most of these experiments showed that during dopamine blockade, animals would not be motivated to eat, for example, even though food may be readily available, and even when their motor capacity to walk, chew, swallow and perform other movements are preserved. It was also recognized that dopamine stimulation in the accumbens increases the motivation for food. Microinjections of dopamine agonists directly into the nucleus accumbens do increase the motivation for natural rewards, such as food: Wise, R.A. and P.P. Rompre, Brain dopamine and reward. Annual Reviews of Psychology, 1989. 40: p. 191225. 1.c. More than 25 years has passed, and the original conclusion that manipulations of the mesolimbic dopamine system inBluences the motivation to seek rewards remains valid: ‐When brain dopamine levels are low, the motivation to seek reward becomes halted. It is the stimulation of the dopamine system, or the presence of a drug that activates the dopamine system, not the absence of dopamine, that energizes and motivates behaviors towards the seeking of rewards. ‐Cellular physiology and pharmacology demonstrates that the action of dopamine in the nucleus accumbens is to inhibit the GABA neurons, which are inhibitory to the next neurons in the chain that instigate motivated behaviors. In other words, dopamine in the accumbens inhibits the inhibitory neurons, thus resulting in behavioral dis inhibition. 1.d. There has been much recent functional neuroimaging work in humans implicating the ventral striatum (including the nucleus accumbens) in a variety of reward processes, including monetary rewards. Although functional magnetic resonance imaging (fMRI) approaches cannot technically address dopamine (or any other brain chemical for that matter), the fact that the neural region receiving these dopamine projections (i.e., the ventral striatum) is implicated in a variety of reward processes (video games, viewing of sexual materials, and monetary rewards, to name a few) validates the large body of evidence that employed a variety of elecrophysiological, microdialysis, or voltammetric techniques, which accumulated over 25 years of research, and which concluded that the mesolimbic dopamine projection to nucleus accumbens plays a key role in reward processes. 2. The conditioned cues evidence: According to the “psychostimulant” anaylsis, the mesolimbic dopamine system plays a key role in mediating the “approach” response elicited by drugs as well as natural rewards. Mesolimbic dopamine strengthens the approach response and motivational arousal elicited by rewards, which are associated with pleasure. The mesolimbic dopamine system, which is clearly critical for reward functions, becomes increasingly responsive to stimuli and cues that predict the delivery of the actual reward (e.g., food): Stewart, J., H. Dewit, and R. Eikelboom, Role of Unconditioned and Conditioned Drug Effects in the SelfAdministration of Opiates and Stimulants. Psychological Review, 1984. 91(2): p. 251268. Schultz, W., P. Dayan, and P.R. Montague, A neural substrate of prediction and reward. Science, 1997. 275: p. 15931599. 3. The “liking” versus “wanting”: Later researchers assert that the process of reward can be further sub‐divided into (1) a “wanting” component, which makes rewards attractive and “wanted”, and which triggers “approach” and pursuit of the reward; and (2) a “liking” component, which involves feeling of pleasure. Although there seem to be additional systems in the brain (which remain unidentiBied) that mediate the “liking” or pleasure component, the mesolimbic dopamine system is critical for speciBically this “wanting” component of the reward: Berridge, K.C. and T.E. Robinson, What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 1998. 28: p. 309369. Robinson, T.E. and K.C. Berridge, The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Research Reviews, 1993. 18: p. 247291. With repeated drug use, the mesolimbic dopamine projections to the nucleus accumbens become sensitized, and eventually lead to excessive incentive salience attribution to the drugs and drug‐related stimuli, which activate this neural circuitry, thus making them highly attractive and pathologically “wanted” or craved. This mesolimbic dopamine sensitization phenomenon does not apply to only drugs, but to all types of rewards. When the mesolimbic dopamine system is activated in the presence of a reward in the environment, the incentive salience of that reward is increased, and the reward becomes more attractive and pathologically “wanted”. 4. The notions of pleasure and dopamine: The evidence that blockade of dopamine nerotransmission in the ncleus accumbens interfered with the motivation to seek rewards prompted Wise (1982) to propose the “anhedonia” hypothesis, that dopamine mediates the pleasure produced by food, sex, or drugs that compulsive drug users seek. ‐However, Wise himself retracted, shortly after, the notion that dopamine blockade reduces pleasure (Wise 1985), and he replaced the anhedonia hypothesis with an incentive‐based theory of motivation, the “psychostimulant” theory (Wise and Bozarth, 1987). ‐The key aspect of that theory is that the mesolimbic dopamine system plays a key role in mediating the “approach” response elicited by drugs as well as natural rewards. In other words, a feeling of pleasure is not necessarily experienced when dopamine is released; mesolimbic dopamine strengthens the approach response and motivational arousal elicited by rewards, which they are clearly associated with pleasure. ‐Despite this consensus in the literature more than a decade ago that dopamine is not synonymous with pleasure, this notion that dopamine is the “pleasure” neurotransmitter of the brain has had an insurmountable appeal; it seems to continue to linger until today in various media reports; even the more recent functional neuroimaging work in humans that addresses the reward mechanisms mediated by the nucleus accumbens often discuss the role of dopamine in this region in a manner that is hardly distinguishable from the notions of pleasure. 5. Dopamine depletion views: DeBiciencies in neurotransmitter activation of dopamine in the mesolimbic reward pathways are the instigators of reward seeking behaviors: ‐This is true in cases of drug‐induced dopamine depletions: e.g., cocaine crash. ‐It is very controversial as to whether this would be true for someone born with low dopamine baseline levels. 6. Habits (implicit) mechanisms of drug reward seeking: While addicted behaviors all start out under some “conscious” control through these ventral striatal motivational neural circuitries, prolonged drug use results in the strengthening of motivation‐relevant associative memories, which promote continued use, and an implicit, or relatively spontaneous (automatic) process begins to govern behavior. ‐Neutral stimuli associated with appetitive behaviors such as drug use come to represent and cue the behavior. As cue‐behavior‐outcome associations are strengthened, patterns of associations signal and drive behavior without the necessary involvement of conscious control processes. ‐ Once a strong habit is formed, cues elicit the habit regardless of anticipated outcomes. ‐ Habits become automatic and difficult to change. Another pivotal feature of habit systems is that participants do not necessarily know what triggers their habits. -At the neural level, there have been numerous neuroscientific demonstrations as what starts out as reward seeking mediated through the ventral striatum can end up as a “habit” automatic behavior and shifts to the dorsal striatum: Everitt, B.J., A. Dickinson, and T.W. Robbins, The neuropsychological basis of addictive behaviour. Brain Research Reviews, 2001. 36: p. 129-138. Everitt, B.J., K.A. Morris, A. Obrien, and T.W. Robbins, The Basolateral Amygdala Ventral Striatal System and Conditioned Place Preference - Further Evidence of Limbic Striatal Interactions Underlying Reward-Related Processes. Neuroscience, 1991. 42(1): p. 1-18. Everitt, B.J., J.A. Parkinson, M.C. Olmstead, M. Arroyo, P. Robledo, and T.W. Robbins, Associative processes in addiction and reward: the role of amygdala and ventral striatal subsystems., in Advancing from the ventral striatum to the extended amygdala, J.F. McGinty, Editor. 1999, Annals of the New York Academy of Science: New York. p. 412-438. Everitt, B. and T.W. Robbins, Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 2005. 8: p. 1481-1489. 2. Inhibitory control (Reflec&ve) system: Prefrontal cortex dependent— a. Decision‐making b. Simple inhibitory control. Dual‐process models of decision‐making, cognition, and associative memory have gained substantial momentum in behavioral research. In characterizing the distinctions between these two cognitive systems, Kahneman summarized that: “…the operations of System 1 are typically fast, automatic, effortless, associative, implicit (not available to introspection), and often emotionally charged; they are also governed by habit and are therefore difBicult to control or modify…” “…Operations of System 2 are slower, serial, effortful, more likely to be consciously monitored and deliberately controlled; they are also relatively Blexible and potentially rule governed…”. According to Kahneman (2003), the implicit or automatic cognitive system (System 1) is the system governing the majority of human decision making, whereas System 2 monitors the operations of System 1. This perspective of relatively automatic processes being a sort of ‘default’ mode guiding behavior, unless overridden by deliberate cognitive processes, has been expressed in social psychological theories, and memory research for over a decade. The distinction between automatic and controlled processes (and similar distinctions) is reinforced further through several independent lines of research in neuroscience, including my own (the impulsive versus reBlective systems for addictive behaviors, Bechara, Nature Neuroscience, 2005). A critical neural region in the reBlective system is the ventromedial prefrontal cortex region (which we have considered as inclusive of the medial orbitofrontal cortex). However, other neural components, including the dorsolateral prefrontal cortex (for working memory) and the cingulate cortex are also a part of this neural circuitry, and are essential for the normal operation of the ventromedial prefrontal cortex: Outline of the Somatic Marker Theory Active vs control (all 10 subjects, all 4 tasks), p(bonf)<0.001 Left hemisphere Right hemisphere Motor/Behavioral Systems AntCingCtx/SupMotArea Striatum Memory Systems : DorsoLatPrefCtx (DLPC) Hippocampus OrbitFrontCtx/VMPC Amygdala Dopamine Serotonin Ach NE Emotion Systems : Insula Posterior Cingulate Hypothalamus PAG Brainstem autonomic centers Computa$on: In the body‐‐‐Body loop In Sensory nuclei and neurotransmiTer cell bodies of brainstem ‐‐‐‐As if body loop Decision‐making versus inhibitory control: There is a distinction between the mechanisms of affective decision‐making and those of simple inhibitory or impulse control. We have argued that, within the “reBlective” system, there is a distinction in functioning between 1) simple inhibitory and impulse control processes (some are mediated by the lateral orbitofrontal and inferior frontal gyrus regions, and some are mediated by the more posterior sectors of the medial prefrontal region, i.e., the anterior cingulate cortex, both dorsal and ventral), and 2) affective decision making (mediated by more anterior regions of medial prefrontal cortex, including the frontal pole), which are highly relevant to behavioral control ability and to the decisions individuals make frequently on a daily basis (Bechara, 2005). Both inhibitory/impulse control function and affective decision‐making are important, speciBic aspects of higher order executive control functioning (Winstanley et al., 2006). 1. Good inhibitory functioning reBlects the ability to actively stop a pre‐potent behavioral response (e.g., drinking or eating in excess) after it has been triggered (Logan et al., 1997; Braver and Ruge, 2006). Individuals with deBicits or failures in these systems have a tendency to act more impulsively. 2. Adequate affective decision‐making reBlects an integration of cognitive and affective systems and the ability to more optimally weigh short term gains against long term losses or probable outcomes of an action (Bechara, 2005). For example, excessive drinking or eating known to have short‐term “reinforcing effects” (but long‐term negative consequences) should be less likely or problematic for individuals scoring higher on tasks that assess this ability. The functional distinction between simple inhibitory/impulse control and affective decision making processes comes from extensive clinical research with patient populations with damage in frontal lobe regions (see (Bechara, 2005) for reviews). However, these two sets of functions are asymmetrically related, i.e., one way (not double) dissociation: Damage that leads to basic inhibitory control deBicits inevitably leads to affective decision‐ making deBicits. However, damage to areas important for affective decision‐making (e.g., anterior ventromedial region) can lead to pure affective decision‐making deBicits, independent of any other simple inhibitory control deBicits. 3. Insular cortex system: responsive to homeosta&c imbalance (depriva&on states), which ac&vity: a. Exacerbates the ac&vity of the habit/impulsive system. b. Disable (or “hijack”) ac&vity of the prefrontal (control/ reflec&ve) system. Based on anatomical connec$ons: Anterior insula: role in integra$on of autonomic and visceral informa$on. Posterior insula: role in somatosensory, ves$bular, and motor integra$on. In 1947, Bonin and Bailey said that “the func$ons and affini$es of the insula are totally unknown”. Since then, although much has been learned about this mysterious lobe, s$ll (with the excep$on of a few) contemporary textbooks of neuroanatomy and func$onal neuroimaging research tend to overlook the func$onal importance of this structure: 1. Earlier studies (mid 80’s), mostly in animals, ascribed a role for the insula in condi$oned aversive learning (e.g. condi$oned taste aversion paradigm). 2. Later studies (late 80’s; e.g Berthier): insula and asymbolia for pain in 6 pa$ents; no emo$onal response to painful s$muli. 3. Few studies throughout these years suggested a role for the insula in language. 4. In the early to mid 1990’s, some neuroscien$sts (e.g., Damasio; Craig) began to say that the insula is a “plaaorm for feelings and emo$on.” More specifically, the idea is that the insular cortex plays an important role in the mapping of bodily states and their transla$on into what we subjec$vely and consciously experience as emo$onal feelings. Perhaps related to conscious feelings of urge to take drugs, including the urge to smoke. I. Case Report: Nathan is a right-handed male who lost interest in smoking after suffering from an ischemic stroke at the age of 28. He was 38 years old when we interviewed him, and he was articulate and insightful about his medical and smoking history. He started smoking at the age of 14. At the time of his stroke he was smoking more than 40 unfiltered cigarettes per day. -Before his stroke, he used to enjoy smoking , experience frequent urges to smoke, and he often found it difficult to abstain from smoking in situations where it was inappropriate, such as at work, or when he was so sick and in the hospital. -He was aware of the nega$ve health consequences of smoking before his stroke, though he was not par$cularly concerned about these consequences; he had never tried to quit and he had no inten$on of quieng at the $me of his stroke. Nathan smoked his last cigarette on the evening before his stroke. When asked about his reason for quitting smoking, he stated: “[his] body forgot the urge to smoke.” -He reported that he felt no urge to smoke during his hospital stay, even though he had the opportunity to smoke. -While he came to believe that his stroke was caused in some way by smoking, this was not the reason why he quit; he never actually tried to quit smoking, but spontaneously, he had completely lost interest in smoking. -He has not felt any urge to smoke since he quit. High-resolution structural magnetic resonance images of his brain revealed: 1 1 2 34 A 2 B A B C 3 C 4 • This suggested that the insula plays a critical role in psychological processes, such as conscious urge, that make it difficult to quit smoking and that promote relapse. Conclusion: -What Hilke said yesterday: We speak different languages! We must translate those languages…How different is what I said about urges and homeostatic signals in relation to the insula from that of “loss aversion” for example? -What I say: you need a “translational” branch for this neuro field to survive in marketing and economics and the social sciences: Basic Neuroscience Basic Neuromarke$ng?? Neuroeconomics?? Transla$onal ‐‐‐‐‐‐‐‐ Clinical Neurosecience Transla$onal Marke$ng or Economics