* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Tonoplast and Vacuoles
Cytokinesis wikipedia , lookup
SNARE (protein) wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Protein (nutrient) wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Cell membrane wikipedia , lookup
Homology modeling wikipedia , lookup
Signal transduction wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein structure prediction wikipedia , lookup
Green fluorescent protein wikipedia , lookup
Magnesium transporter wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Western blot wikipedia , lookup
Proteolysis wikipedia , lookup
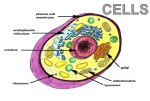
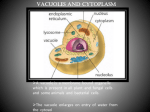
Tonoplast and Vacuoles presented by Tatiana Eremeeva Content 1. 2. 3. 4. 5. 6. Vacuoles. Functions and types. Vacuole biogenesis. Routes towards the vacuole. Tonoplast. Transport processes across the tonoplast. Autophagy and monitoring methods. Vacuoles - (coined from vacuum») – fluid-filled compartments encompassed by a membrane called tonoplast. 10 - Vacuole Plant vacuoles are multifunctional compartments • Storage (proteins, amino acids and organic acids, ions, sugars, pigments) • Digestion (acid hydrolases – proteases, nucleases, glycosidases, lipases) • pH and ionic homeostasis (serve as reservoirs of protons and metabolically important ions) • Defense against microbial pathogens and herbivores (cell wall‐degrading enzymes, phenolic compounds, alkaloids, etc.) • Sequestration of toxic compounds • Pigmentation Plants use vacuoles to produce large cells cheaply • By filling a large volume of the cell with “inexpensive” vacuolar contents plants are able to reduce the cost of making expanded structures such as leaves, which are essentially solar collectors. • The water taken into vacuoles generates turgor pressure which expands the primary cell wall and creates stiff structures in conjunction with the walls. Types of vacuoles Two types of vacuoles are depicted: large protein storage vacuoles (V1) and smaller lytic/autophagic‐type vacuoles (V2) that may be involved in autophagy‐associated programmed cell death. Biochemistry&Molecular Biology of Plants/edited by Buchmann B., Gruissem W., Russel L.J. Routes towards the vacuole • In all eukaryotes, the best described mechanism of exiting the ER is via coat protein complex (COP)II-coated vesicles. The vacuole, together with the plasma membrane, is the most distal point of the secretory pathway, and many vacuolar proteins are transported from the ER through intermediate compartments. • However, past results demonstrate the presence of alternative transport routes from the ER towards the tonoplast, which are independent of Golgi- and post-Golgi trafficking. Moreover, the transport mechanism of the vacuolar proton pumps challenges the current model of vacuole biogenesis, pointing to the ER for being the main membrane source for the biogenesis of the plant lytic compartment. Vacuole biogenesis Corrado Vioti, ER and vacuoles: never been closer (pages 1-5), February 2014 Model for lytic vacuole biogenesis in Arabidopsis. Routes towards the vacuole Comparison of different pathways for the delivery of storage proteins to vacuoles. A – Goldgi-mediated pathway for the delivery of storage proteins to protein storage vacuoles (PSVs) B - Goldgi-mediated pathway in which CCVs bud off the TGN and transfer proteins to the prevacuolar compartment (PVC) before transport to the lytic vacuole C – ER-derived protein bodies filled with prolamins are autophaged by vacuoles. Biochemistry&Molecular Biology of Plants/edited by Buchmann B., Gruissem W., Russel L.J. Tonoplast • - the membrane delimiting plant vacuoles, regulates ion, water and nutrient movement between the cytosol and the vacuolar lumen through the activity of its membrane proteins. • The relative abundance of these proteins and their respective activities/regulation determine the specific function of plant vacuoles. Transport processes across the tonoplast •The regulation of solute passage across the tonoplast can be achieved by modified expression of genes encoding tonoplast proteins. However, also posttranslational modifications of tonoplast proteins represent a fundamental principle in vacular transport regulation and adaptation. •Direct post-translational modifications of the protein allow faster adaptation of protein. Reversible protein phosphorylation by specific protein kinases/phosphatases is a very common post-translational modification. H. Ekkehard, O. Trentmann, Regulation of transport processes across the tonoplast, September 2014 Schematic drawing illustrating the current knowledge on how tonoplast monosaccharide transporters (TMTs) are regulated at the post-translational level. Autophagy • is a degradation pathway that recycles cell materials upon stress conditions or during specific developmental processes (in the lysosome for mammals or in the vacuole for yeast and plants). Assessment of Autophagy Monitoring Methods Model for monitoring autophagy in planta Arabidopsis thaliana roots. Conditions - carbon- and nitrogen-starvation. Goal – evaluate monitoring methods: 1. Green fluorescent protein (GFP)–ATG8 fusion protein. ATG8 (autophagy-related) – protein which is anchored to the autophagosomal membrane, a good marker for the observation of autophagosome movements. 2. Monodansylcadaverine (MDC) - acidotropic fluorescent dye 3. LysoTracker Red (LTR) - acidotropic fluorescent dye Kinetics of autophagic activity can be monitored in planta using GFP–ATG8 transgenic Arabidopsis A.Merkulova, A. Guiboileau, Assessment and Optimization of Autophagy Monitoring Methods in Arabidopsis Roots Indicate Direct Fusion of Autophagosomes with Vacuoles (pages 715-725), February 2014 Monitoring of the induction of autophagy by GFP–ATG8 fusion protein in Arabidopsis roots. Behavior of autophagosomal structures and pre-autophagosomal structures (PAS) revealed by GFP–ATG8 during 24 h of carbon and nitrogen starvation in Arabidopsis. Kinetics of autophagic activity can be monitored in planta using GFP–ATG8 transgenic Arabidopsis •Autophagy is inhibited by wortmannin treatment in Arabidopsis roots. White arrowheads in (B) indicate small dot structures, which are putative pre-autophagosomal structures. •The high level of fluorescence observed at the interstices of root cells in (B) indicates the area of cytoplasm, which is enlarged, probably because the size of the central vacuoles is reduced after the action of wortmannin. A.Merkulova, A. Guiboileau, Assessment and Optimization of Autophagy Monitoring Methods in Arabidopsis Roots Indicate Direct Fusion of Autophagosomes with Vacuoles (pages 715-725), February 2014 Result of Assessment of Autophagy Monitoring Methods • The GFP–ATG8 transgenic line constitutes an excellent method for monitoring autophagy. • These data were compared with plants stained with MDC and LTR. There was no appreciable MDC/LTR staining of small organelles in the root under the induction of autophagy. Extreme caution should therefore be used when monitoring autophagy with the aid of MDC/LTR. Thank you for attention!