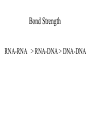* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Method of localizing, either mRNA within the cytoplasm or DNA
Molecular cloning wikipedia , lookup
Comparative genomic hybridization wikipedia , lookup
Gene regulatory network wikipedia , lookup
X-inactivation wikipedia , lookup
Molecular evolution wikipedia , lookup
RNA interference wikipedia , lookup
List of types of proteins wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Non-coding DNA wikipedia , lookup
Polyadenylation wikipedia , lookup
Messenger RNA wikipedia , lookup
Promoter (genetics) wikipedia , lookup
RNA silencing wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Community fingerprinting wikipedia , lookup
Silencer (genetics) wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
SNP genotyping wikipedia , lookup
Gene expression wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epitranscriptome wikipedia , lookup
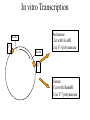
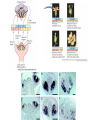
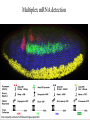
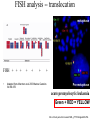
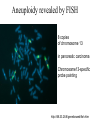
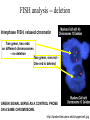
In situ Hybridization (ISH) Method of localizing, either mRNA within the cytoplasm or DNA within the chromosomes, by hybridizing the sequence of interest to a complimentary strand of a nucleotide probe. Nucleic acid hybridization is a fundamental tool in molecular genetics. It takes advantage of the complementary nature of double stranded DNA or RNA to the DNA or even RNA to RNA. Quantitative RNA analysis Technique Advantage Disadvantage In situ hybridization Single cell analysis, In situ spatial analysis, single cell sensitivity Time consuming Single genes Size and quantity Large RNA amounts needed (10-20 µg), single genes Northern Blot Quantitative real-time RTMost quantitative method PCR Single genes, specific primers needed Semi quantitative RT-PCR Relatively quantitative same Laser micro dissection & qRT-PCR Cell specificity Same Poor RNA quality Microarray expression analysis Thousands of genes Thousands of cells analyzed at the same time needed, needs verification Procedure Drea et al. Plant Methods 2005 1:8 doi:10.1186/1746-4811-1-8 Tissue Preparation • Detergents: Triton, SDS (permeabilization) • Proteinase K (permeabilization) • Enzyme neutralization: H2O2 for peroxidase, levamisole for alkaline phosphatase • Acetylation: 0.25 % acetic anhydride in triethanolamine (neutralization of positive charges) • HCl (protein extraction and denaturation of target sequence) Effect of Fixation and Proteinase Digestion 4% paraformaldehyde 2.5 % glutaraldehyde 0.05% 0.02% 0.005% Proteinase K 0.002% Spinal Cord; probe PLP mRNA BM: Non-radioactive in situ hybridization, 1996 Procedure Drea et al. Plant Methods 2005 1:8 doi:10.1186/1746-4811-1-8 Probes • Oligonucleotides: • single stranded DNA (RNase resistant) • Short 20-50 bases (good tissue penetration) • Cover only part of the mRNA, but potentially highly specific • Single stranded DNA (200-600 bases) • Produced by Reverse transcription of RNA or primer amplified • Double stranded DNA • denaturation necessary • only one strand is specific • Less sensitive due to self hybridization • RNA • RNA-RNA hybrids are very stable and RNase resistant • Post hybridization digestion with RNase possible Bond Strength RNA-RNA > RNA-DNA > DNA-DNA • Advantages of RNA probes: – RNA-RNA hybrids are very stable – Tissue can be digested with RNase (dsRNA is not digested) after the hybridization reducing the background – Higher specific activity compared to oligonucleotides – Strand-specific compared to dsDNA probes • Advantages of oligonucleotide probes: – Better tissue penetration – Potentially more specific Procedure Drea et al. Plant Methods 2005 1:8 doi:10.1186/1746-4811-1-8 Probe Labeling • Non-radioactive labeling • Direct: – The use of a nucleotides containing a fluorophore. • Indirect: - Chemical coupling of a modified reporter molecule. The reporter molecule can bind with high affinity to another ligand (Biotin, Digoxigenin). Non-radioactive direct labeling Non-radioactive indirect labeling Non-radioactive indirect labeling • Biotin-streptavidin – Biotin is a naturally occuring vitamin which binds with high affinity (10-14). Highest known interaction in biology. • Digoxigenin – A plant steroid which has a very specific antibody Radioactive indirect labeling Advantage: sensitivity Disadvantage: hazard, long exposure times – – – – S35 P33 P32 H3 medium half-life, good resolution shorter half-life, good resolution short half-life, strong signal, bad resolution long half-life, weak signal/quenching/long exposure times, good cellular resolution Comparison of Labels Radioactive Antigenic (non-radioactive) Cost Availability Storage of label Frequent renewal periodically short lower continuous long Duration of the protocol Storage of probe Sensitivity Long (exposure time) short high rapid Quantification possible long Limited (better with TSA amplification) very difficult Probe labeling • Random primed labeling • PCR • In vitro transcription Probe Labeling • Random prime Labeling In vitro Transcription • Plasmid with T3, T7 or SP6 promoters • Linearization of plasmid DNA by restriction enzyme • In vitro transcription: Plasmid buffer NTP labeled UTP RNA polymerase • DNAse digestion, Phenol/Chloroform extraction and RNA precipitataion In vitro Transcription Antisense: Cut with EcoRI Use T-3 polymerase EcoRI T7 BamHI T3 Sense: Cut with BamHI Use T-7 polymerase Procedure Drea et al. Plant Methods 2005 1:8 doi:10.1186/1746-4811-1-8 Factors Influencing Hybridization • Strand length – The longer the probe the more stable the duplex • Base Composition – The % G:C base pairs are more stable than A:T • Chemical environment – The concentration of Na+ ions stablize – Chemical denaturants (formamide or urea) destablize hydrogen bonds. – Stringency of washes: temperature, salt concentration Controls • Specificity of probe – Sequence analysis – Testing by Northern blot • Negative controls: – – – – RNase treatment pre-hybridization Addition of an excess of unlabeled probe Hybridization with sense probe Tissue known not to express the gene of interest • Positive Controls: – Comparison with protein product – Comparison to probes hybridizing to different part of the same mRNA – Tissue known to express the gene of interest – Poly dT probe or housekeeping gene to check RNA integrity , 23 Jan Ref: Anne Ephrussi, Daniel St Johnston, 2004, Cell, 116 (2), pages 143-152 Multiplex mRNA detection http://superfly.ucsd.edu/%7Edavek/images/quad.html FISH Clinical Applications of FISH 1. Characterization of chromosomal translocations 2. Aneuploidy analysis 3. Cancer specific chromosome deletions FISH analysis -- translocation metaphase FISH + + + • + + + + Adapted from Albertson et al 2003 Nature Genetics 34:369-376 Pre-metaphase acute promyelocytic leukemia Green + RED = YELLOW mti-n.mti.uni-jena.de/~huwww/ MOL_ZYTO/imageAU9.JPG Aneuploidy revealed by FISH 8 copies of chromosome 13 in pancreatic carcinoma Chromosome13-specific probe painting http://68.33.28.8/geneticsweb/fish.htm FISH analysis -- deletion Interphase FISH, relaxed chromatin Two green, two reds on different chromosomes – no deletion Two green, one red – One red is deleted. GREEN SIGNAL SERVE AS A CONTROL PROBE ON A SAME CHROMOSOME. http://lambertlab.uams.edu/images/cell.jpg PRINS-PRimed In Situ labeling • Alternative method for the identification of chromosomes in metaphase spreads or interphase nuclei. • Denatured DNA is hybridized to short DNA fragments, or oligonucleotides followed by primer extension with labeled nucleotides. • Labeling is detected with a fluorescent conjugated antibody. • Limited sensitivity • rapid and low background staining. • Technique can be coupled with PCR (Cycling PRINS) .