* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Pleiotropic effects of the mouse lethal yellow (Ay) mutation
Gene nomenclature wikipedia , lookup
Gene therapy wikipedia , lookup
Human genome wikipedia , lookup
Nucleic acid tertiary structure wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
History of genetic engineering wikipedia , lookup
RNA interference wikipedia , lookup
Non-coding DNA wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genome evolution wikipedia , lookup
SNP genotyping wikipedia , lookup
Gene expression profiling wikipedia , lookup
Frameshift mutation wikipedia , lookup
Gene expression programming wikipedia , lookup
Genomic library wikipedia , lookup
Epitranscriptome wikipedia , lookup
X-inactivation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Molecular Inversion Probe wikipedia , lookup
Genomic imprinting wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
History of RNA biology wikipedia , lookup
Helitron (biology) wikipedia , lookup
RNA silencing wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Microevolution wikipedia , lookup
Non-coding RNA wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Point mutation wikipedia , lookup
Primary transcript wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
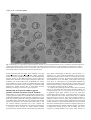
1695 Development 120, 1695-1708 (1994) Printed in Great Britain © The Company of Biologists Limited 1994 Pleiotropic effects of the mouse lethal yellow (Ay) mutation explained by deletion of a maternally expressed gene and the simultaneous production of agouti fusion RNAs David M. J. Duhl1, Mary E. Stevens1, Harry Vrieling1*, Paul J. Saxon2†, Miles W. Miller1, Charles J. Epstein2 and Gregory S. Barsh1‡ 1Department of Pediatrics and Howard Hughes Medical Institute, Stanford University School of Medicine, Stanford, California 94305-5428, USA 2Department of Pediatrics, University of California, San Francisco, California, 94143, USA *Present address: MGC Department of Radiation Genetics and Chemical Mutagenesis, Wassenaarseweg 72, 2333 AL Leiden, The Netherlands †Present address: Department of Laboratory Medicine, Sutter Memorial Hospital, Sacramento, California 95819, USA ‡Author for correspondence SUMMARY Heterozygosity for the mouse lethal yellow (Ay) mutation leads to obesity, increased tumor susceptibility and increased activity of the agouti coat color gene; homozygosity for Ay results in embryonic death around the time of implantation. Although these pleiotropic effects have not been separated by recombination, previous studies have suggested that the dominant and recessive effects result from distinct genetic lesions. Here we use a combination of genomic and cDNA cloning experiments to demonstrate that the Ay mutation is caused by a 120 kb deletion which lies centromere-proximal to the agouti coat color gene. The deletion removes coding but not 5′ untranslated sequences for a ubiquitously expressed gene predicted to encode a protein similar in sequence to an RNA-binding protein, which we named Merc, for maternally expressed hnRNP Crelated gene, but have renamed Raly, since the gene is nearly identical to one reported recently by Michaud et al. (Gene Dev. 7, 1203-1213, 1993). The Ay deletion results in the splicing of Merc/Raly 5′ untranslated sequences to agouti protein-coding sequences, which suggests that ectopic expression of the normal agouti protein by the Ay fusion RNA is responsible for the pleiotropic effects associated with heterozygosity for Ay. We find that Merc/Raly RNA is present in the unfertilized egg and is also transcribed in preimplantation embryos. Using a PCR-based assay to determine the genotype of individual embryos from an Ay/a × Ay/a intercross, we show that, in the absence of zygotic Merc/Raly expression, Ay/Ay embryos develop to the blastocyst stage, but do not hatch from the zona pellucida or form trophoblastic outgrowths. Injection of a Merc/Raly antisense oligonucleotide into non-mutant embryos blocks development prior to the blastocyst stage, and can be rescued by coinjection of a Merc/Raly transgene. These results suggest that maternal expression of Merc/Raly plays an important role in preimplantation development and that its deletion of is sufficient to explain Ay-associated embryonic lethality. INTRODUCTION Pedersen, 1974), an inability of embryos to form outgrowths in culture (Papaioannou, 1988) and failure to hatch from the zona pellucida when placed into diapause (Papaioannou and Gardner, 1992). Although the variety of defects observed may be due to a combination of factors including different genetic backgrounds, variable expressivity of the mutation, and the lack of an independent marker to determine the genotype of individual embryos, these studies suggest that the effects of Ay are most evident around the time of implantation. Because abnormalities are observed in both the TE and the inner cell mass (Papaioannou, 1988), and because Ay/Ay cells cannot be rescued in aggregation chimeras (Barsh et al., 1990), Ay may affect a critical developmental step required prior to implantation. In 1905, mice carrying the lethal yellow mutation (Ay) were used to provide the first evidence that heritable traits could be used to study vertebrate development (Cuénot, 1905). Inviability of Ay/Ay animals, revealed initially by a deviation from Mendelian proportions in the offspring of Ay/− × Ay/− intercrosses, has been the subject of many embryologic studies over the last several decades [reviewed in Magnuson (1986); Silvers (1979)]. Morphologic and ultrastructural abnormalities attributed to the effects of the Ay mutation include degeneration of trophectoderm in the peri-implantation blastocyst (Eaton and Green, 1963), an increased frequency of excluded blastomeres in cleavage-stage embryos (Calarco and Pedersen, 1976; Key words: agouti, lethal yellow, hnRNP, mouse development 1696 D. M. J. Duhl and others Ay is also one of the oldest recognized mutations of the agouti coat color locus, in which alleles associated with the synthesis of yellow pigment in hair follicles are dominant to those associated with the synthesis of black pigment [(reviewed in Silvers (1979)]. Ay in combination with all other agouti locus alleles leads to the exclusive production of yellow pigment and, in addition, has pleiotropic effects, including adult onset obesity and increased somatic growth (Carpenter and Mayer, 1958; Castle, 1941; Danforth, 1927), increased susceptibility to tumor formation (Heston and Deringer, 1947; Heston and Vlahakis, 1961; Vlahakis and Heston, 1963) and premature infertility (Granholm and Dickens, 1986; Granholm et al., 1986). The agouti gene has been cloned recently and is thought to encode a signaling molecule that directs follicular melanocytes to switch from the synthesis of black pigment, eumelanin, to yellow pigment, phaeomelanin (Bultman et al., 1992; Miller et al., 1993). For most agouti genotypes, agouti RNA is expressed only in the skin during the time of phaeomelanin synthesis. However, Ay is associated with the ubiquitous expression of a chimeric RNA that fuses a novel 5′ end, ‘exon 1Ay’, to agouti-coding sequences at its 3′ end (Miller et al., 1993). This suggests that some or all of the pleiotropic effects of Ay are due to ectopic expression of the normal agouti protein. Three separate arguments suggest that lethality of Ay/Ay animals may not result from altered expression of the agouti coat color gene. First, the effects of Ay on coat color are a gainof-function, yet embryonic lethality is most likely a loss-offunction (Barsh and Epstein, 1989; Barsh et al., 1990). Second, the viable yellow (Avy) mutation has similar effects to Ay with regard to obesity and tumor susceptibility, but is not lethal when homozygous (Dickie, 1962; Wolff et al., 1986). Finally, genetic complementation analysis suggests that there are multiple genes close to or within the agouti locus required for embryonic development [(Barsh and Epstein, 1989; Lyon et al., 1985; Russell et al., 1963); reviewed in Siracusa (1991)]. To understand the molecular basis for Ay-associated lethality better, we have performed a combination of molecular and embryologic studies. We find that the Ay chromosome contains a 120 kilobase (kb) deletion that lies centromere-proximal to all previously identified agouti exons. The deletion removes most of the coding sequences for a gene that is likely to encode an RNA-binding protein that we have named Merc, and results in a novel mutational mechanism in which the non-deleted first exon of the Merc gene is spliced to protein-coding sequences of agouti. Based on the developmental defects that we observe both in Ay/Ay embryos and in normal embryos in which expression of the Merc gene has been inhibited experimentally, we conclude that loss of Merc function is responsible for Ayassociated embryonic lethality. MATERIALS AND METHODS Mouse strains and mutations Mice carrying the Ay, AW and A alleles are propagated in our laboratory as C57BL/6J-Ay/a, 129/SvJ-AW/AW and FVB/N-A/A, respectively. The C57BL/6J and 129/SvJ strains were obtained from The Jackson Laboratory and the FVB/N strain was obtained from Taconic (Germantown, NY). Mice carrying the ax mutation were generously provided by Dr Virginia Papaioannou (Tufts University School of Medicine, Boston, MA) as a balanced lethal stock Ay/ax; the ax allele is propagated in our laboratory by backcrossing to C57BL/6J-a/a animals. Genomic cloning, cDNA cloning and DNA probes We have previously described the isolation and structure of cosmid clones that constitute a 60 kb contig containing agouti exons 1B, 1C, 2, 3 and 4 (Miller et al., 1993) (The agouti exon nomenclature used in the present work differs from that used previously; the rationale is summarized in Fig. 1). As it became apparent that alternative isoforms of the agouti cDNAs had 5′ ends that were not contained within these cosmid clones, the contig was extended further 5′ with regard to the direction of agouti transcription by screening a commercial bacteriophage P1 library (Genome Systems, St Louis, MO) with primer pairs derived from agouti cDNAs or genomic clones. The commercial P1 library was constructed from a mouse fibroblast cell line, C127, derived from the RIII mouse strain. The RIII strain carries the A allele, which is consistent with our observation that, in regions of overlap, the P1 clones that we obtained are identical to genomic clones derived from DNA of mice that carry the A allele. The P1 clones P1-24 and P1-25 were isolated using oligonucleotide primers from a subclone of cosD3 (see Fig. 1), 5′-CCTAGGTTTCTCTGTGTCCCC-3′ and 5′-CAGAAGTCCCTGGTAGCTGC-3′, that amplify a 225 base pair fragment containing agouti exon 1B. The P1 clones P1-74 and P1-75 were isolated using oligonucleotide primers from exon 1A, 5′-AGTCTGAGTCCTTGAGCCTC-3′ and 5′-TGGGACCCCCGGTGGTTC-3′, that amplify a 78 base pair fragment. A P1 clone that contains the 5′ end of the Ay-specific cDNA, P1-62, was isolated using the oligonucleotide primers 5′-CCGAGGGGGCGGAAGCGG-3′ and 5′CCGCGCCGAGGTCTGGAG-3′, that lie at the ends of exon 1Ay (see Fig. 1 and Fig. 4) and amplify a 147 base pair fragment. The BamHI restriction maps of the P1 clones were determined by partial digestion and indirect end labeling. Comparison of restriction maps allowed a 160 kb contig to be generated that included all the cosmids and P1-24, P1-25, P1-74, and P1-75 (see Fig. 1). The P1-62 clone is approximately 100 kb in length but does not overlap with any of the cosmid or bacteriophage P1 clones. A 1.0 kb BamHI-EcoRI genomic fragment that contains exon 1Ay was subcloned from P1-62 and is described below as the ‘exon 1Ay probe’. A 1.3 kb XbaI-BamHI genomic fragment that contains exon 1A was subcloned from P1-75 and is described below as the ‘exon 1A probe’. A 1.3 kb XbaI-BamHI fragment subcloned from one of the Merc cDNA clones contains the entire open reading frame and corresponds to residues 403-1720 in Fig. 4A; it is described below as the ‘Merc cDNA probe’. In some experiments, an internal 340 bp SmaI-ClaI fragment was used instead of the 1.3 kb XbaI-BamHI fragment. A 1.0 kb XbaI-EcoRI genomic fragment that contains agouti exon 4 is described below as the ‘exon 4 probe’ [The same probe was described in Miller et al., 1993 as ‘probe d’]. To isolate the Merc cDNA, a commercial cDNA library prepared from the teratocarcinoma cell line PCC4 (Stratagene, San Diego, CA) was screened initially with the exon 1Ay probe. Two clones were recovered, 1.7 kb and 0.8 kb in length; each clone contained exon1Ay sequences followed by an open reading frame at the 3′ end, but neither clone contained a poly(A) sequence or an obvious polyadenylation signal. A 340 basepair (bp) fragment from the 3′ end of the 1.7 kb clone was then used to rescreen the library, and a third clone was isolated, 1.5 kb in length, that contained the entire Merc open reading frame followed by a consensus polyadenylation sequence and a poly(A) tail. RNA expression studies Expression of the Merc RNA in adult tissues was determined by northern hybridization using the 147 bp exon 1Ay fragment as a hybridization probe. For PCR-based studies of embryos, total RNA extracted from approximately 50 mouse eggs, 2-cell embryos, or from Molecular basis of the mouse Ay mutation 1697 25 blastocysts as previously described (Andria et al., 1992) was reverse transcribed using an oligonucleotide primer complementary to sequences in the 3′ end of the Merc open reading frame (see Fig. 4A), 5′-ACCATCATCTCGAATTTG-3′, and then PCR-amplified using the oligonucleotide primers 5′-CAGGGCCGCCTCTTC-3′ and 5′-TGTGCACAGAGCAACCAG-3′. The same group of primers were used to analyze RNA from neonatal skin of AW/AW mice. These primers span at least one Merc intron that separates 5′ untranslated from protein-coding sequences, and produce fragments of 342 bp or 259 bp depending on the presence or absence, respectively, of an alternatively spliced 83 bp exon (see Figs 4, 5). The identity of the products was confirmed both by direct sequencing and by Southern hybridization with the Merc cDNA probe. For embryo expression studies of the Merc/agouti fusion RNA, total RNA was extracted from approximately 50 a/a eggs, or from 25 blastocysts derived from Ay/a males mated to a/a females, in which fusion RNA must arise from the paternal chromosome. The RNA was reverse transcribed using an oligonucleotide primer, 5′-GAGGAATTCACCTTGCCACCTTCTTCATCGA-3′, complementary to sequences within the agouti protein-coding region, and then PCRamplified using the oligonucleotide primers 5′-CAGGGCCGCCTCTTC-3′ and 5′-CGAGTTCATGGAGGAGTTACTCCGC-3′. These primers are located in Merc 5′ untranslated and agouti proteincoding sequence, respectively, and amplify a 386 bp or 229 bp fragment depending on the presence or absence of agouti exons 1A and 1A′ (see Fig. 5). The identity of the products has been confirmed by hybridization with an internal oligonucleotide probe from agouti exon 2, 5′-CAGGAATTCACCATGGATGTCACCCGCCTACTC-3′. Embryologic studies To determine the developmental potential of Ay/Ay embryos, C57BL/6J-Ay/a animals were intercrossed in natural matings, blastocysts were recovered by uterine flushing at 3.5 days post coitum (dpc), and placed into in vitro microdrop culture with DMEM supplemented with 10% fetal calf serum. After 3-4 days, the development of each embryo was assessed with regard to hatching from the zona pellucida, attachment and trophoblastic outgrowth. After phenotypic scoring, the agouti locus genotype of each embryo was determined using a PCRbased assay as previously described (Frohman et al., 1993). The assay determines inheritance of a variant HindIII site, present on the Ay allele and absent from the a allele, located ≤0.2 cM distal to Ay (Siracusa et al., 1987). To determine whether inhibition of Merc gene expression affected development of non-mutant embryos, a sense or antisense oligodeoxynucleotide that spanned the predicted Merc translational initiation site was injected into fertilized 1-cell embryos obtained from superovulated FVB/N or C57BL/6J mice. The oligodeoxynucleotides, 5′-TTCAAGGACATGGTGTTCAC-3′ (antisense) and 5′-GTGAACACCATGTCCTTGAA-3′ (sense), were synthesized with phosphorothioate linkages at the first and last 5 residues to decrease susceptibility to intracellular degradation. An additional control ‘scrambled’ oligodeoxynucleotide with a base composition similar to the antisense oligonucleotide, 5′-TTCTCAGGATGGATGTCACC-3′, was used in some experiments. Based on the experience of Strickland and colleagues with injection of antisense oligodeoxynucleotides into oocytes (Salles et al., 1993), the oligonucleotides were dissolved at 1 µg/µl in 10 mM Tris, pH 7.5; 0.1 mM EDTA, and 3-5 picoliters of the undiluted solution or two serial 50-fold dilutions were microinjected under direct visualization into each embryo, corresponding approximately to 5×108, 1×107, and 2×105 molecules of oligonucleotide injected per embryo. In experiments designed to examine whether expression of Merc could rescue developmental arrest induced by the antisense oligodeoxynucleotide, a Merc or control transgene was coinjected with oligonucleotide at a concentration of 1 µg/ml, which corresponds approximately to 10,000 molecules of 2 kb fragment injected per embryo. The Merc transgene was constructed by fusing the PGK promoter from the vector pNT (Tybulewicz et al., 1991) to a 1.3 kb XbaI-EcoRI fragment that contained the coding sequences of the Merc gene (residues 403-1726 of the Merc cDNA; see Fig. 4); the transgene was then excised as a 1.9 kb EcoRI fragment. A 2 kb control transgene was also used that contained agouti-coding sequences. In a first series of experiments, the embryos were injected with undiluted sense or antisense oligonucleotide, transferred into pseudopregnant foster mothers for 3 days, and then placed into microdrop culture as described above. In subsequent experiments, development of cleavage stage embryos was assessed by transferring injected embryos into microdrop culture and scoring their phenotype with regard to cell number and/or cell lysis on subsequent days. Additional techniques High molecular weight DNA for pulsed field gel electrophoresis was prepared from homogenized spleen tissue and digested with ‘rarecutting’ restriction enzymes as previously described. Contour clamped homogenous electric field (CHEF) electrophoresis was performed using a commercial apparatus (CBS Scientific, San Diego, CA) at 150 V, in 0.5× TBE (45 mM Tris, pH 8, 45 mM boric acic, 1 mM EDTA) and 0.1 µg/ml ethidium bromide, at 6°C. Direction of the electric field was changed according to a ramped program from 40 to 140 sec, using 1 second intervals, and total electrophoresis time was ≈48 hours. Molecular weight estimations were based on a yeast subline derived from strain AB1380, which carries 245 kb, 290 kb, 370 kb, 460 kb, 580 kb, 630 kb, 700 kb, 770 kb, 800 kb, 850 kb and 945 kb chromosomes, and a mouse yeast artificial chromosome (YAC) of 480 kb, which served as a convenient registration marker. In some experiments, ligated concatemers of bacteriophage λ also served as size markers. For the results shown in Table 1, the sizes of individual fragments were first estimated by comparison to yeast markers in adjacent lanes, which provided a ‘window’ of approximately ±40 kb. Exact sizes within this window were then estimated by construction of an internally consistent restriction map for the a and Ay chromosomes. These maps differ by virtue of (1) a 120 kb deletion present only in the Ay allele which is described below; and (2) an approximately 20 kb insertion present only in the a allele between agouti exons 1C and 2 [(Bultman et al., 1992); unpublished observations]. Northern hybridizations, Southern hybridizations and DNA sequence analysis using modified T7 polymerase and dideoxy chain termination were performed according to standard techniques (Sambrook et al., 1989) using radiolabeled nucleotides and hybridization in the presence of 10% dextran sulfate. Reverse transcription and PCR protocols are described in Miller et al. (1993). RESULTS Identification of an Ay-specific first exon of agouti In the course of cloning cDNAs from Ay/ax mice, a chimeric RNA was identified in which a 147 bp Ay-specific sequence (exon 1Ay) was spliced to other exons that contained agouticoding sequences (Miller et al., 1993). The analysis of these chimeric RNAs was complicated by the normally heterogeneous nature of the 5′ end of agouti mRNA, in which we have found three different first exons (1A, 1B, 1C) and one alternatively spliced exon (1A′) in cDNAs from A/A and Aw/Aw mice (Fig. 1). Of 17 cDNA clones isolated from Ay/ax skin RNA, exon 1Ay was fused to agouti exon 1A in six clones, and exon 1Ay was fused to agouti exon 2 in four clones. The remaining seven cDNA clones did not contain exon 1Ay, but instead began with agouti exons 1B or 1C. Fusion of exon 1Ay to agouti exon 2 uses the same splice acceptor used by agouti exons 1A, 1A′, 1B, and 1C. However, fusion of exon 1Ay to agouti exon 1A appears to use a cryptic splice acceptor, 5′- 1698 D. M. J. Duhl and others ccaacatgcag|GAA-3′, since this entire sequence is present normally in exon 1A (see below, Fig. 4). of agouti genotypes Ay/a, Ay/ax, a/a, or a/ax were digested with BssHII, EagI, or SmaI, fractionated on an agarose gel in the 50750 kb size range, transferred to a nylon filter, and hybridized separately with genomic probes that contained exon 1Ay or agouti exons (Fig. 2). The results obtained with a probe that contained agouti exon 1A were identical to those obtained with a probe that contained agouti exon 4 (data not shown). For some enzyme/probe combinations, two fragments were observed with DNA from at The Ay chromosome contains a 120 kb deletion between exon 1Ay and agouti exon 1A To investigate if a chromosomal or subvisible rearrangement caused the Ay mutation, we first determined whether the normal chromosomal location of exon 1Ay lay close to the agouti gene on the distal portion of mouse chromosome 2. Oligonucleotide primers at the ends of exon 1Ay were used to amplify DNA from laboratory mice, a hamster cell line (GM 459), and a mouse/hamster hybrid cell line that carries most of mouse chromosome 2 (ADCT-25). DNA was also amplified from 4 irradiationreduced somatic cell hybrids which have been shown previously to retain mouse DNA markers that lie within approximately 5 cM of the agouti gene (Ollmann et al., 1992). A PCR product specific for exon 1Ay was observed with the laboratory mouse DNA, ADCT-25 DNA, and one radiation hybrid DNA, RH33, but not with GM 459 parent hamster DNA (Fig. 1C). These results indicate that exon 1Ay is derived from a region of mouse chromosome 2 that lies close to the normal agouti gene. Hybridization of exon 1Ay to cloned genomic DNA indicated that exon 1Ay was not located within a 160 kb cosmid and bacteriophage P1 Fig. 1. Genomic and cDNA structure of alternative agouti isoforms and Ay-specific cDNAs. (A) Physical contig (Fig. 1A) that contains location and splicing pattern for agouti isoforms from the wild-type AW allele. Generation of the 160 kb the entire 125 kb agouti gene contig comprising four bacteriophage P1 clones and three cosmid clones is described in Materials and (data not shown). In addition, Methods. Agouti isoforms that begin with exon 1A are expressed only in the ventrum of AW/AW animals a 100 kb mouse genomic P1 but throughout the entire hair growth cycle. In contrast, isoforms that begin with exon 1B or exon 1C are clone that contains exon 1Ay expressed in the dorsum and the ventrum but only during the mid-portion of the hair growth cycle. The did not overlap with the total length of the agouti gene from the beginning of exon 1A to the end of exon 4 is 125 kb. BamHI and agouti contig, suggesting that EcoRI restriction sites are indicated with hash marks above and below the thick horizontal line, exon 1Ay was located at least respectively. Experiments that support this rationale for agouti exon nomenclature including an analysis 150 kb away from agouti exon of cDNAs recovered from AW/AW skin and expression of individual exons in animals that carry different agouti alleles are described in Vrieling et al. (1994). The sequence described as ‘exon 1’ in Miller et al. 1A. To determine more (1993) is described as ‘exon 1C’ in the current work. (B) Exon structure of cDNA clones isolated from precisely the physical relaAy/ax skin. Ten of the seventeen clones began with a 147 bp sequence, ‘exon 1Ay’, specific for the Ay tionship between exon 1Ay mutation. (C) Chromosome mapping of exon 1Ay sequences from DNA of somatic cell hybrid lines. and the agouti gene, we used Genomic DNA from mouse, hamster, a somatic cell hybrid line that contains most of mouse chromosome genomic probes and pulsed 2 (ADCT-25), or different radiation-reduced hybrid lines (RH1, RH33, RH49, and RH150) was PCRfield gel electrophoresis to amplified with exon 1Ay primers as described in Materials and Methods. An aliquot of the amplified construct a long-range restricmaterial was electrophoresed on a 1% agarose gel, which was then stained with ethidium bromide. The tion map of both the Ay and 147 bp exon 1Ay fragment is present in mouse, ADCT-25, and two of the four radiation hybrid lines, non-mutant chromosomes. which demonstrates that exon 1Ay lies on the distal portion of mouse chromosome 2. Characterization of DNA samples from animals the hybrid cell lines is described in Ollman et al. (1992). Molecular basis of the mouse Ay mutation 1699 Fig. 2. Long-range restriction map of the Ay chromosome based on CHEF gel electrophoresis. (A) Restriction map based on the results shown in Fig. 2B and summarized in Table 1. The 120 kb Ay deletion removes an EagI site, but placement of the deletion breakpoints with regard to this site is arbitrary. The locations of exon 1Ay, agouti exon 1A, and agouti exon 4 are indicated with arrows above the corresponding genomic probes, which are described in Materials and Methods. Results obtained for the exon 1A probe and the exon 4 probe were identical. The exon 1Ay probe and the exon 1A probe hybridize to the same Ay-specific fragments whose sizes are listed in Table 1. An EagI site present by sequence analysis in the exon 1Ay probe is not susceptible to digestion in genomic DNA. (B) DNA from Ay/a, Ay/ax, ax/a, or a/a mice was digested with the indicated restriction enzyme, fractionated by CHEF gel electrophoresis as described in Materials and Methods, and a Southern blot was hybridized with the indicated probes. The autoradiogram shown for the Merc cDNA probe was obtained with an internal 340 bp fragment as described in Materials and Methods. Removal of residual probe between hybridizations was checked by autoradiography. The approximately 20 kb insertion present in the a allele between agouti exons 1C and exon 2 does produce a distinguishable difference in mobility compared to fragments from the other alleles. least one heterozygote but not from a/a homozygotes, and comparison of the results allowed us to identify the allelic origin of each fragment (Table 1, Fig. 2). The exon 1Ay and agouti exon 1A probes detect the same a-specific 320 kb and Ay-specific 180 kb BssHII fragments. Similarly, these probes detect the same aspecific 345 kb and Ay-specific 205 kb SmaI fragments. These results indicate that exon 1Ay and agouti exon 1A are located a maximum of 320 kb apart in the a chromosome and a maximum Table 1. High-molecular-weight restriction fragments detected by probes that span the Ay deletion* BssHII probe exon 1Ay Merc cDNA exon 1A; exon 4 Fig. 3. Expression of exon 1Ay sequences in tissues of A/A and Ay/ax mice. Total RNA (30 µg) from the indicated tissues was fractionated by formaldehyde-agarose gel electrophoresis, transferred to a nylon filter, and hybridized with a probe prepared from the 147 bp exon 1Ay sequences. The filter was also hybridized with a Gapd ‘housekeeping’ probe to control for the amount and integrity of the RNA. EagI SmaI a Ay a Ay a Ay 320 320 320 180 − 180 180† 180 220 180† −‡ 180 345 345 345 205 − 205 *Estimated sizes are in kilobases based upon the results shown in Figure 2, and are based on the mobility of yeast chromosomes in adjacent lanes. Probes are described fully in the Materials and Methods and in Figure 2. Comparison of the fragments observed in the a/a and Ay/a DNA samples allowed us to determine their chromosomal origin as “a” or “Ay” with one exception as noted below. †The exon 1Ay probe detects a single EagI fragment of 180 kb in Ay/a DNA. The results obtained with BssHII and SmaI indicate that the probe is present in both alleles. ‡Absence of the 180 kb EagI fragment from the Ay allele is based on the results obtained with BssHII and SmaI. 1700 D. M. J. Duhl and others of 180 kb apart in the Ay chromosome. Furthermore, the difference between the a-specific and Ay-specific fragments is the same for BssHII and SmaI, suggesting that a deletion of 120 kb has occurred in the Ay chromosome. (The deletion is 20 kb less than the difference between the two fragments, 140 kb, due to a 20 kb insertion in the a chromosome that is not present in the Ay chromosome). Similar results were obtained for the enzyme combinations MluI+SalI and NotI+SalI (data not shown). Using a combination of Southern hybridization and DNA Fig. 4. Sequence and splicing pattern of the Merc gene. (A) Composite sequence obtained from three overlapping cDNAs isolated from a teratocarcinoma cell line. The 147 bp exon 1Ay sequence and a consensus polyadenylation signal have a single underline, and the alternatively spliced 83 bp exon has a double underline. The predicted protein sequence as translated from the first methionine codon is shown from residues 483-1370. (B) Diagram of alternative splicing and exon usage in the Ay and non-mutant chromosome as determined by PCRbased studies. The Ay-specific pattern of splicing was determined by comparison of the composite Merc cDNA sequence to the sequences of cDNA clones shown in Fig. 1. Ay-specific cDNAs that contain agouti exon 1A do not contain its entire 5′ end, suggesting that inclusion of exon 1A in Merc/agouti fusion RNAs is due to a cryptic splice acceptor. The non-mutant pattern of splicing was determined by using oligonucleotide primers in 5′ untranslated and potential Merc-coding regions to PCR-amplify cDNA from neonatal AW/AW skin RNA as described in Materials and Methods. Direct sequencing of the gel-purified products (see Fig. 5 for example) revealed two patterns of splicing that differ in their inclusion of an 83 bp region. The genomic sequence following Merc exon 1 is identical to the first 4 nucleotides of this 83 bp region, referred to as Merc exon 1′ in the figure, and contains two potential splice donor sites (see text). (C) Alignment of potential Merc proteincoding sequences with those of the human hnRNP C protein. The RNAbinding domain in hnRNP C consists of a four-stranded beta-pleated sheet with two alpha helices whose positions are indicated. sequence analysis, positions have been determined for the BssHII, EagI, and SmaI sites that are susceptible to cleavage in genomic DNA. The BssHII and EagI sites are located at the 3′ end of agouti exon 4, and the SmaI site is located 25 kb further 3′ (Fig. 2). Applying this information to construct a long-range restriction map indicates that the 120 kb Ay deletion removes a EagI site that lies 200 kb 5′ to agouti exon 4, and that normally separates exon 1Ay and agouti exon 1A (Fig. 2). Molecular basis of the mouse Ay mutation 1701 Exon 1Ay represents the 5′ end of a ubiquitously expressed cDNA that is deleted from the Ay chromosome A simple model to explain both the 120 kb deletion and the chimeric exon 1Ay-agouti mRNA associated with the Ay mutation postulates that the deletion removes the 3′ portion of a gene that begins with exon 1Ay, and juxtaposes the first intron of this gene with 5′ flanking sequences of agouti exon 1A. To determine if exon 1Ay might be contained normally in a gene other than the Ay-specific chimeric mRNA, we used exon 1Ay as a probe to examine RNA from animals of different agouti genotypes by northern hybridization analysis. In most tissues examined of Ay heterozygotes, the exon 1Ay probe detected two RNAs, approximately 1.0 kb and 1.7 kb in length (Fig. 3). In all tissues examined of A/A animals, however, only the 1.7 kb RNA was present. Probes from the agouti cDNA detect only the 1.0 kb RNA in Ay heterozygotes, indicating that the 1.0 kb RNA represents the chimeric Ay-specific RNA, and that the 1.7 kb RNA represents a different gene. To isolate cDNAs representative of the 1.7 kb RNA, we used an exon 1Ay probe to screen a teratocarcinoma cDNA library. Two cDNA clones were identified among 6×105 plaques screened; subsequently, an internal probe from one of these clones was used to rescreen the library and a third clone was isolated. A composite sequence from these three cDNA clones is 1760 bp long including a 35 bp region of poly(A) at the 3′ end (Fig. 4A). The sequence contains a 296 amino acid open reading frame with putative 5′ and 3′ untranslated regions 482 bp and 355 bp in length, respectively. Based on the sequence and expression pattern of the cDNA (see below), we refer to it as Merc, for maternally expressed hnRNP Crelated gene. To investigate the physical relationship of the Merc cDNA to the 120 kb Ay deletion, we hybridized a cDNA fragment that contained the Merc open reading frame to nylon filters in which Ay-specific large restriction fragments had been defined previously (Fig. 2). The Merc open reading frame probe did not hybridize to any Ay-specific fragments, but did detect the same a-specific BssHII, EagI, and SmaI fragments as the exon 1Ay probe, indicating that most or all of the Merc open reading frame was contained in the 120 kb Ay deletion (Fig. 2). The Merc cDNA sequence is alternatively spliced and predicts an RNA-binding protein The entire exon 1Ay sequence, 147 bp in length, is colinear with both the Merc cDNA (residues 244-390) and the corresponding genomic sequence. Surprisingly, the Merc cDNA and the genomic sequence are identical for an additional 4 nucleotides beyond the end of exon 1Ay, at which point they diverge from each other (Fig. 4B). To investigate whether alternative usage of splice donors used normally by the Merc gene might account for this difference, cDNA produced from Aw/Aw skin was PCRamplified with oligonucleotide primers that spanned the interval from exon 1Ay to the Merc open reading frame, and the products were characterized by gel electrophoresis and direct sequencing. Two products were obtained in an approximate 3:1 ratio (see Fig. 5A for example), gel purified and sequenced using an internal primer. The major (≈75%) product was identical to the Merc cDNA; however, the minor (≈25%) product was missing an 83 bp region (described as Merc exon 1′, see Fig. 4B) that began at the end of exon 1Ay and that did not affect the Merc open reading frame. Although further genomic sequence analysis will be required to distinguish whether the major Merc cDNA product is produced by an 83 nt exon that uses the same splice donor as that used by exon 1Ay, or by a 79 nt exon that uses a different splice donor from the one used by exon 1Ay, these results indicate that the Merc gene normally produces two isoforms, at least one of which uses the same splice donor used by exon 1Ay. The methionine codon that initiates the 296 amino acid Merc open reading frame at nucleotide residue 483 is the first ATG in the Merc cDNA sequence, and lies within a region, 5′ACCATGTCC-3′, predicted to function as a site for eukaryotic translational initiation. Sequence similarity searches of computerized databases revealed 43% identity with the human protein heterogeneous nuclear ribonucleoprotein particle C (hnRNP C) (Swanson et al., 1987). A comparison (Fig. 4C) indicates that most of the similarity lies within a 90 residue amino terminal region (80% identity), which includes the RNAbinding domain (Gorlach et al., 1992; Wittekind et al., 1992). In both Merc and hnRNP C, the predicted RNA-binding domain is followed by a proline-rich domain and the carboxyl terminus contains a high proportion of acidic residues. The most striking difference between the two sequences is their predicted isoelectric points, 4.84 for hnRNP C, and 10.26 for Merc. Merc is normally expressed in the unfertilized egg and the blastocyst Because the Merc open reading frame is contained within the Ay-associated 120 kb deletion (see above), we considered 1702 D. M. J. Duhl and others whether failure to express Merc might be responsible for Ayassociated embryonic lethality. Although hnRNP C and other hnRNPs are well characterized biochemically, little is known regarding their expression in development. To investigate whether Merc was expressed at or prior to the time at which Ay/Ay embryos are thought to exhibit morphologic abnormalities, RNA from 25-50 eggs, 2-cell embryos, or blastocysts was reverse transcribed and PCR-amplified with the same oligonucleotide primers used to analyze Merc expression in skin (Fig. 4B). Gel electrophoresis and Southern hybridization of the amplified products indicated that both forms of the Merc RNA (with and without Merc exon 1′, Fig. 4B) were present at relatively high levels in unfertilized eggs and relatively low levels in blastocysts (Fig. 5A). To determine whether the blastocyst RNA was likely to represent embryonic synthesis and/or degradation of maternal stores, expression of the Merc/agouti fusion RNA was examined in embryos derived from an Ay/a male crossed to an a/a female, in which the only source of the fusion RNA is the Ay chromosome provided by the male genome. The fusion RNA was detectable in Ay/a blastocysts but not in a/a blastocysts or in a/a unfertilized eggs (Fig. 5B), suggesting that Merc RNA is expressed embryonically as well as maternally. Ay/Ay embryos fail to hatch in vitro Previous observations of embryos derived from Ay/− × Ay/− intercrosses have reported a wide range of developmental defects in 25% of the animals compared to control crosses (Calarco and Pedersen, 1976; Eaton and Green, 1963; Johnson and Granholm, 1978; Papaioannou, 1988; Papaioannou and Gardner, 1992; Pedersen, 1974). However, most of these studies are complicated by the inability to determine the genotype of individual embryos. To determine if Ay/Ay embryos exhibited morphologic or developmental abnormalities prior to implantation, 3.5 dpc blastocysts were recovered from Ay/a × Ay/a matings, placed into culture and their development was scored after 2-3 days. The genotype of each embryo was determined using a PCR-based assay to evaluate the presence of a variant HindIII site at the Emv-15 locus, which is located less than 0.2 cM distal to Ay (Siracusa et al., 1987). Of 92 embryos evaluated, 65 hatched and formed trophoblastic outgrowths, while 27 never hatched (Table 2). Based on the identity of the variant HindIII site, 14/27 that did not hatch were Ay/Ay, while no Ay/Ay embyros were found among the 65 embryos that did hatch. Inhibition of Merc expression blocks development of cleavagestage embryos Although the expression pattern of the Merc gene is consistent with a role in Ay-associated embryonic lethality, the 120 kb deletion in the Ay chromosome may remove additional genes that contribute to the failure of Ay/Ay embryos to develop past the blastocyst stage. To separate effects due to loss of Merc expression from other effects due to the Ay-associated deletion, we examined the development of non-mutant embryos injected with Merc antisense oligodeoxynucleotides. In a first series of experiments, approximately 5×108 molecules of sense or antisense oligonucleotides that spanned the predicted translational initiation site of Merc were injected into 1-cell fertilized embryos and the embryos were transferred into the oviducts of pseudopregnant females. At 3.5 dpc, embryos were recovered from the uterus, placed into in vitro culture and their development compared to each other and to uninjected embryos. Of 169 1-cell embryos injected with the Merc antisense oligonucleotide, only 76 (45%) were recovered from the uterus compared to 87% and 77% for the uninjected and sense controls, respectively (Fig. 6). Nearly all of the embryos injected with the antisense oligonucleotide appeared morphologically abnormal at 3.5 dpc; most had not developed beyond the 4-cell stage and many appeared to Fig. 5. Embryonic expression of the Merc and the Merc/agouti fusion RNAs. (A) After reverse transcription of RNA from approximately 25-50 C57BL/6J-a/a unfertilized eggs, 2cell embryos, or blastocysts, Merc cDNA was PCR-amplified using oligonucleotide primers as indicated in the diagram and as described in Materials and Methods. Three fragments were evident after ethidium bromide staining or hybridization with the Merc cDNA probe as shown. Direct sequencing of the gel-purified products indicated that the lower fragment was the 255 bp product (which lacked the 83 bp exon shown in Fig. 4B), and that both upper fragments were different forms of the 342 bp product (which contained the 83 bp exon). (B) After reverse transcription of RNA from approximately 25-50 C57BL/6J-a/a unfertilized eggs, blastocysts or Ay/a blastocysts in which the Ay chromosome was provided by the male parent, Merc/agouti fusion cDNA was PCR-amplified using oligonucleotide primers as indicated in the diagram and as described in Materials and Methods. The predominant fragment detected by hybridization to an internal oligonucleotide probe from agouti exon 2 is a 386 bp product that contains agouti exons 1A and 1A′. Molecular basis of the mouse Ay mutation 1703 Table 2. Development in vitro of embryos derived from an Ay/a×Ay/a intercross* Genotype† Hatched Unhatched Total a/a Ay/a Ay/Ay Total 24 4 28 (30%) 41 9 50 (54%) 0 14 14 (15%)‡ 65 27 92 *3.5 dpc blastocysts were obtained as described in Materials and Methods. After 3-4 days in culture, individual embryos were scored with regard to hatching from the zona pellucida and the formation of trophoblastic outgrowths. †The agouti genotype of each embryo was inferred from the identity of a variant HindIII site at the closely linked Emv-15 locus as described in Frohman et al. (1993). ‡The recovery of Ay/Ay embryos is not significantly different from a 1:2:1 distribution at the 95% confidence level (χ2=4.95, 2 degrees of freedom). molecules of antisense oligonucleotide, coinjection of the Merc transgene did not significantly affect the proportion of embryos developing into blastocysts, 9.5%, when compared to antisense oliogonucleotide alone, 2.8% (P=0.24, Table 3). However, with 1×107 molecules of antisense oligonucleotide, coinjection of the Merc transgene resulted in partial rescue; 53% of coinjected embryos developed into blastocysts compared to 27% injected with antisense oligonucleotide alone (P=0.017, Table 3). Finally, coinjection of the Merc transgene completely rescued embryos injected with 2×105 molecules of antisense oligonucleotide; 76% of coinjected embryos developed into blastocysts compared to 12% injected with antisense oligonucleotide alone (P=0.0001, Table 3). DISCUSSION exhibit lysis of individual blastomeres compared to embryos injected with the sense oligonucleotide (Fig. 7). By 5.5 dpc, only 3 of the 76 embryos (4%) injected with the Merc antisense oligonucleotide and recovered from the uterus had developed into blastocysts, compared to 61% and 23% for the uninjected and sense, respectively (Fig. 6). The 3 blastocysts that did develop from embryos injected with the Merc antisense oligonucleotide were probably abnormal, since none of them hatched from the zona pellucida by 6.5 dpc, compared to 87% and 69% for the uninjected and sense controls, respectively (Fig. 6). In a second series of experiments designed to examine the time course and concentration dependence of developmental arrest induced by injection of the Merc antisense oligonucleotide, 1-cell fertilized embryos were injected with approximately 5×108, 1×107, or 2×105 molecules of sense or antisense oligonucleotide, transferred immediately into microdrop culture, and their development was scored with regard to cell number and/or blastomere lysis for the next 4 days. At the two higher concentrations, an effect of the antisense compared to the sense oligonucleotide was apparent 1 day after injection— blastomere lysis was observed in 22% or 19% of the embryos injected with 5×108 or 1×107 molecules of antisense oligonucleotide, respectively, but in none of the embryos injected with the same amount of sense oligonucleotide (Fig. 8). By 4.5 dpc, an effect of the antisense compared to the sense oligonucleotide was apparent in embryos injected with all three oligonucleotide concentrations—only 2.8%, 29%, or 12% of embryos injected with 5×108, 1×107, or 2×105 molecules of antisense oligonucleotide, respectively, had developed into blastocysts compared to 18%, 90%, or 73%, of embryos injected with the corresponding amount of sense oligonucleotide (Fig. 8; Table 3). The effect of the antisense oligonucleotide at 4.5 dpc was also apparent when compared to embryos injected with a control ‘scrambled’ oligonucleotide (Table 3). In a third series of experiments designed to examine whether developmental arrest induced by the Merc antisense oligonucleotide could be rescued by expression of Merc, embryos were coinjected with different amounts of antisense oligonucleotide and a Merc transgene, and their development was compared by scoring the proportion of injected embryos that had developed into blastocysts by 4.5 dpc. With 5×108 The Ay mutation has intrigued developmental biologists for 90 years due to its pleiotropic effects on coat color, regulation of body weight, tumor susceptibility and embryonic development. Here we show that a 120 kb deletion in the Ay chromosome removes potential protein-coding sequences for a maternally expressed gene, Merc, that we have isolated from a teratocarcinoma cDNA library, and which is highly similar to the human hnRNP C gene. The deletion also results in the production of a chimeric RNA in which ubiquitously expressed Merc 5′ untranslated sequences are fused to protein-coding sequences of the agouti gene (Fig. 9). During the preparation of this manuscript, isolation of a gene nearly identical to Merc was reported by Michaud et al. (1993). These investigators used exon 1Ay sequences to isolate a 1.4 kb cDNA from a postimplantation mouse embryo cDNA library which had the potential to encode a gene they named Raly, for RNA-binding Fig. 6. Development of embryos injected with Merc antisense or sense oligonucleotides between 3.5 dpc and 6.5 dpc. Fertilized 1-cell embryos were injected with approximately 5×108 molecules of oligonucleotide as described in Materials and Methods. After 3 days in a pseudopregnant foster mother, the injected embryos and a population of uninjected control embryos were flushed from the uterus, placed into in vitro culture, and their development was scored for the next 3 days. The exact number of embryos in each class are indicated in parentheses. 1704 D. M. J. Duhl and others A (Sense) B (Antisense) Fig. 7. Morphology of 3.5 dpc embryos injected with Merc antisense or sense oligonucleotides at 0.5 dpc. (A) Embryos injected with the sense oligonucleotide exhibit a range of normal development from compacted morula to blastocysts. Approximately 30% of the embryos exhibit developmental abnormalities that may be a non-specific result of the injection (see Table 3). (B) Developmental abnormalities are present in nearly all the embryos injected with the antisense oligonucleotide. protein associated with the lethal yellow mutation. Two differences between the Merc and the Raly cDNA sequences would not produce significant changes in the encoded protein and may be due to the different sources used to isolate the cDNA clones; therefore, we will refer to this gene as Raly, subsequently. Our results confirm those of Michaud et al. (1993) and, in addition, provide a model for understanding the origin of the Merc/agouti fusion (Fig. 9). Finally, our expression and embryologic studies suggest that Ay-associated recessive lethality is caused by loss of Merc gene expression. Relationship of Ay-specific cDNAs to agouti genomic structure and origin of the Ay mutation Initial studies of agouti cDNAs suggested the potential for a complex genomic structure in which alternative isoforms were produced with different 5′ ends, but their significance was not clear until recently. Molecular cloning and expression studies using RNA from animals that carry the wild-type light-bellied agouti (AW) allele have now shown that agouti exons 1A and 1A′ initiate isoforms expressed only in the ventrum but throughout the entire hair growth cycle, and are located 100 kb 5′ of agouti exons 1B and 1C, which initiate isoforms expressed in the dorusm and the ventrum but only during the active phase (mid-anagen) of the hair cycle (see Figs 1, 9; Vrieling et al., 1994). Isoforms produced by the agouti (A) allele always begin with exons 1B or 1C because the ventralspecific promoter is inactive. Of seventeen cDNA clones that we isolated from Ay/ax skin RNA, seven began with agouti exons 1B or 1C. Since there is very little RNA detectable by northern hybridization analysis from the ax allele, these seven cDNA clones are likely to represent normal activity of the hair cycle-specific promoter from the Ay allele. In contrast, the remaining ten cDNA clones that we isolated from Ay/ax skin RNA were Merc/agouti fusions; of these, six contained agouti exon 1A or agouti exons 1A and 1A′ due to an apparent cryptic splice acceptor in exon 1A, while four resulted from splicing of Merc 5′ sequences directly to agouti exon 2. Because none of the cDNA clones that we isolated began with agouti exon 1A, the ventral-specific promoter is probably not active in the Ay allele. The Ay mutation was one of many coat color variants bred by mouse fanciers in the 19th century; therefore its origins and relationships to current inbred strains are somewhat obscure. However, haplotype analysis of closely linked molecular markers indicates that the Ay mutation is related more closely to the A and AW alleles than to the nonagouti (a) allele (Winkes et al., 1993). Thus, absence of Molecular basis of the mouse Ay mutation 1705 ventral-specific promoter activity from the Ay allele may be explained because (1) the 120 kb Ay deletion has removed cisregulatory sequences required for activity of the ventralspecific promoter; or (2) the allele of origin for the 120 kb Ay deletion was A; therefore the ventral-specific promoter was already inactive. Functional analysis of the ventral-specific promoter in combination with physical mapping of the Ay deletion breakpoints will help to distinguish between these possibilities. We detected the Merc RNA in northern hybridization experiments reported here using a probe that contained exon 1Ay. In a similar experiment described in Miller et al. (1993), the 1.7 kb Merc RNA was not evident because the ‘Ay-specific probe’ used in these experiments comprised agouti exons 1A and 1A′. As described above, these exons are found normally only in ventral-specific agouti isoforms; therefore, exons 1A and 1A′ appear specific for the Ay cDNA when analyzed opposite agouti alleles where the ventral-specific promoter is inactive such as A or a. Exons 1A and 1A′ were also present in several Ay-specific cDNAs isolated by Michaud et al. (1993). Pleiotropy associated with heterozygosity for Ay The Merc/agouti fusion RNAs and the normal Merc RNA are both expressed in every adult tissue that we have examined, suggesting that the 120 kb Ay deletion has not altered regulatory elements which normally direct Merc expression. The fusion RNAs are capable of encoding a normal agouti protein, suggesting that ectopic expression of this protein is responsible for the pleiotropic effects associated with heterozygosity for Ay (Carpenter and Mayer, 1958; Castle, 1941; Danforth, 1927; Granholm and Dickens, 1986; Granholm et al., 1986; Heston and Deringer, 1947; Heston and Vlahakis, 1961; Vlahakis and Heston, 1963). Alternative explanations for the pleiotropic effects, including the production of a novel fusion protein or the action of closely linked genes, are unlikely since the pleiotropic phenotype is observed in mice that carry the viable yellow (Avy) mutation (Dickie, 1962; Wolff et al., 1986), in which an agouti fusion RNA is also expressed ubiquitously (Yen et al., 1994), and in which mutant animals can be compared to coisogenic non-mutant littermates. There are several possibilities to explain how ectopic expression of the normal agouti protein could lead to obesity and increased tumor susceptibility, including antagonism of melanocortin receptors sequestration of melanocortins, or activation of an as yet unidentified agouti receptor (Conklin and Bourne, 1993; Jackson, 1993) [reviewed in Yen et al. (1994)]. Experiments with adipose tissue transplants (Meade et al., 1979) and aggregation chimeras (Barsh et al., 1990) suggest Table 3. Development in vitro of 1-cell embryos injected with Merc antisense or control oligonucleotides, with or without a Merc expression vector* Dilution of Oligonucleotide† Injected DNA 1 (1) Control “scrambled” 5/12 (42%) (2) Sense 4/22 (18%) (3) Antisense 1/36 (2.8%) Chi-square vs. (1+2)‡ 6.20; p=0.013 (4) Antisense + Merc 2/21(9.5%) transgene Chi-square vs. (1+2) 1.4; p=0.24 Chi-square vs. (3) 0.24; p=0.63 (5) Antisense + control transgene Fig. 8. Development of embryos injected with Merc antisense or sense oligonucleotides between 0.5 dpc and 4.5 dpc. Fertilized 1-cell embryos were injected with approximately 5×108, 1×107, or 2×105 molecules of sense or antisense oligonucleotide and placed into microdrop culture as described in Materials and Methods. Embryos were scored daily and categorized within the five conditions shown on the right. Bars represent percentages (shown on the abscissa) of embryos in each condition on each day. The results shown represent the sum of two different experiments, in which a total of 150 embryos were injected with 5×108, 1×107, or 2×105 molecules of antisense or sense oligonucleotide. 1:50 1:2500 8/14 (57%) 19/21 (90%) 14/51 (27%) 18.6; p=0.0001 25/47 (53%) 10/12 (83%) 11/15 (73%) 3/25 (12%) 20; p=0.0001 16/21 (76%) 4.0; p=0.046 5.7; p=0.017 7/21 (33%) 0.05; p=0.83 16.8; p=0.0001 *Data are given as No. of embryos developing into blastocysts by 4.5 dpc/No. of embryos injected (percent). The data shown include the results depicted in Figure 8 and represent the results of four separate experiments. †A solution of 10 mM Tris, pH7.5; 0.1mM EDTA that contained either oligonucleotide or oligonucleotide + linear DNA was injected into each embryo. Therefore, dilutions of 1, 1:50, and 1:2500 correspond approximately to 5×108, 1×107, and 2×105 molecules of oligonucleotide injected per embryo. The Merc and control transgenes were maintained at a concentration of 1 µg/ml, which corresponds approximately to 10,000 molecules of 2 kb fragment injected per embryo. ‡The differences between development of embryos injected with control (1) and sense (2) oligonucleotides were not significant at a 95% confidence level, and have therefore been combined in comparison to the antisense (3), and antisense + Merc transgene (4) injections. The Chi-square values are corrected for continuity. 1706 D. M. J. Duhl and others that Ay-induced obesity is cell non-autonomous; however, parabiosis experiments demonstrate that the factors that mediate Ay-induced obesity are not transmitted through the circulation (Wolff, 1963). Thus, like the action of agouti in hair follicles (Silvers, 1958, 1961), agouti-induced obesity may be mediated by a diffusible factor with a limited radius of action. 1988; Papaioannou and Gardner, 1979, 1992; Pedersen, 1974). Although these previous studies were complicated by an inability to determine the genotype of morphologically abnormal embryos, our results confirm that Ay/Ay embryos can develop to the blastocyst stage yet fail to hatch when placed into culture or into diapause. Ay-associated embryonic lethality Function of the Merc gene In contrast to the pleiotropic effects associated with heterozyEmbryos injected with Merc antisense oligonucleotides rarely gosity for Ay, several observations have suggested that lethality develop to the blastocyst stage, and thus are more severely of Ay/Ay embryos is not due to altered expression of the agouti affected than Ay/Ay embryos. Injection of control sense or gene (Barsh and Epstein, 1989; Barsh et al., 1990; Lyon et al., ‘scrambled’ oligonucleotides did not prevent blastocyst devel1985). Our results provide a molecular explanation for these opment, which suggests that the effects that we observed after observations, since the 120 kb Ay deletion produces two injection with Merc antisense oligonucleotides are likely to be distinct genetic lesions; a neomorphic effect caused by ubiqdue to loss of Merc gene expression. Although expression of uitous expression of the Merc/agouti fusion RNA, and an RNA in eggs and in embryos is difficult to quantitate, our amorphic effect due to the loss of Merc. Although the results clearly indicate that Merc RNA is present in unfertilexpression of other genes besides Merc and agouti may be ized eggs, possibly at much higher amounts than in blastocysts. affected by the Ay deletion, injection of 1-cell embryos with Thus, maternal Merc RNA and/or protein in the Ay/Ay embryos Merc antisense oligonucleotides prevents development to the may provide a ‘sparing’ effect compared to non-mutant blastocyst stage, suggesting that loss of Merc gene expression embryos injected with Merc antisense oligonucleotides. These is sufficient to explain Ay-associated lethality. Of three other results are also consistent with an inability to rescue Ay/Ay cells recessive lethal agouti mutations that have been studied, only in aggregation chimeras (Barsh et al., 1990), and suggest that one, nonagouti lethal (al), fails to complement Ay (Lyon et al., loss of Merc gene expression in preimplantation embryos is a 1985). Genetic and molecular studies of al indicate it is a cell-lethal. It remains to be determined, however, whether deletion that removes several genes (Barsh and Epstein, 1989; Merc expression is required for cell viability later in developLyon et al., 1985), and mapping of the al proximal breakpoint ment, especially given the evidence that Ay/Ay inner cell mass may help to refine further the physical boundaries of the Ay can be rescued in blastocyst injection chimeras (Papaioannou recessive lethal complementation group, and to delimit regions and Gardner, 1979). It is possible that the Merc gene product required for normal Merc gene expression. plays a specialized role in metabolism of maternally stored Functional and/or immunologic assays for Merc will be RNAs, and that, later in development, general roles for hnRNA required to determine the degree to which its expression is processing are assumed by other hnRNPs. Although oogenesis affected by injection of antisense oligonucleotides but, in proceeds differently in mammals and invertebrates, it is general, inhibition of gene expression using antisense intriguing that a recently described mutation that interferes approaches is usually not absolute, and preimplantation develwith dorsal/ventral axis formation during Drosophila opment may therefore be sensitive to dosage of the Merc oogenesis, squid (Kelley, 1993), encodes one of the major protein. Heterozygosity for Ay has no obvious effect on Drosophila hnRNPs (Kelley, 1993; Matunis et al., 1992). viability; however, increased expression of agouti in these animals may mask subtle effects on growth caused by a 50% reduction in Merc gene expression since a/al animals are slightly reduced in size compared to a/a animals (unpublished observations). Subtle effects of reduced Merc gene dosage on growth and development may also account for the observation that Ay/a cells are recovered less frequently than expected in aggregation chimeras between A/A embryos and those derived from an Ay/a × Ay/a intercross (Barsh et al., 1990). Previous studies of embryos derived from Ay/− × Ay/− intercrosses have suggested that homozygosity for Ay may lead to a wide Fig. 9. Diagram of the 120 kb Ay deletion and its effects on Merc and agouti expression. The exon variety of developmental defects structure of Merc has not yet been determined and is indicated with dotted lines. As described in (Calarco and Pedersen, 1976; Eaton the text, the Ay allele can give rise to normal agouti mRNAs initiated from the hair cycle-specific and Green, 1963; Johnson and promoter. However, it is not clear whether the absence of agouti mRNAs initiated with the ventral-specific promoter is due to the effects of the deletion or to the allele of origin. Granholm, 1978; Papaioannou, Molecular basis of the mouse Ay mutation 1707 The predicted Merc protein is 80% identical to human hnRNP C over the first 90 amino acids. This region contains a well-characterized RNA-binding domain found in many hnRNPs which assembles into a four-stranded beta-pleated sheet and binds to homopolymeric uridine tracts in vitro (Burd et al., 1989; Gorlach et al., 1992; Wittekind et al., 1992). Although the carboxyl terminus of the predicted Merc protein is very different from hnRNP C, it contains several regions rich in glycine and serine, which are found in other hnRNPs (Burd et al., 1989), and the Merc protein product is likely to function as an hnRNP in cells. In vitro and cell culture experiments suggest that hnRNPs mediate processing and/or transport of newly synthesized nuclear RNA in most eukaryotic cells. Embryonic lethality due to absence of Merc expression in mice is somewhat surprising given the large number of hnRNPs in most organisms, and the potential for functional redundancy. Experiments directed at identification and isolation of Merc homologs in other organisms may help shed light on the possibility that Merc encodes an hnRNP used specifically for the processing and/or transport of maternally expressed RNA. This work was supported in part by HG-00377 from the National Institutes of Health. H. V. is supported by the J.A. Cohen Institute, Leiden, The Netherlands. G. S. B. is an Assistant Investigator of the Howard Hughes Medical Institute. REFERENCES Andria, M. L., Barsh, G. S. and Levy, S. (1992). Expression of TAPA-1 in preimplantation mouse embryos. Biochem. Biophys. Res. Commun. 186, 1201-1206. Barsh, G. S. and Epstein, C. J. (1989). Physical and genetic characterization of a 75-kilobase deletion asssociated with al, a recessive lethal allele at the mouse agouti locus. Genetics 121, 811-818. Barsh, G. S., Lovett, M. and Epstein, C. J. (1990). Effects of the lethal yellow (Ay) mutation in mouse aggregation chimeras. Development 109, 683-690. Bultman, S. J., Michaud, E. J. and Woychik, R. P. (1992). Molecular characterization of the mouse agouti locus. Cell 71, 1195-1204. Burd, C. G., Swanson, M. S., Gorlach, M. and Dreyfuss, G. (1989). Primary structures of the heterogeneous nuclear ribonucleoprotein A2, B1, and C2 proteins: a diversity of RNA-binding proteins is generated by small peptide inserts. Proc. Nat. Acad. Sci. USA 86, 9788-9792. Calarco, P. G. and Pedersen, R. A. (1976). Ultrastructural observations of lethal yellow (Ay/Ay) mouse embryos. J. Embryol. Exp. Morph. 35, 73-80. Carpenter, K. J. and Mayer, J. (1958). Physiologic observations on yellow obesity in the mouse. Am. J. Physiol. 193, 499-504. Castle, W. E. (1941). Influence of certain coat color mutations on body size in mice, rats, and rabbits. Genetics 26, 177-191. Conklin, B. R. and Bourne, H. R. (1993). Mouse coat colour reconsidered. Nature 364, 110. Cuénot, L. (1905). Les races pures et leurs combinasisons chez les souris (4me note). Arch. Zool. Exp. Gen., 4e ser. 3, cxxiii-cxxxii. Danforth, C. H. (1927). Hereditary adiposity in mice. J. Hered. 18, 153-162. Dickie, M. M. (1962). A new viable yellow mutation in the house mouse. J. Hered. 53, 84-86. Eaton, G. J. and Green, M. M. (1963). Giant cell differentiation and lethality of homozygous yellow mouse embryos. Genetica 34, 155-161. Frohman, M. A., Martin, G. R., Cordes, S. P., Halamek, L. P. and Barsh, G. S. (1993). Altered Rhombomere-Specific gene expression and hyoid bone differentiation in the mouse segmentation mutant, kreisler (kr). Development 117, 925-936. Gorlach, M., Wittekind, M., Beckman, R. A., Mueller, L. and Dreyfuss, G. (1992). Interaction of the RNA-binding domain of the hnRNP C proteins with RNA. EMBO J. 11, 3289-3295. Granholm, N. H. and Dickens, G. A. (1986). Effects of reciprocal ovary transplantation on reproductive performance of lethal yellow mice (Ay/a; C57BL/6J). J. Reprod. Fert. 78, 749-753. Granholm, N. H., Jeppesen, K. W. and Japs, R. A. (1986). Progressive infertility in female lethal yellow mice (Ay/a; strain C57BL/6J). J. Reprod. Fert. 76, 279-287. Heston, W. E. and Deringer, M. K. (1947). Relationship between the lethal yellow (Ay) gene of the mouse and susceptibility to spontaneous pulmonary tumors. J. Nat. Cancer Inst. 7, 463-465. Heston, W. E. and Vlahakis, G. (1961). Influence of the Ay gene on mammary-gland tumors, hepatomas, and normal growth in mice. J. Nat. Cancer Inst. 26, 969-982. Jackson, I. J. (1993). Molecular genetics. Colour-coded switches [news]. Nature 362, 587-588. Johnson, L. L. and Granholm, N. H. (1978). In vitro analysis of pre- and early postimplantation development of lethal yellow (Ay/Ay) mouse embryos. J. Exp. Zool. 204, 381-390. Kelley, R. L. (1993). Initial organization of the Drosophila dorsoventral axis depends on an RNA-binding protein encoded by the squid gene. Genes Dev. 7, 948-960. Lyon, M. F., Fisher, G. and Glenister, P. H. (1985). A recessive allele of the mouse agouti locus showing lethality with yellow, Ay. Genet. Res. 46, 95-99. Magnuson, T. (1986) Mutations and chromosomal abnormalities: How are they useful for studying genetic control of early mammalian development? In Experimental Approaches to Mammalian Embryonic Development. (ed. J. Rossant and R. A. Pedersen). pp 437-474. Cambridge: Cambridge University Press. Matunis, E. L., Matunis, M. J. and Dreyfuss, G. (1992). Characterization of the major hnRNP proteins from Drosophila melanogaster. J. Cell Biol. 116, 257-269. Meade, C. J., Ashwell, M. and Sowter, C. (1979). Is genetically transmitted obesity due to an adipose tissue defect? Proc. R. Soc. Lond. B. 205, 395-410. Michaud, E. J., Bultman, S. J., Stubbs, L. J. and Woychik, R. P. (1993). The embryonic lethality of homozygous lethal yellow mice (A(y)/A(y)) is associated with the disruption of a novel RNA-Binding protein. Gene Dev. 7, 1203-1213. Miller, M. W., Duhl, D. M. J., Vrieling, H., Cordes, S. P., Ollmann, M. M., Winkes, B. M. and Barsh, G. S. (1993). Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the Lethal-Yellow mutation. Gene Dev. 7, 454-467. Ollmann, M. M., Winkes, B. M. and Barsh, G. S. (1992). Construction, Analysis, and Application of a Radiation Hybrid Mapping Panel Surrounding the Mouse Agouti Locus. Genomics 13, 731-740. Papaioannou, V. E. (1988). Investigation of the tissue specificity of the lethal yellow (Ay) gene in mouse embryos. Dev. Genet. 9, 155-165. Papaioannou, V. E. and Gardner, R. L. (1979). Investigation of the lethal yellow (Ay/Ay) embryo using mouse chimaeras. J. Embryol. Exp. Morph. 52, 153-163. Papaioannou, V. E. and Gardner, R. L. (1992). Effects of diapause on lethal yellow (Ay/Ay) mouse embryos. J. Exp. Zool. 263, 309-315. Pedersen, R. A. (1974). Development of lethal yellow (Ay/Ay) mouse embryos in vitro. J. Exp. Zool. 188, 307-320. Russell, L. B., McDaniel, M. N. C. and Woodiel, F. N. (1963). Crossing over within a ‘locus’ of the mouse (abstr.). Genetics 48, 907. Salles, F. J., Richards, W. G., Huarte, J., Gubler, P., Vassalli, J. D. and Strickland, S. (1993) Microinjecting antisense sequences into oocytes. In Methods in Enzymology. Guide to Techniques in Mouse Development. (ed. P. M. Wassarman and M. L. DePamphilis). pp 351-360. San Diego: Academic Press. Sambrook, J., Fritsch, E. F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. Silvers, W. K. (1958). An experimental approach to action of genes at the agouti locus in the mouse. II. Transplants of newborn aa ventral skin to ata, Awa, A-, and aa hosts. J. Exp. Zool. 137, 181-188. Silvers, W. K. (1961). Genes and the pigment cells of mammals. Science 134, 368-373. Silvers, W. K. (1979) The agouti and extension series of alleles, umbrous and sable. In The Coat Colors of Mice. pp. 6-44. New York: Springer-Verlag. Siracusa, L. D. (1991). Genomic organization and molecular genetics of the agouti locus in the mouse. Ann. N. Y. Acad. Sci. 642, 419-430. Siracusa, L. D., Russell, L. B., Eicher, E. M., Corrow, D. J., Copeland, N. G. and Jenkins, N. A. (1987). Genetic organization of the agouti region of the mouse. Genetics 117, 93-100. Swanson, M. S., Nakagawa, T. Y., LeVan, K. and Dreyfuss, G. (1987). Primary structure of human nuclear ribonucleoprotein particle C proteins: 1708 D. M. J. Duhl and others conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol. Cell Biol. 7, 1731-1739. Tybulewicz, V. L., Crawford, C. E., Jackson, P. K., Bronson, R. T. and Mulligan, R. C. (1991). Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153-1163. Vlahakis, G. and Heston, W. E. (1963). Increase of induced skin tumors in the mouse by the lethal yellow gene (Ay). J. Natl. Cancer Inst. 31, 189-195. Vrieling, H., Duhl, D. M. J., Millar, S. E., Miller, K. A. and Barsh, G. S. (1994). Differences in dorsal and ventral pigmentation result from regional expression of the mouse agouti gene. Proc. Natn. Acad. Sci. USA (In press). Winkes, B.M., Ollmann, M. M. and Barsh, G. S. (1993) Association os Xmv10 and the nonagouti (a) mutation explained by close linkage instead of causality. Mammalian Genome, in press. Wittekind, M., Gorlach, M., Friedrichs, M., Dreyfuss, G. and Mueller, L. (1992). 1H, 13C, and 15N NMR assignments and global folding pattern of the RNA-binding domain of the human hnRNP C proteins. Biochemistry 31, 6254-6565. Wolff, G. L. (1963). Growth of inbred yellow (Aya) and non-yellow (aa) mice in parabiosis. Genetics 48, 1041-1058. Wolff, G. L., Roberts, D. W. and Galbraith, D. B. (1986). Prenatal determination of obesity, tumor susceptibility, and coat color pattern in viable yellow (Avy/a) mice. The yellow mouse syndrome. J. Heredity 77, 151-158. Yen, T. T., Gill, A. M., Frigeri, L. G., Barsh, G. S. and Wolff, G. L. (1994). Obesity, diabetes, and neoplasia in yellow Avy/- mice: Ectopic expression of the agouti gene. The FASEB J. (in press). (Accepted 25 March 1994)