* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download How to Find and Participate in a GIST clinical trial
Compounding wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug design wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacokinetics wikipedia , lookup
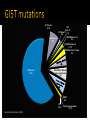
(aka---how to know where to look and who to call) Rare disease, 5000-7000 new cases of GIST every year Compare 230,000 new invasive breast CA cases/year GIST studies tend to be opened at fewer sites OHSU(Portland), Dana Farber (Boston), Fox Chase (Philadelphia), MD Anderson (Houston), Memorial Sloan Kettering (New York) 9 12 2 4 12 1 1 1 2 1 1 1 1 1 Oregon - 12 California -12 Washington - 9 Colorado - 4 Nebraska - 2 Alaska Arkansas Hawaii Idaho -2 Illinois Indiana Michigan South Carolina Tennessee Nigeria Phase I: Researchers test a new drug or treatment in a small group of people for the first time to evaluate its safety, determine a safe dosage range, and identify side effects. Phase II: The drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety. Phase III: The drug or treatment is given to large groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely. Phase IV: Studies are done after the drug or treatment has been marketed to gather information on the drug's effect in various populations and any side effects associated with long-term use Source: http://www.nlm.nih.gov/services/ctphases.html Check with your insurance Does your insurance allow you to participate? Myth: Clinical trials do NOT “pay for everything” As of January 1, 2014, the Affordable Care Act (ACA) includes the requirement that private insurers cover routine costs during participation in a clinical trial At OHSU we will check this for you prior to study participation KIT (exon 9/11/13/17) PDGFRA D842V (exon 12/14/18) Wild Type--- what does that mean? Recommend getting tissue mutation testing ASAP (with first biopsy or surgery) At a minimum, mutation testing after Imatinib (Gleevec) failure Do not usually need another biopsy/surgery to get this done KIT Exon 8 0.1% KIT Exon 9 8% KIT Exon 17 KIT Exon 13 1% 2% PDGFRA Exon 12 2% PDGFRA Exon 14 0.1% PDGFRA Exon 18 other 3% KIT Exon 11 65% SHDA/B/C/D Mutation (Carney-Stratakis) 6% BRAF 2% NF1 0.2% Source: Michael Heinrich, MD RAS gene mutation 0.1% Date of diagnosis Keep a copy of your pathology report(s) Date(s) of surgery(ies) and location(s) where surgery was done Previous treatments and dates (why did you come off treatment?)---be specific (was it for progression or for symptoms?) https://www.molecularmatch.com/ https://clinicaltrials.gov/ BLU-285 Phase 1 oral TKI drug Several sites open around the world Visits weekly for the first month then roughly monthly KTN-158 Phase 1 intravenous antibody drug OHSU can enroll 1-3 patients/month for now Requires infusion every 3 weeks indefinitely SARC 029 trial Likely to open later this summer Phase II study combining two different oral drugs Tracy Walker, RN, BSN OHSU Knight Cancer Institute [email protected] 503-346-1183