* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Altered fear learning across development in both mouse and human
Metastability in the brain wikipedia , lookup
Neural oscillation wikipedia , lookup
NMDA receptor wikipedia , lookup
Neuroeconomics wikipedia , lookup
Mirror neuron wikipedia , lookup
Development of the nervous system wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neural coding wikipedia , lookup
State-dependent memory wikipedia , lookup
Central pattern generator wikipedia , lookup
Neurogenomics wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroanatomy wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Environmental enrichment wikipedia , lookup
Conditioned place preference wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Visual extinction wikipedia , lookup
Synaptic gating wikipedia , lookup
Channelrhodopsin wikipedia , lookup
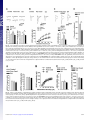
Altered fear learning across development in both mouse and human Siobhan S. Pattwella,b, Stéphanie Duhouxb, Catherine A. Hartleyb, David C. Johnsonb,c, Deqiang Jinga, Mark D. Elliotta, Erika J. Ruberryb, Alisa Powersb, Natasha Mehtab, Rui R. Yanga, Fatima Solimana,b, Charles E. Glatta, B. J. Caseya,b,c,1, Ipe Ninand,1, and Francis S. Leea,e,1 a Department of Psychiatry, cDepartment of Neurology and Neuroscience, eDepartment of Pharmacology, and bSackler Institute for Developmental Psychobiology, Weill Cornell Medical College of Cornell University, New York, NY 10065; and dDepartment of Psychiatry, New York University School of Medicine, New York, NY 10016 F ear learning is a highly adaptive, evolutionarily conserved process that allows one to respond appropriately to cues associated with danger. In the case of psychiatric disorders, however, fear may persist long after an environmental threat has passed. This unremitting and often debilitating form of fear is a core component of many anxiety disorders, including posttraumatic stress disorder (PTSD), and involves exaggerated and inappropriate fear responses. Existing treatments, such as exposure therapy, are based on principles of fear extinction, during which cues previously associated with threat are presented in the absence of the initial aversive event until cues are considered safe and fear responses are reduced. Extinction-based exposure therapies have the strongest empirical evidence for benefitting adult patients suffering from PTSD (1), yet a comparative lack of knowledge about the development of fear neural circuitry prohibits similarly successful treatment outcomes in children and adolescents (2). Adolescence, in particular, is a developmental stage when the incidence of anxiety disorders peaks in humans (3–6), and it is estimated that over 75% of adults with fear-related disorders met diagnostic criteria as children and adolescents (7, 8). Because of insufficient or inaccurate diagnoses and a dearth of pediatric and adolescent specialized treatments, fewer than one in five children or adolescents are expected to receive treatment for their anxiety disorders (9), leaving a vast number with inadequate or no treatment (2, 10). The increased frequency of anxiety disorders in pediatric and adolescent populations highlights the importance of understanding neural mechanisms of fear regulation from a developmental perspective, as existing therapies directly rely upon principles of fear-extinction learning. Converging evidence from human and rodent studies suggests that insufficient top-down regulation of subcortical structures (11–14), such as the amygdala, may coincide with diminished prototypical extinction learning (15), as well as ongoing fine-tuning of excitatory–inhibitory balance in the prefrontal cortex that may coincide with diminished prototypical extinction learning (16). Because top-down prefrontal regulation www.pnas.org/cgi/doi/10.1073/pnas.1206834109 has been postulated to mediate extinction learning and may determine the efficacy of exposure therapy often used as part of cognitive behavioral therapy, it is important to discern how changes in the development of prefrontal circuitry influences fear extinction. Studying the development of fear learning and memory in humans, while examining, in parallel, the underlying neural mechanisms in rodent models, may offer insights into optimizing treatment strategies for developing populations by clarifying when, during development, a particular intervention or treatment may be more or less effective. Results Behavioral Results. Human fear-extinction learning. Because immature functional connectivity between the ventromedial prefrontal cortex (vmPFC) and amygdala in adolescents has previously been shown in tasks of emotion regulation (13), we initially sought to investigate age-dependent differences in fear-extinction learning in humans. Using age delineations for children, adolescents, and adults, we assessed skin conductance response across development in humans to measure prototypical physiological fear responses during conditioned fear acquisition and fear memory extinction (17–21) (Fig. 1A). A two-way ANOVA on skin conductance response during late acquisition (the last of three acquisition runs) with main factors of age group (children, adolescents, adults) and stimulus type [paired conditioned stimulus (CS+) or unpaired (CS−) with an aversive noise] showed a main effect of stimulus type (CS+ > CS−) [F(1, 79) = 10.786, P = 0.002] and no Group × Stimulus type interaction [F(2, 79) = 0.032, P = 0.968] (Fig. S1A), demonstrating that all subjects learned to discriminate between the threat cue and the safety cue. Furthermore, there was no main effect of age group on responses to either stimulus type [CS+: F(2,79) = 0.581, P = 0.562; CS−: F(2, 79) = 0.655, P = 0.522] or the differential acquisition measure [CS+ − CS−: F(2, 79) = 0.021, P = 0.979] during late acquisition. Thus, any subsequent group effects in extinction learning are not related to differences in fear acquisition. In contrast, analysis of extinction indices revealed a main effect of age group for humans [F(2, 80) = 3.228, P = 0.038], such that adolescents showed attenuated fear-extinction learning compared with children [t(56) = 2.34, P = 0.023] and adults [t(51) = 1.802, P = 0.078] (Fig. 1B). This effect of age group on fear extinction was present when sex and trait anxiety are entered Author contributions: S.S.P., S.D., D.J., F.S., B.J.C., I.N., and F.S.L. designed research; S.S.P., S.D., D.J., M.D.E., E.J.R., A.P., N.M., R.R.Y., and I.N. performed research; S.S.P., B.J.C., I.N., and F.S.L. contributed new reagents/analytic tools; S.S.P., S.D., C.A.H., D.C.J., M.D.E., and I.N. analyzed data; and S.S.P., C.A.H., D.C.J., C.E.G., B.J.C., I.N., and F.S.L. wrote the paper. The authors declare no conflict of interest. This article is a PNAS Direct Submission. 1 To whom correspondence may be addressed. E-mail: [email protected], ninani01@ med.nyu.edu, or [email protected]. This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10. 1073/pnas.1206834109/-/DCSupplemental. PNAS Early Edition | 1 of 6 PSYCHOLOGICAL AND COGNITIVE SCIENCES The only evidence-based behavioral treatment for anxiety and stress-related disorders involves desensitization techniques that rely on principles of extinction learning. However, 40% of patients do not respond to this treatment. Efforts have focused on individual differences in treatment response, but have not examined when, during development, such treatments may be most effective. We examined fear-extinction learning across development in mice and humans. Parallel behavioral studies revealed attenuated extinction learning during adolescence. Probing neural circuitry in mice revealed altered synaptic plasticity of prefrontal cortical regions implicated in suppression of fear responses across development. The results suggest a lack of synaptic plasticity in the prefrontal regions, during adolescence, is associated with blunted regulation of fear extinction. These findings provide insight into optimizing treatment outcomes for when, during development, exposure therapies may be most effective. NEUROSCIENCE Edited by Bruce S. McEwen, The Rockefeller University, New York, NY, and approved August 29, 2012 (received for review April 23, 2012) Fig. 1. Cued extinction learning and spontaneous recovery across development in mice and humans. (A) Behavioral paradigms for parallel fear conditioning experiments in humans and mice. (B) Analysis of extinction indices [(averaged first two extinction trials) − (averaged last two extinction trials)] reveals a main effect of age group for humans, such that adolescents display attenuated fear extinction learning compared with children and adults, (adolescent 0.05916 ± 0.06904; children 0.25435 ± 0.04839; adults 0. 22510 ± 0.05931). (C) A lack of extinction learning and retention of extinction memory in is observed in adolescent mice, as displayed by a significantly decreased differential extinction indices [(Day 1, Tone 1) − (Day 4, Tone 5)] compared with older and younger ages, (P23 66.5% ± 2.75; P29 14.72% ± 4.79; P70 35.17% ± 4.89). (D) Adolescent mice display attenuated extinction learning over the course of four days compared with preadolescent and older adult mice. (E) Preadolescent mice demonstrate a lack of spontaneous recovery [(Day 2, Tone 1) − (Day 1, Tone 5)] compared with older ages. All results are presented as a mean ± SEM ***P < 0.001. See also Table S1 and Fig. S1. as covariates [F(2, 73) = 3.086, P = 0.052] (see SI Materials and Methods). There was no significant difference in extinction learning between children and adults (P = 0.701). Mouse extinction learning. To examine the mechanistic basis of this altered fear-extinction learning, we performed parallel studies in mice across comparable postnatal ages. Using classic Pavlovianbased fear conditioning, fear-learning studies were conducted in preadolescent postnatal day (P)23, adolescent (P29), and adult (P70) mice to assess developmental differences in conditioned fear acquisition and extinction learning at ages comparable to human children, adolescents, and adults (22, 23). Freezing behavior was assessed in mice as the prototypical species-specific fear response. First, consistent with previous reports delineating erasure of conditioned fear memories in very young mice because of immature perineuronal net framework in the lateral amygdala (24), we found that preadolescent mice exhibit rapid reductions in freezing behavior and a lack of spontaneous recovery of fear responses between sessions, demonstrating persistent attenuation of fear memories with multiple extinction trials [F(2, 21) = 11.563, P < 0.001] (Fig. 1 D and E). However, preadolescent fear memory does not degrade solely with the passage of time but requires extinction sessions to weaken the memory trace (Fig. S1C). Second, adolescent mice, like the human subjects, display significantly attenuated fear-extinction learning compared with their preadolescent and adult counterparts (Fig. 1 C and D). Analysis of extinction indices revealed a main effect of age for mice [F(2, 22) = 25.426, P < 0.001], demonstrating attenuated extinction learning in adolescent mice. 2 of 6 | www.pnas.org/cgi/doi/10.1073/pnas.1206834109 These findings are consistent with previous rodent studies that show adolescent rats require twice as many extinction trials as adults, a pharmacological manipulation such as D-cycloserine, or CS presentations of prolonged duration to achieve comparable reductions in conditioned fear behavior (23, 25, 26). This lack of extinction in adolescent mice was not a result of higher baseline activity levels or heightened auditory sensitivity (Fig. S1B). Physiological Correlates. Having demonstrated attenuated adolescent extinction learning in both humans and rodents, we sought to perform detailed immunohistochemical and electrophysiological experiments across development in mice to examine potential underlying synaptic changes in the neural circuitry implicated in fear learning. Strong cross-species preservation of the neural circuitry implicated in fear-extinction learning is supported by human and nonhuman animal extinction studies, further bolstering the translational credibility of rodent experiments to explore mechanistic underpinnings that are otherwise precluded from experiments with human subjects (18, 27). Immunohistochemical results. Given the critical role of the vmPFC in fear-extinction learning and retention of extinction memory, we hypothesized that there would be alterations in vmPFC synaptic plasticity across development. We focused on two subregions of the vmPFC, the dorsally located prelimbic cortex (PL), which is associated with production of conditioned-fear responses and expression of conditioned-fear behaviors (28), and the more ventrally located infralimbic cortex (IL), which is associated with suppression of conditioned-fear responses typically seen during Pattwell et al. Fig. 2. Fear extinction modifies glutamatergic synaptic transmission in IL L5 pyramidal neurons of P23 mice. (A) Paradigm design. (B) Frequency and amplitude of sEPSCs in IL L5 pyramidal neurons from control (n = 17 neurons, 6 mice), fear-conditioned (Fear Cond, n = 17 neurons, 6 mice), and fear-extinguished P23 mice (Ext, n = 16 neurons, 6 mice). Both the frequency and amplitude of sEPSCs were significantly increased in fear extinguished mice. (Upper) Examples of sEPSCs. (C) EPSC amplitude in IL L5 pyramidal neurons from control (n = 10 neurons, 5 mice), fear-conditioned (n = 9 neurons, 5 mice), and fear-extinguished P23 mice (n = 11 neurons, 5 mice). EPSC amplitude was significantly increased in fear extinguished mice compared with control group. (Upper) Examples of EPSCs evoked by 100-, 200-, and 300-μA stimulation of L2/3. (D) AMPA/NMDA ratio in IL L5 pyramidal neurons from control (n = 13 neurons, 5 mice), fearconditioned (n = 8 neurons, 5 mice), and fear-extinguished P23 mice (n = 11 neurons, 5 mice). AMPA/NMDA ratio was significantly increased in fear extinguished mice. (Upper) Examples of EPSCs evoked at −60 and +40 mV by 150-μA stimulation of L2/3. (E) Histograms showing the average density of c-Fos– labeled nuclei in the IL across behavioral treatment group reveal increased density of c-Fos expression in the IL of P23 fear-extinguished mice compared with fear-conditioned mice (P23 Fear Cond 1,557 ± 34.15; P23 Ext 2,169 ± 56.06), Student t test, *P < 0.01. See also Figs. S2–S10. Pattwell et al. PNAS Early Edition | 3 of 6 NEUROSCIENCE evoked excitatory postsynaptic currents (EPSCs), and AMPA/ NMDA ratio in both the IL and PL layer 5 (L5) pyramidal neurons after fear acquisition and extinction (see Fig. S2 for corresponding behavioral data and Fig. S7 for representative images of electrode placement). Although we did not observe any modification of the sEPSCs, EPSCs, or AMPA/NMDA ratio in the IL L5 pyramidal neurons from fear-conditioned P23 mice (Fig. 2 B–D), fear-extinguished P23 mice exhibited a significant increase in frequency [F(2, 47) = 6.9, P < 0.01] and amplitude [F(2, 47) = 14, P < 0.001] of sEPSCs, EPSC amplitude [F(2, 27) = 11, P < 0.001], and AMPA/NMDA ratio [F(2, 29) = 7.8, P < 0.01] in the IL L5 pyramidal neurons (Fig. 2 B–D), suggesting that fear extinction involves an enhancement of glutamatergic synaptic transmission in the IL. An increase in AMPA/NMDA ratio [F(2, 29) = 7.8, P < 0.01] suggests that the enhanced excitatory synaptic transmission is primarily mediated by AMPA receptors (Fig. 2D). Unlike the IL neurons, the PL L5 pyramidal neurons from fear-conditioned P23 mice exhibited a significant increase in sEPSC amplitude [F(2, 46) = 6.9, P < 0.01], EPSC amplitude [F(2, 29) = 5.8, P < 0.01], and AMPA/NMDA ratio [F(2, 27) = 3.4, P < 0.05] compared with the control group (Fig. S8 A–C). The fear-extinguished P23 mice showed a significant decrease in sEPSC amplitude, EPSC amplitude, and AMPA/ NMDA ratio compared with the fear-conditioned group, suggesting a depotentiation of glutamatergic synaptic transmission after fear extinction (Fig. S8 A–C). Although the PL L5 pyramidal neurons from fear-conditioned P23 mice showed an increase in sEPSC frequency, which was depotentiated in extinguished mice, these effects did not reach statistical significance (Fig. S8A). In contrast to the synaptic plasticity in the IL and PL of P23 mice after fear acquisition and extinction, neither fear conditioning nor extinction trials affected sEPSCs, EPSCs, or AMPA/NMDA ratio in P29 mice (Fig. 3 A–C and Fig. S9 A–C). The lack of modification of glutamatergic synapses in the vmPFC of P29 mice is consistent with the absence of fear extinction during this PSYCHOLOGICAL AND COGNITIVE SCIENCES successful extinction learning and upon recall of extinction memory (29–32). To investigate neuronal activity levels in each of these regions, we used immunohistochemical techniques to measure c-Fos protein levels in vmPFC of P23, P29, and adult mice after fear-extinction learning. Downstream of the immediate-early gene c-fos, c-Fos activity has been shown to be associated with successful fear-extinction learning in the IL of adult rodents (33). Consistent with previous studies, density of c-Fos–labeled cells in the IL of fear-extinguished mice was significantly higher than nonextinguished, fear-conditioned controls in both P23 and adult mice (Fig. 2E; see also Fig. 4D), whereas there was no change in density of c-Fos labeling in adolescent mice (Fig. 3D), suggesting that neural activity in the vmPFC of adolescent mice differs from the prototypical adult neural activity observed during fear extinction. These immunohistochemical results suggesting enhanced activity in IL of P23 and adult mice, compared with adolescents, parallel the lack of extinction learning in adolescent mice (as shown in Fig. 1 C and D). In addition to increased c-Fos in the IL of P23 and adult mice, decreased c-Fos expression was observed in the PL of P23 (Fig. S8D) and adult (Fig. S10D) fear-extinguished mice compared with age-matched fear-conditioned mice, with no change in c-Fos expression pattern for either group during adolescence (Fig. S9D) (see Fig. S3 for corresponding behavioral data and Figs. S4–S6 for representative images). Electrophysiological results. To probe developmental influences on fear-associated synaptic plasticity within the PL and IL, we performed electrophysiological recordings in vmPFC brain slices of mice after both fear acquisition and fear extinction. An earlier study showed that fear conditioning involved a decrease in intrinsic excitability of IL neurons, whereas fear extinction reversed this decrease in excitability (34). However, the specific synaptic mechanisms in the vmPFC that are involved in fear conditioning or extinction have not been explored. Therefore, we measured spontaneous excitatory postsynaptic currents (sEPSCs), Fig. 3. Absence of modification of glutamatergic synaptic transmission in IL L5 pyramidal neurons of P29 mice after fear acquisition and extinction trials. (A) Frequency and amplitude of sEPSCs in IL L5 pyramidal neurons from control (n = 13 neurons, 8 mice), fear-condition (n = 15 neurons, 8 mice), and fearextinction groups (n = 15 neurons, 8 mice). (B) EPSC amplitude in IL L5 pyramidal neurons from control (n = 9 neurons, 5 mice), fear-condition (n = 14 neurons, 7 mice), and fear-extinction groups (n = 13 neurons, 7 mice). (C) AMPA/NMDA ratio in IL L5 pyramidal neurons from control (n = 11 neurons, 4 mice), fearcondition (n = 9 neurons, 3 mice), and fear-extinction groups (n = 10 neurons, 3 mice). Fear acquisition and extinction trials did not affect sEPSCs, EPSCs, and AMPA/NMDA ratio in IL L5 pyramidal neurons of P29 mice. (D) Histograms showing the average density of c-Fos–labeled nuclei in the IL across the behavioral treatment group reveal no change in density of c-Fos expression in the IL of P29 fear-extinguished mice compared with fear-conditioned mice (P29 Fear Cond 1,468 ± 51.25; P29 Ext 1,506 ± 40.49). See also Figs. S2–S10. narrow developmental window. Next, we examined glutamatergic synaptic transmission in the IL and PL L5 pyramidal neurons of adult mice after fear acquisition and extinction. Similar to P23 mice, we did not observe any modification of sEPSCs, EPSCs, or AMPA/NMDA ratio in the adult IL L5 pyramidal neurons after fear conditioning (Fig. 4 A–C). However, we observed an increase in sEPSC amplitude [F(2, 43) = 3.8, P < 0.05], EPSC amplitude [F(2, 24) = 3.7, P < 0.05], and AMPA/NMDA ratio [F(2, 31) = 8.2, P < 0.01] without any effect on sEPSC frequency in fear-extinguished adult mice (Fig. 4 A–C). Unlike the IL neurons, we observed a significant increase in frequency [F(2, 50) = 4.9, P < 0.05] and amplitude [F(2, 50) = 12.7, P < 0.001] of sEPSCs, EPSC amplitude [F(2, 21) = 5.4, P < 0.05], and AMPA/ NMDA ratio [F(2, 31) = 4.8, P < 0.05] in the PL L5 pyramidal neurons from the fear-conditioned adult mice, and these potentiating effects were reversed in fear-extinguished mice (Fig. S10 A–C). These results suggest that fear extinction involves an enhancement of glutamatergic synaptic transmission in the IL L5 pyramidal neurons in P23 and adult mice, but not adolescent mice. In addition, fear acquisition involves an enhancement of glutamatergic synaptic transmission at the PL glutamatergic synapses in preadolescent (P23) and adult mice but not in adolescent (P29) mice. The potentiated glutamatergic synapses in the PL undergo depotentiation during fear extinction. Although the basal sEPSC amplitude and EPSC amplitude showed a tendency to increase in P29 mice compared with P23 and adult mice, only sEPSC amplitude in the PL L5 pyramidal neurons reached statistical significance [F(2, 48) = 5, P < 0.05]. Although the mechanism is unclear, this enhanced basal excitatory synaptic transmission might contribute to a lack of plasticity in the P29 mice. Furthermore, the IL sEPSC frequency in the adult mice was significantly higher compared with both the P23 and P29 mice, suggesting a protracted development of spontaneous glutamate release mechanism in the IL [F(2, 41) = 9.3, P < 0.001] (Figs. 2B, 3A, and 4A). In summary, these results suggest that synaptic plasticity in the vmPFC is involved in fear regulation, and the lack of synaptic plasticity in the vmPFC may play a role in altered fear extinction in adolescent mice. Discussion Adolescence is a highly conserved developmental stage, both neurobehaviorally and physiologically, during which all mammals must meet evolutionary pressures associated with sexual emergence and transition from dependence on parents to independence (35). Fear learning plays a critical adaptive role in this process as the adolescent leaves the relatively protected and stable family environment and explores a novel and highly variable outside environment. Conservation of the behavioral demands associated with adolescence provides face and construct validity for translational studies of fear learning in this age group. Defining the unique attributes of fear acquisition and extinction during adolescence may have wide clinical implications, as the Fig. 4. Fear extinction modifies glutamatergic synaptic transmission in IL L5 pyramidal neurons of adult mice. (A) Frequency and amplitude of sEPSCs in IL L5 pyramidal neurons from control (n = 14 neurons, 7 mice), fear-conditioned (n = 17 neurons, 8 mice), and fear-extinguished adult mice (n = 15 neurons, 8 mice). The amplitude of sEPSCs was significantly increased in fear-extinguished mice. (B) EPSC amplitude in IL L5 pyramidal neurons from control (n = 8 neurons, 5 mice), fear-conditioned (n = 10 neurons, 5 mice), and fear-extinguished adult mice (n = 9 neurons, 5 mice). EPSC amplitude was significantly increased in fearextinguished mice. (C) AMPA/NMDA ratio in IL L5 pyramidal neurons from control (n = 9 neurons, 5 mice), fear-conditioned (n = 12 neurons, 5 mice), and fearextinguished adult mice (n = 13 neurons, 5 mice). AMPA/NMDA ratio was significantly increased in fear-extinguished mice. (D) Histograms showing the average density of c-Fos–labeled nuclei in the IL across behavioral treatment group reveal increased density of c-Fos expression in the IL of adult fearextinguished mice compared with fear-conditioned mice (Adult Fear Cond 1,650 ± 29.08; Adult Ext 2,257 ± 26.13), Student t test, *P < 0.01. See also Fig. S2–S10. 4 of 6 | www.pnas.org/cgi/doi/10.1073/pnas.1206834109 Pattwell et al. Pattwell et al. across age, with the poorest response in adolescents, highlighting the importance of optimizing treatment strategies based on age. Materials and Methods Animals. Male, C57BL6/J mice were used for all experiments. To eliminate potential developmentally sensitive, shipping-induced stress effects, breeding pairs of C57BL6/J wild-type mice from Charles River were set up in the colony and monitored daily. Litters were weaned at P21 and males from various litters were randomly combined to eliminate any litter-driven effects on behavior. Mice were housed five per cage in a temperature and humidity controlled vivarium maintained on a 12-h light/dark cycle. Mice had ad libitum access to food and water. Separate cohorts of mice (aged P23–P70) were used for all fear-conditioning, retrieval, and object-placement tasks. All procedures regarding animal care and treatment were in compliance with guidelines established by Weill Cornell Medical College’s Institutional Animal Care and Use Committee and the National Institutes of Health. Rodent Fear Conditioning. The fear-conditioning apparatus consisted of a mouse shock-chamber (Coulbourn Instruments) placed in a sound-attenuated box. After a 2-min acclimation period to the conditioning chamber [scented with 0.1% peppermint in 70% (vol/vol) EtOH], mice were fearconditioned with three tone-shock pairings, consisting of a 30-s presentation of a (5 kHz, 70 dB) tone (CS) that coterminated with a 0.7-mA foot shock (unconditioned stimulus, US) during the last 1.0 s of the tone with an intertrial interval (ITI) of 30 s. After the final tone-shock pairing, mice remained in the conditioning chamber for 1 min before being returned to their home cages. Twenty-four hours after fear conditioning, the extinction procedure began in which mice were exposed to five presentations of the CS in the absence of the US. To eliminate any confounding interactions of contextual fear, tones were presented in a novel context, consisting of a green cylindrical arena [scented with 0.1% lemon in 70% (vol/vol) EtOH]. Tone presentations lasted for 30 s with an ITI of 30 s. After the final tone presentation, mice remained in the conditioning chamber for 1 min before being returned to their home cages. Fear-extinction trials were repeated daily for a total of 4 d of extinction training. Experiments were controlled by a computer using Graphic State software. Mice were videotaped for subsequent analysis by raters blind to behavioral groups. Freezing was defined as the absence of visible movement except that required for respiration (56). The freezing during the initial acclimation period was measured and used as an assay for unconditioned effects on general locomotor activity. Percent time spent freezing was calculated by dividing the amount of time spent freezing during the 30-s tone presentations by the duration of the tone. Extinction trials were binned into early and late trials, with the early trials representing the average of the trials on day 1 of extinction (24 h postconditioning), and late trials representing the average of the trials on day 4 of extinction (96 h postconditioning). Electrophysiology. Mice were anesthetized by pentobarbital, perfused intracardially for 2 min with ice-cold artificial cerebrospinal fluid (ACSF) containing: NaCl (118 mM), KCl (2.5 mM), CaCl2 (1 mM), MgSO4 (2 mM), NaH2PO4 (1 mM), and D-glucose (10 mM), pH adjusted to 7.4 with NaHCO3, osmolarity adjusted to 325 mOsm, and aerated by 95% O2/5% CO2. Mice brains were removed and 300-μM slices were prepared using a vibratome (Campden Instruments). Brain slices were kept submerged in a brain slice keeper (Scientific Systems Design) at room temperature for at least 1 h. For the recording of EPSCs, brain slices were placed in a recording chamber continuously perfused with ACSF containing NaCl (118 mM), KCl (2.5 mM), CaCl2 (2 mM), MgSO4 (2 mM), NaH2PO4 (1 mM), and D-glucose (10 mM), pH adjusted to 7.4 with NaHCO3, osmolarity adjusted to 325 mOsm and bubbled with 95% O2/5% CO2 (vol/vol), at 2 mL/min. Recording temperature was maintained at 32 °C using a TC324B in-line solution heater (Warner Instruments). Using video-enhanced infrared differential interference contrast microscopy (Hamamatsu C5405), with an Olympus BX50WI upright microscope fitted with a 40× long working distance water-immersion PNAS Early Edition | 5 of 6 NEUROSCIENCE Human Participants. Before participating in the study, subjects were screened for exclusion criteria, which included hearing impairment and neurological and psychiatric disorders. All subjects gave written informed consent approved by the Weill Cornell Medical College Institutional Review Board. In addition to the consent given by their legal guardian, minor subjects gave a written assent. All participants were compensated for their participation. Age, sex, and number of subjects are further defined in SI Materials and Methods. Experimental design and behavioral paradigms are further defined in the SI Materials and Methods and as shown previously (18, 56). PSYCHOLOGICAL AND COGNITIVE SCIENCES most common and validated treatment for anxiety disorders involves exposure-based therapy, which relies heavily on extinction principles for reevaluating existing contingencies (1). Although the ontogeny of conditioned fear expression and extinction has primarily been focused on infant and juvenile models (36–39), rodent models have recently started to incorporate older, more intermediate, adolescent ages (23, 25, 40, 41). Previous studies have shown that a temporary suppression of hippocampal-dependent contextual fear memory occurs during adolescence (17), as acquisition and expression of amygdala-dependent cue fear memory remain intact, highlighting a developmental dissociation between contextual and cue fear learning. Developmental differences in fear learning have been previously shown in very young rats and mice. During very early development in preweanling rodents, (<P21), odor-shock conditioning can be modulated by maternal presence (37), and mechanisms of cued extinction learning differ from adult-like extinction via alterations in NMDA receptor requirement (42) and mPFC activity (43). Furthermore, in early development (<P24), auditory cued extinction learning appears to be permanent, resulting in little spontaneous recovery and leading to persistent attenuation of a fragile memory trace, suggestive of memory degradation and permanent memory erasure (24, 44, 45). Collectively, our studies have demonstrated attenuated extinction learning, at a defined developmental stage, in both mice and humans. Through performing parallel human and mouse studies examining fear acquisition and extinction, we have uncovered similar developmental patterns in fear-extinction learning, lending credibility to the use of a developmental mouse-model system for examining human adolescent fear and anxiety. After confirming that similar developmental patterns in fear-extinction behavior exist for both mice and humans, we were able exploit the mouse model system to probe underlying physiological mechanisms responsible for the attenuated fear-extinction learning observed in adolescence. Earlier studies have shown changes in intrinsic properties of the vmPFC neurons after fear acquisition and extinction (31, 34, 46). However, the specific involvement of the vmPFC excitatory synapses in fear learning or extinction was unclear. Our findings showing potentiation of the PL excitatory synapses after fear acquisition in P23 and adult mice, and their subsequent depotentiation upon extinction, suggest that the PL excitatory synapses dynamically regulate fear expression. More importantly, the simultaneous potentiation of the IL excitatory synapses in adult fear-extinguished mice provides an additional mechanism by which vmPFC excitatory synapses mediate extinction. The PL projects to the basolateral amygdala and might exert excitatory effects on the central amygdala to enhance fear (47, 48), but on depotentiation, the PL L5 pyramidal neurons might reverse this fear-enhancing effect. In addition, the enhanced glutamatergic IL output during fear extinction might facilitate the intercalated cell-mediated feed-forward inhibition of the central amygdala, resulting in decreased fear response (49–53). However, these synaptic plasticity changes in the PL and IL observed in P23 and adult mice are absent in adolescent mice, suggesting that the vmPFC is not similarly engaged in the regulation of learned fear at this age. Given the delayed development of cortical GABAergic transmission, it is plausible that an imbalance in inhibitory synaptic transmission during adolescence interferes with synaptic plasticity in the mPFC (54, 55). These experiments identify unique synaptic properties in the vmPFC that underlie developmentally regulated differences in fear extinction. During adolescence, there is altered vmPFC synaptic activity and decreased fear-extinction behavior compared with younger and older ages, which may provide insights into the efficacy of treatments for anxiety disorders that rely on extinction mechanisms during this developmental period. In particular, these data suggest that treatment response to exposure-based cognitive behavioral therapy would vary nonlinearly objective, the PL and IL L5 pyramidal neurons were identified and the stimulating electrodes were placed at the L2/3. sEPSCs and EPSCs were recorded at −60 mV in the presence of GABAA receptor blocker bicuculline (10 μM) using patch electrodes (2-4 MΩ) filled with an intracellular pipette solution consisted of: CsCl (145 mM), Hepes (10 mM), EGTA (0.5 mM), QX314 (5 mM), GTP (0.2 mM), and MgATP (5 mM), with osmolarity adjusted to 290 mOsm with sucrose, and pH adjusted to 7.4 with CsOH. The AMPA/ NMDA ratio was calculated by dividing the peak EPSC at −60 mV by the NMDA receptor current measured at 50 ms after the peak at +40 mV. EPSCs and sEPSCs were analyzed by pClamp 10 (Molecular Devices) and Mini Analysis (Synaptosoft). Recordings were rejected when holding current or series resistance changed by 10% or more. 1. Rothbaum BO, Davis M (2003) Applying learning principles to the treatment of posttrauma reactions. Ann N Y Acad Sci 1008:112–121. 2. Liberman LC, Lipp OV, Spence SH, March S (2006) Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther 44:1491–1502. 3. Monk CS, et al. (2003) Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 20:420–428. 4. Kessler RC, et al. (2005) Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 352:2515–2523. 5. Merikangas KR, et al. (2011) Service utilization for lifetime mental disorders in U.S. adolescents: Results of the National Comorbidity Survey-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 50:32–45. 6. Newman DL, et al. (1996) Psychiatric disorder in a birth cohort of young adults: Prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol 64:552–562. 7. Pollack MH, et al. (1996) Relationship of childhood anxiety to adult panic disorder: Correlates and influence on course. Am J Psychiatry 153:376–381. 8. Kim-Cohen J, et al. (2003) Prior juvenile diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry 60:709–717. 9. Merikangas KR, et al. (2010) Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49:980–989. 10. Keller MB, et al. (1992) Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 31:595–599. 11. Lévesque J, et al. (2004) Neural basis of emotional self-regulation in childhood. Neuroscience 129:361–369. 12. Galvan A, et al. (2006) Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci 26:6885–6892. 13. Hare TA, et al. (2008) Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry 63:927–934. 14. Casey BJ, et al. (2010) The storm and stress of adolescence: Insights from human imaging and mouse genetics. Dev Psychobiol 52:225–235. 15. Milad MR, Quirk GJ (2012) Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol 63:129–151. 16. Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888. 17. Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS (2011) Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci USA 108:1182–1187. 18. Soliman F, et al. (2010) A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327:863–866. 19. Laviola G, Adriani W, Terranova ML, Gerra G (1999) Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev 23:993–1010. 20. Adriani W, Chiarotti F, Laviola G (1998) Elevated novelty seeking and peculiar damphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci 112:1152–1166. 21. LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. 22. Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. 23. McCallum J, Kim JH, Richardson R (2010) Impaired extinction retention in adolescent rats: Effects of D-cycloserine. Neuropsychopharmacology 35:2134–2142. 24. Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261. 25. Kim JH, Li S, Richardson R (2011) Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex 21:530–538. 26. Lai CS, Franke TF, Gan WB (2012) Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483:87–91. 27. Gottfried JA, Dolan RJ (2004) Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 7:1144– 1152. 28. Corcoran KA, Quirk GJ (2007) Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27:840–844. 29. Milad MR, Vidal-Gonzalez I, Quirk GJ (2004) Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci 118:389–394. 30. Hefner K, et al. (2008) Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci 28:8074–8085. 31. Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ (2009) Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29:8474–8482. 32. Knapska E, Maren S (2009) Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 16:486–493. 33. Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ (2004) Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24: 5704–5710. 34. Santini E, Quirk GJ, Porter JT (2008) Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28:4028–4036. 35. Spear LP (2004) Adolescent brain development and animal models. Ann N Y Acad Sci 1021:23–26. 36. Rudy JW (1993) Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci 107:887–891. 37. Moriceau S, Sullivan RM (2006) Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci 9:1004–1006. 38. Kim JH, Richardson R (2008) The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: Unlearning as a potential mechanism for extinction early in development. J Neurosci 28:1282–1290. 39. Rudy JW, Morledge P (1994) Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci 108:227–234. 40. Hefner K, Holmes A (2007) Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res 176:210–215. 41. Shen H, et al. (2010) A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science 327:1515–1518. 42. Langton JM, Kim JH, Nicholas J, Richardson R (2007) The effect of the NMDA receptor antagonist MK-801 on the acquisition and extinction of learned fear in the developing rat. Learn Mem 14:665–668. 43. Kim JH, Hamlin AS, Richardson R (2009) Fear extinction across development: The involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci 29:10802–10808. 44. Kim JH, Richardson R (2010) Extinction in preweanling rats does not involve NMDA receptors. Neurobiol Learn Mem 94:176–182. 45. Kim JH, Richardson R (2007) A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem 88:48–57. 46. Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53:871–880. 47. Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. 48. Vertes RP (2006) Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142: 1–20. 49. Paré D, Quirk GJ, Ledoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9. 50. Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. 51. Quirk GJ, Garcia R, González-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343. 52. Likhtik E, Pelletier JG, Paz R, Pare D (2005) Prefrontal control of the amygdala. J Neurosci 25:7429–7437. 53. Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004) Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43:897–905. 54. Chattopadhyaya B, et al. (2004) Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci 24:9598–9611. 55. Kilb W (2011) Development of the GABAergic system from birth to adolescence. Neuroscientist, 10.1177/1073858411422114. 56. Fanselow MS (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15:177–182. 6 of 6 | www.pnas.org/cgi/doi/10.1073/pnas.1206834109 ACKNOWLEDGMENTS. This work was supported by the Sackler Institute (B.J.C.); the DeWitt-Wallace Fund of the New York Community Trust (F.S.L.); the Irma T. Hirschl/Monique Weill-Caulier Trust (F.S.L.); the International Mental Health Research Organization (F.S.L.); the Burroughs Wellcome Foundation (F.S.L.); the Pritzker Consortium (F.S.L. and C.E.G.); National Institutes of Health Grants HD055177 (to B.J.C. and S.S.P.), MH079513 (to B.J.C. and F.S.L.), NS052819 (to F.S.L.); and Swiss National Science Foundation Grant PBGEP3-123643 (to S.D.). Pattwell et al. Supporting Information Pattwell et al. 10.1073/pnas.1206834109 SI Materials and Methods Human Participants. Eighty-three healthy volunteers, including 30 children (5–11 y old; 14 male, mean age = 8.8 y), 28 adolescents (12–17 y old; 15 male; mean age = 13.9 y), and 25 adults (18–28 y old; 12 male, mean age = 22.8 y), completed the study (Table S1). An additional 42 participants (13 children, 16 adolescents, 13 adults), were excluded from analysis because they either didn’t show measurable skin conductance response (SCR) (n = 20, 5 children, 10 adolescents, 5 adults), failed to differentiate between the threat and safety cues [i.e., no difference in mean galvanic skin response to conditioned stimulus (CS)+ compared with CS− in any run of the acquisition session or in the first run of the extinction session] (n = 17, three children, six adolescents, eight adults) or requested to stop participating before the conclusion of the study (five children). In addition, the four youngest females in the adult cohort were excluded to balance the sex ratios within each group. Trait anxiety was assessed using the Spielberg State-Trait Anxiety Inventory trait subscale (STAI-T) (1). Trait anxiety ratings were collected from all but three participants (one child, one adolescent, and one adult). Pubertal development was assessed through self-report or parent reports for two standardized scales (2, 3), resulting in an averaged Tanner staging correspondence of Tanner stage 1 to preadolescence, Tanner stages 2, 3, and 4 to adolescence, and Tanner stage 5 to postadolescence. Experimental Design. Participants underwent a partial-reinforcement discriminative fear-conditioning paradigm spanning 2 d. Two colored squares were used as stimuli. On day 1, one square (CS+) was paired on 50% of the trials with an aversive sound [unconditioned stimulus (US)]. The other square was never paired with the aversive sound (CS−). The color of the CS+ and CS− was counterbalanced across subjects. The partial pairing of the US with the CS+ allowed us to analyze the response to the CS+ independently of the response triggered by the tone. One day later, all participants underwent extinction training wherein the CS+ and CS− were presented repeatedly without the US. The paradigm was adapted from one previously used (4). Participants were not informed about the goals of the study; they were told to attend to the stimuli, to press a key when each square appeared on the screen (to ensure attention), and that they may occasionally hear a loud noise. Each CS was presented for 3 s each, with a 13-s intertrialinterval (ITI). The duration of the ITI was set to ensure that SCR responses could return to baseline and allow responses to be decoupled between trials. On reinforced CS+US trials, the CS+ coterminated with the US. The conditioning session consisted of 12 CS+US, 12 nonreinforced CS+, and 24 CS− trials. Extinction consisted of 24 CS− and 24 nonreinforced CS+ trials. Stimuli were presented in pseudorandomized order, avoiding consecutive CS+USs during the conditioning session and allowing no more than two consecutives squares of the same color in either session. Both the conditioning and extinction sessions were divided into three runs of 16 trials each, with each run lasting 4 min 26 s; a brief break occurred between each run. The auditory stimulus was a white noise combined with a 1,000-Hz tone, which was intensity tiered for smooth onset and offset. It was created using the software Audacity 1.2.6 (http://audacity.sourceforge.net) and was rated as aversive in an independent experiment (5). The sound was delivered in both ears, through headphones. Sound intensity was tested before each experiment using an audiometer, and subjects chose a sound level that was very unpleasant but not painful. The sound intensity level ranged from 85 dB to 95 dB (mean ± SD; Pattwell et al. www.pnas.org/cgi/content/short/1206834109 92.5 ± 2.9) in children, from 94.5 dB to 107 dB in adolescents (96.5 ± 2.4), and from 94 dB to 104 dB in adults (96.5 ± 2.4). The duration of the auditory stimulus was 1 s. Human physiological assessment and analysis of SCR is further defined herein and as previously described in ref. 6. Human Physiological Assessment and Analysis. SCR was acquired using disposable electrodermal (Biopac) electrodes attached to the distal phalanx of the second and third digits of the left hand. The signal was amplified and recorded using a skin conductance recording system (MP35; Biopac), together with the AcqKnowledge (Biopac) software. E-prime software (Psychology Software Tools) controlled the presentation of visual and auditory stimuli and sent timemarkers to the skin conductance module for each stimulus. The SCR was sampled at a rate of 200 Hz and a 1Hz filter was applied (Gain 2 μmho/V). SCR was analyzed offline using Matlab. For each subject, data were smoothed and peaks were located within a time window of 1–10 s following stimulus onset, and considered as stimulus related if the difference between trough to peak was greater than or equal to 0.02 (6). The amplitude of the largest SCR detected in each time window was included in the analysis. When no peak was detected, a zero value was included in the analysis. SCR scores (magnitudes) were square root-transformed to normalize the distribution and were then scaled according to the subject’s maximal square roottransformed peak detected across the whole experiment. These magnitudes were then averaged for each stimulus type separately (CS+, CS−) for each of the three runs of the two phases (conditioning, extinction), for each subject. The first trial of each run was excluded from analysis to remove the orienting response that typically occurs at the start of a session. Fear acquisition was assessed by comparing mean SCR response to CS+ and CS− in the final run of acquisition (“late acquisition”), during which all groups exhibited the greatest discrimination between the CS+ and CS−. We conducted a two-way ANOVA with main factors of age group (children, adolescents, and adults) and stimulus type (CS+, CS−). Extinction learning was assessed by subtracting the mean skin conductance response to the last two CS+ trials from the first two CS+ trials of extinction. This measure closely parallels the extinction index used in the mouse; however, two trials were included to obtain a more stable index of extinction, given the significant amount of signal noise that is characteristic of skin conductance data. Extinction was entered into a one-way ANOVA, testing for any group effects (children, adolescent, adult). Effects of sex, pubertal developmental stage, and trait anxiety upon extinction were also analyzed. Post hoc least-squares difference tests were performed to further assess the direction of any significant effect. An α-level of 0.05 was used for all statistical comparisons. c-Fos Immunohistochemistry. All experiments were carried out at room temperature unless otherwise specified. Ninety minutes after exposure to experimental factors, the mice were killed by intraperitoneal injection of Sleep-Away and perfused transcardially with 30 mL of saline followed by 90 mL of 4% paraformaldehyde (wt/vol) in 0.1 M sodium phosphate (pH 7.4) at a flow rate of 30 mL/min. Brains were removed and postfixed overnight in 4% paraformaldehyde before transferred to 30% sucrose (wt/vol) in 0.1 M sodium phosphate (pH 7.4) for 48 h at 4 °C. Brains were frozen in powdered dry ice and stored at −20 °C until sectioning. Coronal sections (40 μm) of whole brain were cut by using sliding microtome frozen by powdered dry ice. Six 1 of 7 sets of serial sections were collected in Ependorf tubes each containing 2 mL cryoprotectant [30% glycerol (vol/vol) and 30% ethylene glycol (vol/vol) in 0.1 M sodium phosphate, pH 7.4] and stored at −20 °C. Free-floating serial sections (taken one every third) were washed (three times for 10 min each) in TBS, incubated for 30 min in a blocking solution containing 4% normal horse serum (vol/vol), 1% BSA in TBS with 0.2% Triton X-100 (TBS-Tx), and incubated overnight at 4 °C with rabbit anti–c-Fos primary antibody (c-Fos sc-52, Lot F2209; Santa Cruz Biotechnology; sc-52 antibody was raised against amino acids 3–16 of human c-Fos: SGFNADYEASSSRC) diluted 1:1,000 in the blocking solution mentioned above. Sections were then washed in TBS and incubated for 2 h with Alexa Fluor-labeled donkey anti-rabbit IgG secondary antibody (Alexa Fluor-488 or -555) diluted 1:500 in TBS-Tx. Sections were again washed mounted onto chrom-alum/gelatin-coated slides, and air-dried for 2 h in dark. Slides were cover-slipped by water-soluble glycerol-based mounting medium containing DAPI, and sealed with nail polish. Estimation of cell density of c-fos+ neurons in the PL and IL was performed with StereoInvestigator 9.0 (Microbrightfied). Briefly, randomly selected serial sections in an interval 120 μm (taken once every third section) on the slide were numbered by rostracaudal order, and contours of the prelimbic cortex (PL) and infralimbic cortex (IL) were traced by referring to the Allen Brain Map (Allen Company). Systemic random sampling was achieved by applying 150 × 150-μm grids to each section, and fractionator probe with a 20 × 20 × 30-μm counting frame was used for sampling to count cell numbers by abiding by general fractionator counting guidelines (7). Individual cell density was calculated for each mouse by dividing the total sampled cell numbers by the total volumes of fractionators. Supporting Human Behavioral Results. Although there was a significant linear increase in trait anxiety as a function of age (r = 0.231, P = 0.039), trait anxiety was unrelated to both fear acquisition (r = −0.02, P = 0.862) and extinction (r = −0.012, P = 0.914). Furthermore, entering trait anxiety as a covariate in an ANCOVA analysis of age group effects on extinction showed a similar effect of age group [F(2, 76) = 3.038, P = 0.054], but no significant effect of trait anxiety on extinction learning [F(1, 76) = 1. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, Palo Alto, CA). 2. Petersen AC, Crockett L, Richards M, Boxer A (1988) A self-report measure of pubertal status: Lessons from research on anxiety. J Youth Adolesc 17:117–133. 3. Tanner JM (1962) Growth at Adolescence (Blackwell Scientific Publication, Oxford, UK), 2 Ed. 4. Soliman F, et al. (2010) A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327:863–866. Pattwell et al. www.pnas.org/cgi/content/short/1206834109 0.029, P = 0.864]. This result suggests that observed group differences in extinction learning are independent of group differences in trait anxiety. Entering pubertal stage into a one-way ANOVA revealed no significant effects on either acquisition [F(4, 78) = 1.224, P = 0.307] or extinction [F(4, 78) = 0.821, P = 0.516], suggesting that altered extinction in adolescents is better accounted for by an age. Decibel levels were recorded for all but 1 of the 83 participants in our analysis. Mean decibel levels were lower in children (mean = 92.60, SE = 0.53) than in both adolescents [mean = 96.48, SE = 0.48; t(55) = 5.45, P < 0.00001] and adults (mean = 96.60, SE = 0.49; t(52) = 5.52, P < 0.00001]. However, as there were no group differences in fear acquisition, this suggests that conditioning was not influenced by sound intensity, but simply that a lower decibel noise might have been sufficiently aversive to children to function as an effective reinforcer. Furthermore, there was no correlation across participants of all ages between decibel level and either fear acquisition (r = −0.092, P = 0.41) or fear extinction (r = −0.03, P = 0.80). A two-way ANOVA revealed no significant effect of sex on extinction learning [F(1, 79) = 0.653, P = 0.422)], nor any age × sex interaction [F(2, 79) = 1.063, P = 0.35]. Differences in extinction learning might also have been linked to differences in anxiety. We performed one-way ANOVAs on the trait anxiety with Age as a between-subject factor, and found a main effect of Age on the trait anxiety ratings [F(2, 77) = 3.144, P = 0.049], with the adults showing higher trait anxiety than the children [t(50) = 2.266, P = 0.028]. Despite these age-related differences in anxiety, there was no correlation between participants’ extinction learning and self-reported trait anxiety levels (Pearson’s correlation, r = 0.047, P = 0.678). Thus, the age-related differences in the ability to extinguish conditioned fear in healthy individuals reported here is unrelated to self-reported anxiety. If we restrict our analysis of the human data to males only, for the sake of comparison with the male-only mouse sample, we see a similar age effect on extinction [one-way ANOVA F(2,38) = 2.273, P = 0.117] to that observed in the mouse, as well as in the complete human sample. 5. Fanselow MS (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15:177–182. 6. Labar KS, Phelps EA (2005) Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav Neurosci 119(3):677–686. 7. Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147(Pt 3):229–263. 2 of 7 Fig. S1. Additional behavioral data for corresponding human and mouse data presented in Fig. 1. (A) There were no developmental differences in fear acquisition for human subjects. (B) The lack of extinction learning observed in postnatal day (P)29 was not because of enhanced auditory sensitivity or a general increased freezing response to tone, because freezing increased in a linear manner during fear acquisition, with low baseline freezing during tone 1 (the 29 s before first shock delivery at 30 s). (C) Preadolescent cued fear memory does not degrade with mere passage of time, as evidenced by high freezing response on day 5 in the absence of extinction learning, demonstrating that extinction trials are necessary for degradation of the fragile memory trace. Fig. S2. Corresponding behavioral data for electrophysiology experiments presented in Figs. 2–4. (A) Behavioral paradigm schematic. (B–D) Cue test demonstrates decreased freezing behavior in P23 and adult fear-extinguished mice compared with fear conditioned mice, but no difference between P29 fear extinguished and fear conditioned mice, suggestive of a lack of fear extinction learning. Pattwell et al. www.pnas.org/cgi/content/short/1206834109 3 of 7 Fig. S3. Corresponding behavioral data for c-Fos immunohistochemical experiments presented in Figs. 2–4. (A) Behavioral paradigm schematic. (B–D) Cue test demonstrates decreased freezing behavior in P23 and adult fear extinguished mice compared with fear conditioned mice, but no difference between P29 fearextinguished and fear-conditioned mice, suggestive of a lack of fear-extinction learning. Fig. S4. Corresponding images for c-Fos histograms presented in Fig. 2. Representative images for c-Fos density in the IL and PL of preadolescent (P23) mice. (Magnification: 10×.) Pattwell et al. www.pnas.org/cgi/content/short/1206834109 4 of 7 Fig. S5. Corresponding images for c-Fos histograms presented in Fig. 3. Representative images for c-Fos density in the IL and PL of adolescent (P29) mice. (Magnification: 10×.) Fig. S6. Corresponding images for c-Fos histograms presented in Fig. 4. Representative images for c-Fos density in the IL and PL of adult mice. (Magnification: 10×.) Fig. S7. Corresponding images of brain slices from P23, P29 and adult mice showing the positions of stimulating and recording electrodes in PL and IL cortices. Pattwell et al. www.pnas.org/cgi/content/short/1206834109 5 of 7 Fig. S8. Fear acquisition and extinction modify glutamatergic synaptic transmission in PL L5 pyramidal neurons of P23 mice. (A) Frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) in PL L5 pyramidal neurons from control (n = 17 neurons, 6 mice), fear-conditioned (n = 16 neurons, 6 mice), and fear-extinguished P23 mice (n = 13 neurons, 6 mice). sEPSC amplitude was significantly increased in fear conditioned mice. Fear-extinguished mice showed a significant reversal of sEPSC amplitude. (Upper) Examples of sEPSCs. (B) Evoked excitatory postsynaptic current (EPSC) amplitude in PL L5 pyramidal neurons from control (n = 10 neurons, 6 mice), fear-conditioned (n = 11 neurons, 6 mice), and fear-extinguished P23 mice (n = 11 neurons, 6 mice). EPSC amplitude was significantly increased in fear-conditioned mice. Fear-extinguished mice showed a significant reversal of EPSC amplitude. (Upper) Examples of EPSCs evoked by 100, 200 and 300-μA stimulation of L2/3. (C) AMPA/NMDA ratio in PL L5 pyramidal neurons from control (n = 8 neurons, 5 mice), fearconditioned (n = 12 neurons, 5 mice), and fear-extinguished P23 mice (n = 10 neurons, 5 mice). AMPA/NMDA ratio was significantly increased in fear conditioned mice. Fear-extinguished mice showed a significant reversal of AMPA/NMDA ratio. (Upper) Examples of EPSCs evoked at −60 and +40 mV by 150-μA stimulation of L2/3. (D) Histograms showing the average density of c-Fos–labeled nuclei in the PL across behavioral treatment groups reveal decreased density of c-Fos expression in the PL of P23 fear extinguished mice compared with fear-conditioned mice (P23 Fear Cond 1,823 ± 52.94; P23 Ext 1,253 ± 62.40), Student t test, *P < 0.01. Fig. S9. Absence of modification of glutamatergic synaptic transmission in PL L5 pyramidal neurons of P29 mice after fear acquisition and extinction trials. (A) Frequency and amplitude of sEPSCs in PL L5 pyramidal neurons from control (n = 16 neurons, 8 mice), fear-condition (n = 19 neurons, 9 mice), and fear-extinction groups (n = 18 neurons, 9 mice). (B) EPSC amplitude in PL L5 pyramidal neurons from control (n = 10 neurons, 5 mice), fear-conditioned (n = 12 neurons, 6 mice), and fear-extinction groups (n = 12 neurons, 6 mice). (C) AMPA/NMDA ratio in PL L5 pyramidal neurons from control (n = 7 neurons, 3 mice), fearcondition (n = 7 neurons, 3 mice), and fear-extinction groups (n = 7 neurons, 3 mice). (D) Histograms showing the average density of c-Fos–labeled nuclei in the PL across behavioral treatment group reveal no change in density of c-Fos expression in the PL of P29 fear-extinguished mice compared with fear-conditioned mice (P29 Fear Cond 1,737 ± 33.84; P29 Ext 1,685 ± 29.30). Pattwell et al. www.pnas.org/cgi/content/short/1206834109 6 of 7 Fig. S10. Fear acquisition and extinction modify glutamatergic synaptic transmission in PL L5 pyramidal neurons of adult mice. (A) Frequency and amplitude of sEPSCs in PL L5 pyramidal neurons from control (n = 17 neurons, 8 mice), fear-conditioned (n = 20 neurons, 9 mice), and fear-extinguished adult mice (n = 16 neurons, 8 mice). sEPSC frequency and amplitude were significantly increased in fear conditioned mice. Fear-extinguished mice showed a significant reversal of both frequency and amplitude of sEPSCs. (B) EPSC amplitude in PL L5 pyramidal neurons from control (n = 8 neurons, 5 mice), fear-conditioned (n = 8 neurons, 5 mice), and fear-extinguished adult mice (n = 8 neurons, 5 mice). EPSC amplitude was significantly increased in fear conditioned mice. Fear-extinguished mice showed a significant reversal of EPSC amplitude. (C) AMPA/NMDA ratio in PL L5 pyramidal neurons from control (n = 11 neurons, 5 mice), fear-conditioned (n = 13 neurons, 5 mice), and fear-extinguished adult mice (n = 12 neurons, 5 mice). AMPA/NMDA ratio was significantly increased in fear-conditioned mice. Fearextinguished mice showed a significant reversal of AMPA/NMDA ratio. (D) Histograms showing the average density of c-Fos–labeled nuclei in PL across behavioral treatment group reveal decreased density of c-Fos expression in the PL of adult fear-extinguished mice compared with fear-conditioned mice (Adult Fear Cond 1,881 ± 45.88; Adult Ext 1,365 ± 57.24), Student t test, *P < 0.01. Table S1. Participant information for human fear-conditioning experiments presented in Fig. 1 Variable and score Sex Age Range, mean(SE) STAI-T, mean(SE) Children (n = 30) Adolescents (n = 28) Adults (n = 25) 14 male, 16 female 5–11 mean = 8.8 (0.323) 42.57 (1.858) 15 male, 13 female 12–17 13.89 (0.279) 45.46 (1.271) 12 male, 13 female 18–28 22.8 (0.513) 48.71 (1.964) Table shows ages, sex, and average Spielberg Trait Anxiety scores for all participants. Pattwell et al. www.pnas.org/cgi/content/short/1206834109 7 of 7