* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Different adrenal sympathetic preganglionic
Psychophysics wikipedia , lookup
Central pattern generator wikipedia , lookup
Electrophysiology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Axon guidance wikipedia , lookup
Neural oscillation wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Response priming wikipedia , lookup
Neuroanatomy wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Multielectrode array wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Transcranial direct-current stimulation wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Perception of infrasound wikipedia , lookup
Neuroregeneration wikipedia , lookup
Synaptic gating wikipedia , lookup
Development of the nervous system wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Neural coding wikipedia , lookup
Microneurography wikipedia , lookup
Neurostimulation wikipedia , lookup
Optogenetics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Evoked potential wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
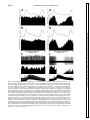
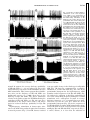
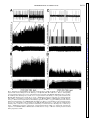
Am J Physiol Regulatory Integrative Comp Physiol 279: R1763–R1775, 2000. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion SHAUN F. MORRISON AND WEI-HUA CAO Department of Physiology, Northwestern University Medical School, Chicago, Illinois 60611 Received 24 April 2000; accepted in final form 10 July 2000 have provided histochemical differentiation of the populations of adrenal medullary chromaffin cells secreting epinephrine and those secreting norepinephrine (13, 15, 23, 25). Several lines of evidence have suggested that the two populations of adrenal chromaffin cells are regulated by distinct neural pathways to the adrenal medulla. First, nerve terminals on epinephrine-secreting chromaffin cells are morphologically different from those on norepinephrinesecreting cells (24). More direct evidence for the existence of distinct populations of sympathetic preganglionic neurons (SPNs) innervating cat epinephrine- and norepinephrine-synthesizing chromaffin cells comes from an elegant anatomic study showing that adrenal SPNs that contain the calcium-binding protein calretinin terminate in the vicinity of noradrenergic chromaffin cells and are located more caudally than adrenal SPNs, which do not contain calretinin and whose terminals are located preferentially among adrenergic chromaffin cells (18). Whether this is also the case in the rat remains to be determined. Second, stimulation at different hypothalamic sites in the cat (21, 42) or stimulation of the splanchnic nerve at different frequencies (17, 29) can evoke selective or differential secretion of adrenal epinephrine or norepinephrine, suggesting that medullary chromaffin cells secreting epinephrine and those that secrete norepinephrine are controlled by different descending and preganglionic pathways. Similar data are available in the rat. Third, certain stimuli in the rat, such as the hypoglycemia induced by insulin or mimicked by 2-deoxyglucose (2-DG), produce a response consistent with a selective activation of epinephrine-secreting adrenal chromaffin cells (22, 34, 43, 48, 53, 54), while, in contrast, acute cold exposure in the rat results in a preferential secretion of norepinephrine (54). In the present study, we sought to provide electrophysiological evidence for the independent regulation of adrenergic and noradrenergic chromaffin cells by characterizing the physiological and stimulus-evoked responses of adrenal SPNs. Because the chromaffin cells of the adrenal medulla are the sole sympathetic target tissue directly innervated by SPNs, the adrenal medulla provides a unique opportunity to study antidromically identified SPNs controlling a known target tissue. We reasoned that adrenal SPNs regulating adrenergic chromaffin cells could be differentiated from those regulating noradrenergic chromaffin cells by their excitatory responses to the glucopenia after 2-DG, which produces a selective increase in adrenal epinephrine secretion (31, 34, 48). In addition, we determined the responses of adrenal SPNs to stimulation in the rostral ventrolateral medulla (RVLM), a location of adrenal sympathetic premotor neurons (47, 57), and to acute activation of the baroreceptor reflex, a potent, Address for reprint requests and other correspondence: S. F. Morrison, Dept. of Physiology (M211), Northwestern Univ. Medical School, 303 E. Chicago Ave., Chicago, IL 60611 (E-mail: s-morrison2 @northwestern.edu). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. adrenal medulla; sympathetic nerve activity; hypoglycemia; rostral ventrolateral medulla; 2-deoxyglucose; vasoconstrictor neurons; intermediolateral nucleus; blood pressure; splanchnic nerve activity ANATOMIC STUDIES http://www.ajpregu.org 0363-6119/00 $5.00 Copyright © 2000 the American Physiological Society R1763 Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Morrison, Shaun F., and Wei-Hua Cao. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regulatory Integrative Comp Physiol 279: R1763–R1775, 2000.—Brain stimulation or activation of certain reflexes can result in differential activation of the two populations of adrenal medullary chromaffin cells: those secreting either epinephrine or norepinephrine, suggesting that they are controlled by different central sympathetic networks. In urethan-chloralose-anesthetized rats, we found that antidromically identified adrenal sympathetic preganglionic neurons (SPNs) were excited by stimulation of the rostral ventrolateral medulla (RVLM) with either a short (mean: 29 ms) or a long (mean: 129 ms) latency. The latter group of adrenal SPNs were remarkably insensitive to baroreceptor reflex activation but strongly activated by the glucopenic agent 2-deoxyglucose (2-DG), indicating their role in regulation of adrenal epinephrine release. In contrast, adrenal SPNs activated by RVLM stimulation at a short latency were completely inhibited by increases in arterial pressure or stimulation of the aortic depressor nerve, were unaffected by 2-DG administration, and are presumed to govern the discharge of adrenal norepinephrine-secreting chromaffin cells. These findings of a functionally distinct preganglionic innervation of epinephrine- and norepinephrine-releasing adrenal chromaffin cells provide a foundation for identifying the different sympathetic networks underlying the differential regulation of epinephrine and norepinephrine secretion from the adrenal medulla in response to physiological challenges and experimental stimuli. R1764 DIFFERENTIATION OF ADRENAL SPNS short-term regulator of cardiac and vasoconstrictor sympathetic outflows that has also been shown to modulate adrenal nerve activity in the rat (26, 51). Our results provide strong support for the existence of a unique population of adrenal SPNs controlling the secretion of epinephrine and suggest that the adrenal SPNs regulating noradrenergic chromaffin cells behave in a manner similar to that expected of SPNs controlling vasoconstriction. METHODS RESULTS Antidromic identification of adrenal SPNs. In 81 rats, 139 neurons in the IML region of thoracic segments T8-T10 (located beneath thoracic vertebrae T7T9) responded to stimulation of the adrenal nerve at a constant latency and followed paired adrenal nerve stimuli separated by ⬍5 ms. The antidromic nature of the constant latency responses of these neurons was confirmed with the collision test. In the example in Fig. 1, adrenal SPN action potentials were evoked at a constant onset latency (51 ms) when adrenal nerve stimuli were delivered at 53.5 ms after a spontaneous discharge of the SPN (Fig. 1, trace 1) but collided when adrenal nerve stimuli were applied at 52 ms after a Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 General procedures. Male Sprague-Dawley rats (300–400 g) were anesthetized with intravenous injections of urethane (800 mg/kg) and ␣-chloralose (70 mg/kg). The trachea, femoral vein, and femoral artery were cannulated for artificial ventilation, drug administration, and measurement of arterial pressure, respectively. Rectal temperature was maintained at 37°C with a thermostatically controlled heating lamp and table. Animals were placed in a stereotaxic apparatus and spinal investigation unit with the incisor bar at ⫺12 mm below the interaural line and the spinal clamp on the T10 and T11 vertebrae. An occipital craniotomy and T7-T9 laminectomy were performed, and exposed nervous tissue was covered with a warmed mixture of mineral oil and silicone grease. After paralysis with d-tubocurarine (0.8 mg/kg iv), animals were respired with 100% oxygen at a minute volume of 140–180 ml. Respiratory rate or tidal volume was adjusted to maintain expired CO2 between 3.5 and 4.5%. A bilateral pneumothorax reduced respiratory pump-related movements of tissue near the recording electrode. After 4 h, supplemental doses of ␣-chloralose (20 mg/kg iv) were given every 2 h. Recording and identification of adrenal SPNs. The extracellular action potentials of adrenal SPNs were recorded (capacity coupled, 300–3,000 Hz) with glass microelectrodes containing a carbon filament. The indifferent electrode was inserted in muscle adjacent to the vertebral column. The region between 700 and 1,100 m ventral to the dorsal surface of the spinal cord and along the dorsal root entry zone was explored for adrenal SPNs that were antidromically identified with stimuli (20–200 A, 1 ms, 0.5 Hz) applied to the left adrenal nerve just proximal to its entrance to the adrenal capsule. Three criteria were used to establish the antidromic nature (35, 37) of the responses of spinal neurons to adrenal nerve stimulation: 1) constant onset latency, 2) high following frequency, and 3) collision with spontaneous action potentials or those evoked orthodromically by stimulation in the RVLM. To perform time-controlled collision tests, individual unit action potentials were selected from ongoing activity and used to trigger an adrenal nerve stimulus at a specified delay. At the end of some experiments, current (15 A, 1 min) was passed through the carbon filament to make a small lesion marking the SPN recording site. These were consistently located within the principal nucleus of the intermediolateral (IML) cell column. RVLM stimulation. A tungsten microelectrode with a 50-m exposed tip was used to deliver paired (6-ms interpulse interval) stimuli (50–300 A, 1 ms, 0.5 Hz) to the region of the RVLM containing spinally projecting, sympathetic premotor neurons, including those projecting monosynaptically to adrenal SPNs (47, 57). The stimulating electrode was positioned 2.6 mm rostral, 1.9 mm lateral, and 2.4 mm ventral to the calamus scriptorius. In some experiments, the stimulating microelectrode was replaced with a micropipette (20-m tip diameter) containing the excitatory amino acid L-glutamate (10 mM), from which microinjections (60 nl) were made to determine the effect on adrenal SPN discharge of exciting only neurons in RVLM. At the conclusion of the experiment, the position of the stimulating electrode and/or microinjection pipette was marked with an electrophoretic deposit of fast green dye from a micropipette stereotaxically positioned at the same RVLM site. After perfusion with fixative, the brain stem was sliced (50 m), and the locations of dye marks were plotted on drawings from a rat atlas (39). Activation of the baroreceptor reflex. To determine the effect of activation of baroreceptor afferent nerves on the discharge of adrenal SPNs, the left aortic depressor nerve (ADN) was dissected in the neck and separated from the cervical vagus nerve, and the sectioned central end was placed on platinum hook electrodes for stimulation with square wave pulses. The following two stimulus paradigms were used: 1) a short burst of three pulses, 6-ms interpulse interval, 20–100 A, 1 ms duration, 0.25 Hz and 2) highfrequency train of 20 Hz for 3 s, 1 ms duration, 20–100 A. Natural stimulation of the baroreceptors was produced by the rise in arterial pressure after an intravenous bolus injection of phenylephrine (0.1 ml, 50–100 g/ml). Adrenal SPN response to glucopenia. To determine the response of adrenal SPNs to simulated hypoglycemia [a stimulus that produces selective release of adrenal epinephrine (22, 43, 53, 54)], spontaneous SPN activity was recorded before and for at least 15 min after an intravenous bolus of 2-DG (250 mg/kg). Histograms (1-s bins) of unit activity were computed, and differences between control activity levels and those at various intervals after the 2-DG administration were determined. Data analysis. Action potentials of adrenal SPNs and the arterial pressure were digitized at 22 kHz and recorded on VCR tape along with 5-volt pulses coincident with stimuli applied to the adrenal or aortic nerves or to the RVLM. Computer-aided data analysis consisted of stimulus- or arterial pulse-triggered histograms of the discharges of adrenal SPNs. To identify poststimulus periods of increased or decreased probability of discharge of adrenal SPNs, the mean discharge rate during the 100-ms period before stimulus delivery was determined from peristimulus time histograms of adrenal SPN discharge. The onsets of excitatory or inhibitory responses of adrenal SPNs were identified as the first bin in peristimulus time histograms that was followed by at least three bins whose values were greater than (or less than, for inhibitory responses) the mean control discharge rate and whose values continued to increase (or decrease, for inhibitory responses). The results are expressed as means ⫾ SE. Significant differences between groups were determined with the Student’s t-test. DIFFERENTIATION OF ADRENAL SPNS spontaneous SPN action potential (Fig. 1, trace 2). As previously described (35, 37), the SPN axonal refractory period was determined by delivering twin pulses to the adrenal nerve at an interval (40 ms; Fig. 1, traces 3 and 4) after a spontaneous action potential, resulting in collision of the first antidromic spike. The SPN recording showed that a second antidromically evoked action potential was generated when the interpulse interval was 2.4 ms (Fig. 1, trace 3), i.e., equal to or greater than the axonal refractory period, but not when the interpulse interval was 2.2 ms (Fig. 1, trace 4). RVLM-evoked discharge of adrenal SPNs. All of the adrenal SPNs were excited by paired stimuli applied to the region of the RVLM containing sympathetic premotor neurons. Most of the SPNs responded to each RVLM stimulus with a single action potential. Threshold responses in which only 10–20% of the RVLM stimuli evoked an SPN spike typically occurred at stimulus currents between 40 and 80 A. Maximal responses in which an SPN action potential occurred for nearly every RVLM stimulus were attained at a mean current of 225 A. Splanchnic SPNs, which would include those with axons in the adrenal nerve, were previously divided into four groups on the basis of the patterns and latencies of their responses to RVLM stimulation (37). The antidromically identified adrenal SPNs in the present study exhibited RVLM stimulusevoked responses characteristic of the splanchnic SPNs in either group I (short onset latency excitation) or group IV (early inhibition and late excitation). The adrenal SPNs with the characteristics of splanchnic group IV SPNs were designated Epi Adr SPNs, and adrenal SPNs with RVLM stimulus-evoked responses similar to those of splanchnic group I SPNs were designated NE Adr SPNs. Epi Adr SPN responses to RVLM stimulation. In 71 (51%) adrenal SPNs, RVLM stimulation produced a biphasic response (Fig. 2A) consisting of an early reduction in unit action potential probability followed by a period of increased discharge probability during which the unit could fire more than one time to a paired RVLM stimulus. This response, characteristic of Epi Adr SPNs, is shown in the peristimulus time histogram in Fig. 2A, top. The probability of unit discharge fell below control levels for ⬃80 ms after the RVLM stimulation (mean control discharge rate in the 100 ms before the stimulation: 4.9 Hz; mean discharge rate between 15 and 80 ms after the stimulation: 1.8 Hz). This was followed by a period of increased discharge probability lasting from 110 to 180 ms after the RVLM stimulus, during which the 100 RVLM stimuli evoked 86 spikes with a mean latency of 139 ms and a modal latency of 150 ms. These two phases of the RVLM stimulus-evoked response of Epi Adr SPNs are clearly seen in the mean peristimulus time histogram (Fig. 2A, bottom) derived from the individual response histograms of 71 Epi Adr SPNs. The early period of inhibition lasted for 68 ms after the stimulus, and during this time (i.e., from the end of the stimulus blanking at 12–68 ms) the mean discharge rate averaged 64% of control. The late period of excitation of Epi Adr SPNs lasted for 108 ms, between 76 and 184 ms, during which the mean response latency was 129 ⫾ 2.8 ms and the modal response latency was 136 ms. As shown in Fig. 3, top, the antidromic latencies of Epi Adr SPNs ranged from 6 to 60 ms (mean: 35 ⫾ 1.5 ms), corresponding to approximate conduction velocities between 0.55 and 5.5 m/s, based on an average distance from the spinal recording site to the adrenal nerve stimulation site of 33 ⫾ 2 mm (n ⫽ 31 rats in which this distance was measured). Epi Adr SPNs were excited by activation of RVLM neurons with microinjections of glutamate. In the example in Fig. 2B, the discharge of an adrenal SPN with an RVLM stimulus-evoked response pattern characteristic of Epi Adr SPNs (see Fig. 2A) was increased from a basal rate of 5.5 spikes/s to a peak rate of 12 spikes/s after an ipsilateral microinjection of glutamate (10 mM, 60 nl) in the RVLM, which also resulted in an Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 1. Antidromic activation and collision test for an adrenal sympathetic preganglionic neuron (SPN). Trace 1: single pulse stimulation of the adrenal nerve (2 mA) delivered 53.5 ms after a spontaneous action potential results in a constant-onset latency response (51 ms) in the SPN; trace 2: adrenal nerve stimuli at 52 ms after a spontaneous action potential fail to elicit an SPN response; trace 3: a single constant-onset latency response is recorded from the SPN after twin adrenal nerve stimuli 2.4 ms apart delivered 40 ms after spontaneous SPN spikes; trace 4: no response is recorded from the SPN after twin adrenal nerve stimuli 2.2 ms apart delivered 40 ms after spontaneous SPN spikes. All traces are superpositions of 4 stimulation trials. Vertical calibration is 125 V. R1765 R1766 DIFFERENTIATION OF ADRENAL SPNS increase in mean arterial pressure of 60 mmHg. Similar stimulation of neuronal cell bodies in the RVLM produced an increase in discharge rate of 89 ⫾ 5.6% in three Epi Adr SPNs and an increase in mean arterial pressure of 47 ⫾ 10.7 mmHg. In none of these cases was an early inhibition (see the histograms of the electrically evoked responses in Fig. 2A) observed, although the temporally diffuse nature of the glutamate microinjection stimulus and the presence of RVLM neurons with excitatory inputs to Epi Adr SPNs could have masked such an effect. NE Adr SPN responses to RVLM stimulation. Sixtyeight (49%) adrenal SPNs responded to RVLM stimulation with single action potentials that all had latencies ⬍50 ms. The peristimulus time histogram for a representative NE Adr SPN (Fig. 2C, top) indicates a dramatic, short-lasting increase in discharge probabil- ity between 20 and 40 ms after the RVLM stimuli (mean response latency: 28.8 ms). This excitation was usually followed by a period of decreased discharge or silence often lasting for 100–200 ms. The mean peristimulus time histogram derived from the responses of 68 NE Adr SPNs (Fig. 2C, bottom) shows the consistency of this early and remarkable excitation, which occurred from 12 ms (the end of the stimulus-blanking period) to 52 ms, with a mean response latency of 29 ⫾ 0.9 ms (range: 16–47 ms). As shown in Fig. 3, bottom, the antidromic latencies of NE Adr SPNs ranged from 7 to 70 ms (mean: 37 ⫾ 2.0 ms), corresponding to approximate conduction velocities between 0.47 and 4.7 m/s. These did not differ from those for Epi Adr SPNs. NE Adr SPNs were also excited by activation of RVLM neurons with microinjections of glutamate. In Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 2. Responses of adrenal SPNs to activation of the rostral ventrolateral medulla (RVLM). A: top, peristimulus time histogram of the activity of adrenal SPNs with early inhibition and late excitation (Epi Adr SPN) during 100 stimuli (120 A, twin pulses) to the ipsilateral RVLM. Bin width was 5 ms. Bottom, average peristimulus time histogram of the responses of 71 Epi Adr SPNs. Each bin represents the mean ⫹ SE of the values (normalized for no. of RVLM stimuli delivered) for that bin from all of the individual peristimulus time histograms. Bin width is 4 ms. B: response of an Epi Adr SPN to a microinjection (arrowhead, 60 nl) of glutamate (10 mM) in the ipsilateral RVLM. Top, extracellular action potentials of the SPN; middle, rate histogram (500-ms bin width) of the SPN activity; bottom, arterial pressure (AP). C: top, peristimulus time histogram of the activity of SPNs with short onset latency excitation (NE Adr SPN) during 30 stimuli (80 A, twin pulses) to the ipsilateral RVLM. Bin width was 5 ms. Bottom: average peristimulus time histogram of the responses of 68 NE Adr SPNs. Bin width is 4 ms. D: response of an NE Adr SPN to a microinjection (arrowhead, 60 nl) of glutamate (10 mM) in the ipsilateral RVLM. Traces are as in B. DIFFERENTIATION OF ADRENAL SPNS the example in Fig. 2D, the discharge of an adrenal SPN with an RVLM stimulus-evoked response pattern characteristic of NE Adr SPNs (see Fig. 2C) was increased from a basal rate of 2 spikes/s to a peak rate of 10 spikes/s after an ipsilateral microinjection of glutamate (10 mM, 60 nl) in the RVLM, which also resulted in an increase in mean arterial pressure of 51 mmHg. Similar stimulation of neuronal cell bodies in the RVLM produced an increase in discharge rate of 171 ⫾ 14.8% in four NE Adr SPNs and an increase in mean arterial pressure of 54 ⫾ 2.2 mmHg. Baroreceptor reflex influences on the discharge of Adr SPNs. Adrenal SPNs with RVLM stimulus-evoked responses characteristic of Epi Adr SPNs (see Fig. 2A) had a mean spontaneous discharge frequency of 5.6 ⫾ 0.3 Hz at a mean arterial pressure of 99 ⫾ 3.0 mmHg. Perisystolic averaging indicated that the spontaneous discharge of Epi Adr SPNs was not synchronized to the cardiac cycle, suggesting that the activity of these neurons was not strongly influenced by the baroreceptor reflex. In the example in Fig. 4A, there is no significant alteration in the average spikes per bin for an Epi Adr SPN over the course of 1.5 cardiac cycles, including two systolic pressure increases of ⬃70 mmHg. The average perisystolic time interval histogram (Fig. 4B) for 15 Epi Adr SPNs indicates that, although there was a tendency for a reduced discharge probability during middiastole, this modulation represented an inhibition of only 18% of the normalized maximum firing rate over the course of the cardiac cycle (average mean arterial pressure: 108 mmHg). NE Adr SPNs had a mean discharge frequency of 3.5 ⫾ 0.3 Hz, which was significantly slower (P ⬍ 0.01) than that of Epi Adr SPNs at an equivalent mean arterial pressure of 99 ⫾ 2.4 mmHg. In contrast to those of Epi Adr SPNs, the perisystolic time interval histograms of the spontaneous discharge of all NE Adr SPNs exhibited prominent peaks and troughs indicating a strong influence of the baroreceptor reflex on the networks governing the activity of NE Adr SPNs. In the example in Fig. 4D, the discharge probability of this NE Adr SPN fell precipitously from 16 to 40 ms after the onset of systole and then rose throughout diastole. The mean perisystolic time interval histogram constructed for 10 NE Adr SPNs (Fig. 4E) indicated a similarly large reduction in mean discharge probability in middiastole (average mean arterial pressure: 118 mmHg). This resulted in an inhibitory modulation representing 83% of the maximum discharge, which was significantly (P ⬍ 0.01) greater than that for Epi Adr SPNs, although the average mean arterial pressures were not different between the groups. The effect of natural activation of the baroreceptor reflex indicated a similarly reduced sensitivity of the discharge of Epi Adr SPNs to baroreceptor reflex-mediated sympathoinhibition. As shown in Fig. 4C, the mean discharge rate of an Epi Adr SPN was changed from 3.8 spikes/s at a mean arterial pressure of 90 mmHg to 3.6 spikes/s at a mean arterial pressure of 183 mmHg during the pressor response to a bolus injection of phenylephrine (5 g in 0.1 ml iv). In contrast, increasing arterial pressure to 186 mmHg resulted in a complete inhibition of the discharge of an NE Adr SPN (Fig. 4F). In a group of eight Epi Adr SPNs, an average phenylephrine-induced increase in arterial pressure of 71 ⫾ 6.4 mmHg resulted in a mean reduction in discharge frequency of 18 ⫾ 3.9%, whereas a similar increase in arterial pressure (70 ⫾ 5.2 mmHg) evoked a complete inhibition (100% reduction) of the discharge of 10 NE Adr SPNs. The responses of adrenal SPNs to electrical stimulation of baroreceptor afferents in the ADN also indicated a differential sensitivity of the medullary networks governing the discharge of Epi Adr SPNs and of NE Adr SPNs to baroreceptor reflex inputs. Averaged responses to three pulse stimuli applied to the ADN showed no consistent period of reduced discharge probability in Epi Adr SPNs but a dramatic reduction in discharge probability in NE Adr SPNs. Figure 5A, top, which shows superimposed records from 30 trials of ADN stimulation for an Epi Adr SPN, shows no period of unit discharge inhibition comparable to that in a typical NE Adr SPN whose superimposed records of 30 trials of ADN stimulation (Fig. 5D) indicate a reduced discharge probability between 65 and 190 ms after the ADN stimulus onset. The mean peristimulus time interval histogram for 37 Epi Adr SPNs (Fig. 5A, bottom) indicates that, on average, Epi Adr SPNs experienced a reduced discharge probability (to 72% of control) between 230 and 390 ms after the onset of the ADN Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 3. Histograms of the antidromic onset latencies of adrenal SPNs putatively regulating adrenal medullary epinephrine secretion (Epi Adr SPN, top) and those regulating adrenal medullary norepinephrine secretion (NE Adr SPN, bottom). R1767 R1768 DIFFERENTIATION OF ADRENAL SPNS Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 4. Relationship of adrenal SPN discharge to activation of the baroreceptor reflex by arterial pressure. A: top, systolic-triggered average of the arterial pressure. Bottom, perisystolic time interval histogram of the spontaneous activity of an Epi Adr SPN during 1,077 cardiac cycles. Horizontal axis is expressed as % of the cardiac cycle length, which was 149 ms. Vertical axis in the histogram is normalized to the maximum no. of counts in the modal bin. B: top, mean (solid line) and mean ⫹ SE (dotted line) systolic-triggered average of the arterial pressure. Bottom, average perisystolic time interval histogram derived from analysis of the spontaneous discharge of 15 Epi Adr SPNs. In the latter, each bin contains the mean ⫹ SE of the values for that bin from all of the individual perisystolic time interval histograms, after normalizing to the modal bin value. Horizontal axis is expressed as % of the cardiac cycle length to normalize for differing cardiac cycle lengths among animals. Bin width is 5% of the cardiac cycle. C: response of an Epi Adr SPN to the pressor response after iv bolus of phenylephrine (PE, 5 g). Top, extracellular action potentials of the SPN; middle, corresponding rate histogram (1-s bin width) of the SPN activity; bottom, arterial pressure. D: top, systolic-triggered average of the arterial pressure. Bottom, perisystolic time interval histogram of the spontaneous activity of an NE Adr SPN. Analysis is from 500 cardiac cycles, and cardiac cycle length was 159 ms. E: top, mean (solid line) and mean ⫹ SE (dotted line) systolic-triggered average of the arterial pressure. Bottom, average perisystolic time interval histogram derived from analysis of the spontaneous discharge of 10 NE Adr SPNs. F: response of NE Adr SPN to the pressor response after iv bolus of phenylephrine (PE, 5 g). Top, extracellular action potentials of the SPN; middle, corresponding rate histogram (1-s bin width) of the SPN activity; bottom, arterial pressure. Horizontal calibration bar is 8 s for C and 10 s for F. Vertical calibration is 40 V for C, top, and 30 V for F, top. DIFFERENTIATION OF ADRENAL SPNS R1769 stimuli. In contrast, the average discharge probability of NE Adr SPNs (n ⫽ 32) was reduced to 16% of the control discharge rate between 50 and 260 ms after the ADN stimulation. This baroreceptor-mediated inhibitory effect on the discharge of NE Adr SPNs was significantly greater (P ⬍ 0.001) than that on the discharge of Epi Adr SPNs. The slower conduction velocity in the pathway from the RVLM mediating the excitation of Epi Adr SPNs (see Fig. 2A) may have contributed to the longer onset latency and longer period of reduced discharge probability in Epi Adr SPNs. High-frequency stimulation of baroreceptor afferents in the ADN that produced equivalent reductions in arterial pressure (Fig. 5, B and E, bottom) resulted in no perceptible change in the discharge of an Epi Adr SPN (Fig. 5B) but was accompanied by a complete inhibition of an NE Adr SPN (Fig. 5E). The mean peristimulus histogram for the high-frequency, ADN stimulus-evoked responses of 19 Epi Adr SPNs (Fig. 5C) indicated a reduction in discharge probability of 11% of the control firing frequency during the 3-s ADN stimulation and a subsequent elevation in average discharge frequency (⫹5% of control) that was maintained for at least the next 10 s. From the mean peristimulus histogram (Fig. 5F) for the responses of 13 NE Adr SPNs to similar ADN stimulations (maximum reductions in mean arterial pressure of 23 and 20 mmHg for Epi and NE Adr SPNs, respectively; Fig. 5, C and F, bottom), it is clear that the reduction in Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 5. Responses of adrenal SPNs to stimulation of the aortic depressor nerve (ADN). A: top, superposition of 30 records of the discharges of an Epi Adr SPN during stimulation of the ADN (200 A, 3 pulses). Bottom, mean peristimulus time interval histogram for 37 Epi Adr SPNs in which each bin contains the mean ⫹ SE of the values for that bin from all of the individual peristimulus time interval histograms. Bin width is 10 ms. Counts from stimulus artifacts were eliminated by zeroing the two corresponding bins. B: top, extracellular action potentials of an Epi Adr SPN (and smaller stimulus artifacts). Bottom, arterial pressure during a high-frequency stimulation of the ADN (20 A, 20 Hz, 3 s). C: top, mean peristimulus time interval histogram of the responses of 19 Epi Adr SPNs. Bottom, mean peristimulus histogram of the arterial pressures. Each bin of these histograms contains the mean ⫹ SE of the values for that bin from all of the individual peristimulus histograms. Bin width is 1 s. D: top, superposition of 30 records of the discharges of an NE Adr SPN during stimulation of the ADN (200 A, 3 pulses). Bottom: mean peristimulus time interval histogram for 32 NE Adr SPNs. E: top, extracellular action potentials of an NE Adr SPN (and smaller stimulus artifacts). Bottom, arterial pressure during a high-frequency stimulation of the ADN (100 A). Horizontal calibration is 4 s for B and E. Vertical calibration is 80 V for A and D, 40 V for B, and 50 V for E. F: top, mean peristimulus time interval histogram of the responses of 13 NE Adr SPNs. Bottom, mean peristimulus histogram of the arterial pressures. Each bin of these histograms contains the mean ⫹ SE of the values for that bin from all of the individual peristimulus histograms. Bin width is 1 s. R1770 DIFFERENTIATION OF ADRENAL SPNS in the RVLM on a drawing (39) through the rat medulla 2.5 mm rostral to the calamus scriptorius. Each of the recovered sites is within the vasomotor region of the RVLM, which contains sympathetic premotor neurons regulating vasoconstrictor sympathetic outflow (36) and adrenal medullary catecholamine secretion (47, 57). DISCUSSION The principal new finding from these experiments is that adrenal SPNs can be divided into two distinct populations based of their evoked and reflex responses. Epi Adr SPNs were markedly excited during the glucopenia evoked by intravenous 2-DG administration, consistent with SPNs that regulate the secretion from adrenal, epinephrine-producing chromaffin cells, whereas NE Adr SPNs were unaffected by 2-DG, consistent with SPNs regulating the discharge of adrenal, norepinephrine-secreting chromaffin cells. Epi Adr SPNs consistently responded to electrical stimulation of the RVLM with a biphasic change in discharge probability: an early inhibition followed by a long latency excitation. In contrast, the response of NE Adr SPNs to RVLM stimulation was always a dramatic, short latency excitation. Epi Adr SPNs exhibited little or no sensitivity to the baroreceptor reflex, as indicated by the relative absence of a cardiac cycle-related modulation of their spontaneous discharge and only a minimal inhibition of their discharge during large, phenylephrine-evoked pressor responses or in response to stimulation of baroreceptor afferents in the ADN. The discharge of NE Adr SPNs, by comparison, was exquisitely sensitive to baroreceptor reflex activation: the perisystolic time histograms of NE Adr SPNs contained bins with zero counts, and natural (increased arterial pressure) or electrical activation of the baroreceptor reflex produced a prompt and complete inhibition of NE Adr SPN discharge. These data provide the first functional differentiation among populations of SPNs, thereby supporting the existence of functional specificity at the level of the SPN (1, 18, 40). These results also form a foundation for determining the organization and properties of the networks selectively controlling adrenal epinephrine secretion (21, 52, 54), and they suggest that the central pathways determining adrenal norepinephrine release may be indistinguishable from those controlling sympathetic tone to vasoconstrictor targets. Several lines of evidence indicate that the SPNs from which we recorded innervated the epinephrine- and the norepinephrine-secreting adrenal chromaffin cells. The SPNs reported in this study were found in thoracic segments T8-T10, which are within the T5-T11 distribution containing the majority of adrenal SPNs retrogradely labeled after adrenal medullary injection of tracer in the rat (2, 28, 44, 46, 47) and other species (18, 27). Jensen et al. (27) have shown that the population of SPNs innervating the rabbit adrenal medulla is separate from that innervating ganglion cells in the aorticorenal ganglion, indicating that the axons of ad- Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 discharge probability during the ADN stimulation (84% of control frequency) is significantly (P ⬍ 0.001) greater than that for Epi Adr SPNs and that the mean discharge frequency after the inhibition (3.4 ⫾ 0.1 spikes/s) is not different from the control firing rate (3.5 ⫾ 0.1 spikes/s). Responses of Adr SPNs to 2-DG-induced glucopenia. Because plasma levels and adrenal secretion rates of epinephrine and norepinephrine have been shown to be differentially sensitive to reductions in blood glucose (53, 54), we reasoned that adrenal SPNs regulating the discharge of epinephrine-secreting chromaffin cells should be excited during the glucopenia produced by 2-DG, while little or no change in discharge frequency would be expected of adrenal SPNs regulating the activity of chromaffin cells secreting norepinephrine. We tested the response of 20 adrenal SPNs with RVLM stimulus-evoked responses characteristic of Epi Adr SPNs (see Fig. 2A) and 17 adrenal SPNs with RVLM stimulus-evoked responses characteristic of NE Adr SPNs (see Fig. 2C) to an intravenous bolus of 2-DG (250 mg/kg). In the example shown in Fig. 6A, the discharge rate of an Epi Adr SPN increased from 5.2 spikes/s during the control preinjection and early postinjection period (inset on left) to 8.6 spikes/s during the sustained activation (inset on right) after the 2-DG injection. The mean post-2-DG time interval histogram for Epi Adr SPNs (Fig. 6B) shows that 2-DG produced a significant (P ⬍ 0.05) increase in discharge frequency beginning ⬃30 s after the 2-DG injection. This excitation reached a plateau (mean discharge frequency: 143 ⫾ 0.7% of control) between 1 and 2 min after the 2-DG injection and remained at this level for at least 5 min (mean discharge frequency: 144 ⫾ 12.4% of control at 180 s; 149 ⫾ 10.3% of control at 240 s; 148 ⫾ 14.9% of control at 300 s). In contrast, the discharge frequency of the NE Adr SPN shown in Fig. 6C was slightly reduced from 0.5 spikes/s during the control preinjection and early postinjection period (inset on left) to 0.3 spikes/s during the period ⬃90–110 s (inset on right) after the 2-DG injection. The mean post-2-DG time interval histogram for the NE Adr SPNs (Fig. 6D) suggests a slight, short-lasting increase in SPN discharge between 5 and 15 s after the 2-DG injection; thereafter, mean SPN discharge frequency was reduced, averaging 90 ⫾ 0.5% of control in the period between 1 and 2 min after the 2-DG injection. This level of activity was maintained for at least 5 min (mean firing frequency: 86 ⫾ 6.8% of control at 180 s, 89 ⫾ 6.4% of control at 240 s, 88 ⫾ 3.3% of control at 300 s). Location of stimulation and microinjection sites in the RVLM. Figure 7, top, shows two histological sections at the level of the RVLM containing fast green dye spots indicating the positions of the stimulating electrode in two experiments in which Epi and NE Adr SPNs were recorded. These stimulation sites were located ventral to the compact division of the nucleus ambiguus, close to the ventral surface of the medulla and within 500 m of the caudal end of the facial nucleus. Figure 7, bottom, shows the stimulation sites DIFFERENTIATION OF ADRENAL SPNS R1771 Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 6. Responses of adrenal SPNs to iv injection of 2-deoxyglucose (2-DG, 250 mg/kg, time 0). A: top, post-2-DG injection time interval histogram of the discharge of an Epi Adr SPN. Bin width is 1 s. Bottom: corresponding record of arterial pressure. Inset on left: extracellular action potentials of the Epi Adr SPN during the 5 s just after the 2-DG injection (discharge rate: 5.2 Hz). Inset on right: similar record during 5 s of the peak activation of the Epi Adr SPN (discharge rate: 8.6 Hz). B: top, mean ⫹ SE discharge frequency (expressed as %control) histogram (bin width: 1 s) obtained by averaging the responses of 20 Epi Adr SPNs to administration of 2-DG. Bottom: average ⫹ SE mean arterial pressure recorded during responses to 2-DG. C: top, post-2-DG injection time interval histogram of the discharge of an NE Adr SPN. Bin width is 1 s. Bottom: corresponding record of arterial pressure. Inset on left, extracellular action potentials of the NE Adr SPN during the 5 s just after the 2-DG injection (discharge rate: 0.5 Hz). Inset on right, similar record during a 5-s interval at 110 s (discharge rate: 0.3 Hz). D: top, mean ⫹ SE discharge frequency (expressed as %control) histogram (bin width: 1 s) obtained by averaging the responses of 17 NE Adr SPNs to administration of 2-DG. Bottom: average ⫹ SE mean arterial pressure recorded during responses to 2-DG. R1772 DIFFERENTIATION OF ADRENAL SPNS renal SPNs project selectively to the adrenal medulla. It seems unlikely that our antidromic stimulus would have activated SPN axons other than those of adrenal SPNs, since the adrenal nerve was always well isolated and the stimulating electrode hooks were positioned close to the entrance of the adrenal nerve in the adrenal capsule. Although adrenal SPNs may innervate adrenal medullary ganglion cells and adrenal chromaffin cells (14), the former appear to be a minor cell population in the adrenal medulla, and it is not known whether or when they receive a unique preganglionic input. In view of the conclusion, based on anatomic data, that there are ⬃2.7 times as many epinephrinecontaining as norepinephrine-secreting chromaffin cells in the adult rat medulla (50), we were initially surprised to record from nearly equal numbers of Epi Adr SPNs and NE Adr SPNs. However, given the potential for different degrees of convergence of adrenal SPN inputs onto chromaffin cells (24) and our small sample size in each rat, we might not expect a close Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 Fig. 7. Localization of stimulation sites in the RVLM. Top: fast green dye deposits (arrows) at the positions of the RVLM stimulating electrode in 2 experiments. NA, compact division of the nucleus ambiguus. Bottom: filled circles on a drawing through the medulla ⬃2.5 mm rostral to the calamus scriptorius indicate the positions of the RVLM stimulating electrode in 15 representative experiments. NTS, nucleus tractus solitarius; PrH, prepositus hypoglossal nucleus; MVe, medial vestibular nucleus; SpVe, spinal vestibular nucleus; SpV, spinal trigeminal nucleus; IO, inferior olive; PYR, pyramidal tract. correlation between the relative numbers of each type of adrenal SPN and chromaffin cells. The simulated hypoglycemia induced by the glucopenic agent 2-DG produces a strong stimulus for the release of adrenal epinephrine, with a relatively minor increase in plasma norepinephrine, an indeterminate fraction of which arose as “spillover” from sympathetic postganglionic terminals (7). This result parallels that seen with insulin-induced hypoglycemia (11, 16, 53) and with chronic 2-DG administration (41). Because only one of the populations of adrenal SPNs was excited by 2-DG, we conclude that 2-DG administration was an effective stimulus for distinguishing adrenal SPNs projecting to epinephrine-secreting chromaffin cells from those regulating adrenal norepinephrine release. Our results extend those indicating a large increase in preganglionic adrenal nerve activity after 2-DG administration (7) to suggest that this increase was comprised predominantly of the activation of adrenal SPNs innervating epinephrine-secreting chromaffin cells. Both electrical and chemical stimulation of the population of sympathetic premotor neurons in the RVLM activated Epi Adr SPNs and NE Adr SPNs, consistent with the anatomic finding that the RVLM contains adrenal sympathetic premotor neurons (47, 56, 57). Although the characteristics of the excitatory responses of Epi Adr SPNs and NE Adr SPNs to chemical excitation of RVLM neurons were not markedly different, there were significant differences in the latencies of the action potentials evoked in Epi Adr SPNs and in NE Adr SPNs after electrical stimulation of RVLM. The mean response latency of NE Adr SPNs (29 ms, Fig. 2C) to RVLM stimulation corresponds to an average conduction velocity of ⬃2.4 m/s, which falls within the range of “rapidly conducting” RVLM spinal, sympathetic premotor neurons with strong baroreceptor reflex sensitivity (6, 32, 36, 45). In combination with the high degree of baroreceptor reflex sensitivity of NE Adr SPNs, these results suggest that NE Adr SPNs receive baroreceptor-modulated, premotor excitatory inputs from RVLM neurons similar to those that provide the tonic excitation to vasoconstrictor SPNs. In contrast, RVLM stimulation excited Epi Adr SPNs with a mean latency to peak of 129 ms (Fig. 2A), indicating an average conduction velocity of RVLM premotor neurons to Epi Adr SPNs of ⬃0.5 m/s, which is within the range of “slowly conducting” sympathetic premotor neurons (6, 36, 45). This observation raises several points. The absence of baroreceptor modulation of the discharge of Epi Adr SPNs suggests that their excitatory premotor inputs from RVLM would arise from an as-yet-undescribed population of RVLM neurons with slowly conducting spinal axons, but without the strong inhibitory responses to increases in arterial pressure that constitute a hallmark of sympathetic premotor neurons. The recent finding that all slowly conducting sympathetic premotor neurons (albeit barosensitive) in the RVLM are C1 cells (45) leads to the interesting speculation that a subpopulation of the phenylethanolamine-N-methyltransferase-containing DIFFERENTIATION OF ADRENAL SPNS micity, whereas 50% of the latter had a weak cardiac rhythmicity (9). Curiously, the same group indicated subsequently (8) that preganglionic adrenal nerve activity was decreased (although not as much as renal or postganglionic adrenal nerve activity) during a phenylephrine-evoked increase in arterial pressure. Because the adrenal nerves contain not only the axons of SPNs innervating epinephrine-synthesizing chromaffin cells but also the preganglionic axons of SPNs innervating the norepinephrine-synthesizing chromaffin cells and postganglionic axons innervating cortical cells and arteriolar smooth muscle, it is not possible to determine from these data whether such responses are indicative of baroreceptor control of the discharge of SPNs specifically controlling adrenal epinephrine secretion. Although determinations of catecholamine levels in adrenal venous blood could provide more specific information on the baroreflex regulation of the secretion of the two adrenal catecholamines, the sensitivity of the adrenal chromaffin cells to blood-borne agents, including ANG II (4, 12, 20, 33, 55), could produce significant changes in catecholamine secretion (e.g., during severe hypotension) independent of changes in neural input to chromaffin cells. In this regard, the responses of Epi Adr SPNs to hypotensive stimuli were not tested in this study. Finally, the differences between our results and those indicating a reduction in epinephrine in adrenal venous blood during a phenylephrine-evoked pressor response (26) may have arisen from a marked reduction in adrenal blood flow (not reported) leading to a compromised recovery of epinephrine during the pressor response. In summary, these results provide a characterization of adrenal SPNs that has allowed us to distinguish those regulating adrenal chromaffin cells secreting epinephrine from those controlling adrenal norepinephrine release. The dramatic differences between these two populations of adrenal SPNs in their reflex- and centrally evoked responses suggest that their discharge is controlled by different central neural circuits, including unique populations of adrenal sympathetic premotor neurons in the RVLM. Our findings support the distinct functional organization of the networks governing the activity of Epi Adr SPNs and NE Adr SPNs that has been proposed (18, 52) to account for the differential regulation of epinephrine and norepinephrine secretion from the adrenal medulla in response to physiological challenges and experimental stimuli. REFERENCES 1. Appel NM and Elde RP. The intermediolateral cell column of the thoracic spinal cord is comprised of target-specific subnuclei: evidence from retrograde transport studies and immunohistochemistry. J Neurosci 8: 1767–1775, 1988. 2. Bacon SJ and Smith AD. Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J Auton Nerv Syst 24: 97–122, 1988. 3. Bacon SJ, Zagon A, and Smith AD. Electron microscopic evidence of a monosynaptic pathway between cells in the caudal raphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Exp Brain Res 79: 589–602, 1990. Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 neurons in RVLM without baroreceptor sensitivity may provide a premotor input to Epi Adr SPNs. Although most studies of sympathetic premotor neurons in the RVLM focus on those that are strongly inhibited by baroreceptor input, slowly conducting, RVLM spinal neurons without baroreceptor sensitivity have been described (5). It is also possible that the RVLM stimulus-evoked excitation of Epi Adr SPNs is not direct, but rather it may involve a synaptic relay in which a rapidly conducting RVLM neuron activates a more slowly conducting, medullary or spinal input to Epi Adr SPNs. In this regard, adrenal SPNs receive a direct input, which may be serotonergic (2), from medullary raphe nuclei (3, 47, 56) that contain neurons with slowly conducting axons and that receive inputs from the RVLM. Finally, considering the short latency inhibition of Epi Adr SPNs seen with electrical stimulation in RVLM, it is worth noting that the period of the RVLM stimulus-evoked inhibition of Epi Adr SPNs overlaps that of the simultaneously evoked excitation of NE Adr SPNs, indicating that this inhibitory input is selective for Epi Adr SPNs, leading to the speculation that the descending inputs to NE Adr SPNs may activate a spinal inhibitory interneuronal input to Epi Adr SPNs. Although this inhibition of Epi Adr SPNs may have been mediated by activation of local axons within RVLM, since it was not seen with glutamate stimulation of the RVLM, the latter also activated an excitatory input to Epi Adr SPNs that may have masked an inhibition. Our conclusion that the discharge of Epi Adr SPNs is not significantly influenced by the baroreceptor reflex is derived from their consistently weak responses using the following three different approaches to activation of the baroreceptor reflex: modulation of the SPNs’ spontaneous discharge probability relative to the occurrence of the systolic pressure rise, baroreceptor loading during the pressor response to ␣-adrenergic receptor stimulation, and electrical stimulation of the baroreceptor afferents in the ADN. The effectiveness of each of these was indicated by the dramatic reductions in discharge frequency of NE Adr SPNs to the same stimulus, often within the same experiment. In combination with the responses of Epi Adr SPNs to 2-DG administration, this result suggests that sympathetically regulated secretion of adrenal epinephrine is determined more by signals pertaining to metabolic homeostasis (such as plasma glucose levels) than by those involved in the short-term regulation of arterial pressure (19), although this has not been explicitly tested in rats. Just the opposite appears to be true for the supraspinal sympathetic pathways controlling the activity of NE Adr SPNs, whose discharge could easily be completely inhibited by baroreceptor activation but was little affected by the 2-DG stimulus. Several reports have demonstrated some baroreceptor reflex regulation of adrenal nerve activity in the rat (8, 10, 26, 30, 38, 49). However, when ganglionic blockade was used to differentiate adrenal nerves that contained predominantly preganglionic vs. postganglionic axons, the former were found to have no cardiac rhyth- R1773 R1774 DIFFERENTIATION OF ADRENAL SPNS 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. ferase, dopa-decarboxylase and dopaminehydroxylase. Experientia 27: 951–952, 1971. Grynszpan-Winograd O. Adrenaline and noradrenaline cells in the adrenal medulla of the hamster: a morphological study of their innervation. J Neurocytol 3: 341–361, 1974. Hillarp NA and Hokfelt B. Evidence of adrenaline and noradrenaline in separate adrenal medullary cells. Acta Physiol Scand 25: 1–134, 1953. Ito K, Sato A, Shimamura K, and Swenson RS. Reflex changes in sympatho-adrenal medullary functions in response to baroreceptor stimulation in anesthetized rats. J Auton Nerv Syst 10: 295–303, 1984. Jensen I, Pilowsky P, Llewellyn-Smith I, Minson J, and Chalmers J. Sympathetic preganglionic neurons projecting to the adrenal medulla and aorticorenal ganglion in the rabbit. Brain Res 586: 125–129, 1992. Kesse WK, Parker TL, and Coupland RE. The innervation of the adrenal gland. I. The source of pre- and postganglionic nerve fibres to the rat adrenal gland. J Anat 157: 33–41, 1988. Klevans LR and Gebber GL. Comparison of differential secretion of adrenal catecholamines by splanchnic nerve stimulation and cholinergic agents. J Pharmacol Exp Ther 172: 69–76, 1970. Kurosawa M, Sato A, Sato Y, and Suzuki H. Undiminished reflex responses of adrenal sympathetic nerve activity to stimulation of baroreceptors and cutaneous mechanoreceptors in aged rats. Neurosci Lett 77: 193–198, 1987. Kuzmin AI, Pogorelov VM, Zaretsky DV, Medvedev OS, and Chazov EI. Comparison of the effects of 2-deoxyglucose and immobilization on secretion and synthesis rate of catecholamines in the adrenal gland: A microdialysis study in conscious rats. Acta Physiol Scand 155: 147–155, 1995. Lipski J, Kanjhan R, Kruszewska B, and Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study “in vivo.” J Physiol (Lond) 490: 729–744, 1996. MacLean MR and Ungar A. Effects of the renin-angiotensin system on the reflex response of the adrenal medulla to hypotension in the dog. J Physiol (Lond) 373: 343–352, 1986. Medvedev OS, Selivanov VN, and Kuzmin AI. Selective activation of adrenaline secretion by the rat adrenal in neuroglycopenia detected via microdialysis. Fiziol Zh 76: 1172–1178, 1996. Morrison SF. Raphe pallidus excites a unique class of sympathetic preganglionic neurons. Am J Physiol Regulatory Integrative Comp Physiol 265: R82–R89, 1993. Morrison SF, Milner TA, and Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J Neurosci 8: 1286–1301, 1988. Morrison SF and Reis DJ. Responses of sympathetic preganglionic neurons to rostral ventrolateral medullary stimulation. Am J Physiol Regulatory Integrative Comp Physiol 261: R1247– R1256, 1991. Nijima A. Baroreceptor effects on renal and adrenal nerve activity. Am J Physiol 230: 1733–1736, 1976. Paxinos G and Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 1986. Pyner S and Coote JH. Evidence that sympathetic preganglionic neurones are arranged in target-specific columns in the thoracic spinal cord of the rat. J Comp Neurol 342: 15–22, 1994. Rappaport EB, Young JB, and Landsberg L. Effects of 2-deoxy-D-glucose on the cardiac sympathetic nerves and the adrenal medulla in the rat: further evidence for a dissociation of sympathetic nervous system and adrenal medullary responses. Endocrinology 110: 650–656, 1982. Robinson RL, Culberson JL, and Carmichael SW. Influence of hypothalamic stimulation on the secretion of adrenal medullary catecholamines. J Auton Nerv Syst 8: 89–96, 1983. Scheurink A and Ritter S. Sympathoadrenal responses to glucoprivation and lipoprivation in rats. J Neurochem 50: 1302– 1308, 1993. Schramm LP, Adair JR, Stribling JM, and Gray LP. Preganglionic innervation of the adrenal gland of the rat: a study using horseradish peroxidase. Exp Neurol 49: 540–553, 1975. Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 4. Breidert M, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA, and Holst JJ. Angiotensin II regulates both adrenocortical and adrenomedullary function in isolated perfused pig adrenals. Peptides 17: 287–292, 1996. 5. Brown DL and Guyenet PG. Cardiovascular neurons of brain stem with projections to spinal cord. Am J Physiol Regulatory Integrative Comp Physiol 247: R1009–R1016, 1984. 6. Brown DL and Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985. 7. Carlsson S, Skarphedinsson JO, Delle M, Hoffman P, and Thoren P. Differential responses in post- and pre-ganglionic adrenal sympathetic nerve activity and renal sympathetic nerve activity after injection of 2-deoxy-D-glucose and insulin in rats. Acta Physiol Scand 145: 169–175, 1992. 8. Carlsson S, Skarphedinsson JO, Delle M, Hoffman P, and Thoren P. Reflex changes in post- and preganglionic sympathetic adrenal nerve activity and postganglionic sympathetic renal nerve activity upon arterial baroreceptor activation and during severe haemorrhage in the rat. Acta Physiol Scand 144: 317–323, 1992. 9. Carlsson S, Skarphedinsson JO, Jennische E, Delle M, and Thoren P. Neurophysiological evidence for and characterization of the post-ganglionic innervation of the adrenal gland in the rat. Acta Physiol Scand 140: 491–499, 1990. 10. Claassen DE, Morgan DA, Hirai T, and Kenney MJ. Nonuniform sympathetic nerve responses after sustained elevation in arterial pressure. Am J Physiol Regulatory Integrative Comp Physiol 271: R1264–R1269, 1996. 11. Cohen WR, Piasecki GJ, Cohn HE, Susa JB, and Jackson BT. Sympathoadrenal responses during hypoglycemia, hyperinsulinemia, and hypoxemia in the ovine fetus. Am J Physiol Endocrinol Metab 261: E95–E102, 1991. 12. Corwin EJ, Seaton JF, Hamaji M, and Harrison TS. Central role for angiotensin in control of adrenal catecholamine secretion. Am J Physiol Regulatory Integrative Comp Physiol 248: R363–R370, 1985. 13. Coupland RE, Pyper AS, and Hopwood D. A method for differentiating between noradrenaline- and adrenaline-storing cells in the light and electron microscope. Nature 201: 1240– 1242, 1964. 14. Dagerlind Pelto-Huikko M, Diez M and Hókfelt T. Adrenal medullary ganglion neurons project into the splanchnic nerve. Neuroscience 69: 1019–1023, 1995. 15. Dorsey DA and Schmidt RE. Correlation of GAP-43 immunoreactivity with subpopulations of chromaffin cells in rat adrenal medulla. Neurosci Lett 162: 29–33, 1993. 16. Drake K, Gateva E, Deutsch J, and Cohen WR. Sex differences in the adrenal catecholamine response to hypoglycemia in rats. Metabolism 47: 121–124, 1998. 17. Edwards AV and Jones CT. Autonomic control of adrenal function. J Anat 183: 291–307, 1993. 18. Edwards SL, Anderson CR, Southwell BR, and McAllen RM. Distinct preganglionic neurons innervate noradrenaline and adrenaline cells in the cat adrenal medulla. Neuroscience 70: 825–832, 1996. 19. Fater DC, Sundet WD, Schultz HD, and Goetz KL. Arterial baroreceptors have minimal physiological effects on adrenal medullary secretion. Am J Physiol Heart Circ Physiol 244: H194–H200, 1983. 20. Feuerstein G, Boonyaviroj P, Gutman Y, Khosla MC, and Bumpus FM. Adrenal catecholamine response to haemorrhage abolished by an angiotensin antagonist. Eur J Pharmacol 41: 85–86, 1977. 21. Folkow B and von Euler US. Selective activation of noradrenaline and adrenaline producing cells in the cat’s adrenal gland by hypothalamic stimulation. Circ Res 2: 191–195, 1954. 22. Gagner JP, Gauthier S, and Sourkes TL. Descending spinal pathways mediating the responses of adrenal tyrosine hydroxylase and catecholamines to insulin and 2-deoxyglucose. Brain Res 325: 187–197, 1985. 23. Goldstein M, Fuxe K, Hokfelt T, and Joh TH. Immunohistochemical studies on phenylethanolamine-N-methyltrans- DIFFERENTIATION OF ADRENAL SPNS 52. 53. 54. 55. 56. 57. during hemorrhagic hypotension in rats. Circ Res 64: 686–694, 1989. Vollmer RR. Selective neural regulation of epinephrine and norepinephrine cells in the adrenal medulla— cardiovascular implications. Clin Exp Hypertens 18: 731–751, 1996. Vollmer RR, Balcita JJ, Sved AF, and Edwards DJ. Adrenal epinephrine and norepinephrine release to hypoglycemia measured by microdialysis in conscious rats. Am J Physiol Regulatory Integrative Comp Physiol 273: R1758–R1763, 1997. Vollmer RR, Baruchin A, Kolibal-Pegher SS, Corey SP, Stricker EM, and Kaplan BB. Selective activation of norepinephrine- and epinephrine-secreting chromaffin cells in rat adrenal medulla. Am J Physiol Regulatory Integrative Comp Physiol 263: R716–R721, 1992. Vollmer RR, Corey SP, Meyers SA, Stricker EM, and Fluharty SJ. Angiotensin augments epinephrine release in pithed rats fed a low-sodium diet. Am J Physiol Regulatory Integrative Comp Physiol 258: R187–R192, 1990. Wesselingh SL, Li YW, and Blessing WW. PNMT-containing neurons in the rostral medulla oblongata (C1, C3 groups) are transneuronally labelled after injection of herpes simplex virus type 1 into the adrenal gland. Neurosci Lett 106: 99–104, 1989. Zagon A and Smith AD. Monosynaptic projections from the rostral ventrolateral medulla oblongata to identified sympathetic preganglionic neurons. Neuroscience 54: 729–743, 1993. Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on November 4, 2016 45. Schreihofer AM and Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997. 46. Strack AM, Sawyer WB, Marubio LM, and Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988. 47. Strack AM, Sawyer WB, Platt KB, and Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 491: 274–296, 1989. 48. Sun CL, Thoa NB, and Kopin IJ. Comparison of the effects of 2-deoxyglucose and immobilization on plasma levels of catecholamines and corticosterone in awake rats. J Endocrinol 105: 305–311, 1979. 49. Tochihara M. Reflex control of cardiac sympathetic nerve activity in anesthetized rats. Hokkaido Igaku Zasshi 71: 247–258, 1996. 50. Tomlinson A and Coupland RE. The innervation of the adrenal gland. IV. Innervation of the rat adrenal medulla from birth to old age A descriptive and quantitative morphometric and biochemical study of the innervation of chromaffin cells and adrenal medullary neurons in Wistar rats. J Anat 169: 209–236, 1990. 51. Victor RG, Thoren P, Morgan DA, and Mark AL. Differential control of adrenal and renal sympathetic nerve activity R1775