* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The circadian visual system, 2005
Signal transduction wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Haemodynamic response wikipedia , lookup
Multielectrode array wikipedia , lookup
Synaptogenesis wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Subventricular zone wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Synaptic gating wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuroanatomy wikipedia , lookup
Circumventricular organs wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
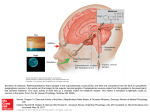
Hypothalamus wikipedia , lookup
Optogenetics wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Channelrhodopsin wikipedia , lookup
BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 a v a i l a b l e a t w w w. s c i e n c e d i r e c t . c o m w w w. e l s e v i e r. c o m / l o c a t e / b r a i n r e s r e v Review The circadian visual system, 2005 L.P. Morina,⁎, C.N. Allenb a Department of Psychiatry and Graduate Program in Neuroscience, Stony Brook University, Stony Brook, NY 11794, USA Department of Physiology and Pharmacology and Centre for Research on Occupational and Environmental Toxicology, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA b A R T I C LE I N FO AB S T R A C T Article history: The primary mammalian circadian clock resides in the suprachiasmatic nucleus (SCN), a Accepted 9 August 2005 recipient of dense retinohypothalamic innervation. In its most basic form, the circadian Available online 5 December 2005 rhythm system is part of the greater visual system. A secondary component of the circadian visual system is the retinorecipient intergeniculate leaflet (IGL) which has connections to Keywords: many parts of the brain, including efferents converging on targets of the SCN. The IGL also Circadian provides a major input to the SCN, with a third major SCN afferent projection arriving from Suprachiasmatic the median raphe nucleus. The last decade has seen a blossoming of research into the SCN anatomy and function of the visual, geniculohypothalamic and midbrain serotonergic Intergeniculate leaflet systems modulating circadian rhythmicity in a variety of species. There has also been a IGL substantial and simultaneous elaboration of knowledge about the intrinsic structure of the SCN. Many of the developments have been driven by molecular biological investigation of the circadian clock and the molecular tools are enabling novel understanding of regional function within the SCN. The present discussion is an extension of the material covered by the 1994 review, “The Circadian Visual System.” © 2005 Elsevier B.V. All rights reserved. ⁎ Corresponding author. Fax: +1 631 444 7534. E-mail address: [email protected] (L.P. Morin). 0165-0173/$ – see front matter © 2005 Elsevier B.V. All rights reserved. doi:10.1016/j.brainresrev.2005.08.003 2 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 Abbreviations: 5-HT, serotonin CALB, calbindin CALR, calretinin CCK, cholecystokinin ENK, enkephalin GABA, gamma amino butyric acid GRP, gastrin releasing hormone IGL, intergeniculate leaflet NPY, neuropeptide Y NT, neurotensin PACAP, pituitary adenylate cyclase-activating polypeptide SCN, suprachiasmatic nucleus SP, substance P SS, somatostatin VIP, vasoactive intestinal polypeptide VP, vasopressin Contents 1. 2. 3. 4. 5. 6. Introduction and caveats . . . . . . . . . . . . . . . . . . . . . . . . . . . . Psychophysics of the circadian rhythm system . . . . . . . . . . . . . . . . . Organization of the suprachiasmatic nucleus . . . . . . . . . . . . . . . . . 3.1. Nomenclature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.2. Distribution of cell phenotypes . . . . . . . . . . . . . . . . . . . . . 3.3. Intra-SCN connections . . . . . . . . . . . . . . . . . . . . . . . . . . Retinal photoreceptors and projections . . . . . . . . . . . . . . . . . . . . 4.1. Melanopsin ganglion cell characteristics . . . . . . . . . . . . . . . . 4.2. Retinal ganglion cell projections and bifurcation . . . . . . . . . . . 4.3. Classical and ganglion cell photoreceptor contribution to regulation light reflex and melatonin suppression . . . . . . . . . . . . . . . . 4.3.1. Circadian rhythms . . . . . . . . . . . . . . . . . . . . . . . 4.3.2. Masking, pupillary light reflex and melatonin suppression. . . Neuromodulators of the retinohypothalamic tract . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . of circadian . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . rhythms, masking, . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . the . . . . . . . . . . . . 3 . . . . 4 . . . . 5 . . . . 5 . . . . 5 . . . . 8 . . . . 8 . . . . 8 . . . . 9 pupillary . . . 11 . . . 11 . . . 11 . . . 12 5.1. Glutamate . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.2. N-Acetylaspartyl glutamate . . . . . . . . . . . . . . . . . . . 5.3. Glutamate receptors . . . . . . . . . . . . . . . . . . . . . . . 5.4. Nitric oxide and RHT signal transduction . . . . . . . . . . . 5.5. PACAP. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.6. Substance P . . . . . . . . . . . . . . . . . . . . . . . . . . . . SCN structure–function relationships . . . . . . . . . . . . . . . . . 6.1. Function of cellular neuromodulators in the SCN . . . . . . . 6.1.1. GABA . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.1.2. VIP and VIP/PACAP receptors. . . . . . . . . . . . . . 6.1.3. Calbindin and the central subnucleus of the hamster 6.1.4. Bombesin and gastrin releasing peptide. . . . . . . . 6.1.5. Neuromedin U and neuromedin S . . . . . . . . . . . 6.1.6. Vasopressin . . . . . . . . . . . . . . . . . . . . . . . 6.1.7. Diurnality–nocturnality . . . . . . . . . . . . . . . . . 6.2. Acetylcholine . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.3. Neurophysiology of the SCN action potential rhythm . . . . . 6.4. Inter-SCN differences in gene expression . . . . . . . . . . . 6.5. Regional expression of Fos . . . . . . . . . . . . . . . . . . . 6.5.1. Hamster . . . . . . . . . . . . . . . . . . . . . . . . . 6.5.2. Rat . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.6. Regional clock gene expression . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . SCN . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12 12 13 13 14 15 17 18 18 18 19 21 21 21 22 22 23 25 25 25 25 26 3 BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 6.7. 7. 6.8. SCN 7.1. 6.6.1. Mouse. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.6.2. Rat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.6.3. Hamster . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Stimulus induction of gene expression . . . . . . . . . . . . . . . . . . 6.7.1. Photic stimuli . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.7.2. Nonphotic stimuli . . . . . . . . . . . . . . . . . . . . . . . . . 6.7.3. Inconsistencies between phase response and gene expression Polysialic acid and SCN function . . . . . . . . . . . . . . . . . . . . . connectivity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Humoral factors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.2. Efferent anatomy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.3. Afferent anatomy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8. Intergeniculate leaflet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.1. Nomenclature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.2. Development . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.3. Anatomy. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.3.1. Geniculohypothalamic tract. . . . . . . . . . . . . . . . . . . . . . 8.3.2. Connectivity and relationship to sleep, visuomotor and vestibular 8.4. Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.4.1. Neurophysiology . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.4.2. Nonphotic regulation of rhythmicity . . . . . . . . . . . . . . . . . 8.4.3. Photic regulation of rhythmicity . . . . . . . . . . . . . . . . . . . 9. Serotonin and midbrain raphe contribution to circadian rhythm regulation . . . 9.1. Effects of serotonin-specific neurotoxins . . . . . . . . . . . . . . . . . . . 9.2. Serotonin receptor function . . . . . . . . . . . . . . . . . . . . . . . . . . 9.3. Regulation and consequences of serotonin release . . . . . . . . . . . . . 9.4. Serotonergic potentiation of light-induced phase shifts . . . . . . . . . . . 10. Failure to re-entrain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11. Masking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12. The future . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Introduction and caveats There have been a large number of new developments with respect to knowledge about the anatomy and physiology of circadian rhythm regulation since publication of our 1994 review, “The Circadian Visual System” (Morin, 1994). As is nearly always the case with such projects, many of the developments were well underway, and in some instances completed, prior to the actual publication of that review. Most notably, much of the presentation in the 1994 review concerning the serotonergic system was incomplete or wrong and understanding of the issues changed substantially soon after publication of the review. Other issues emerged as unanticipated major developments. One of these has been the discovery of the photopigment, melanopsin, in retinal ganglion cells and the subsequent establishment of those ganglion cells as a special class of photoreceptors. The steady growth of molecular biological contributions to the understanding of circadian clock mechanisms in invertebrates has been translated into parallel evaluation of clock mechanisms in mammals. This development has created new avenues for architectural analysis of the suprachiasmatic nucleus (SCN) function according to the regional distributions of cell phenotypes comprising the circadian clock. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26 27 27 28 28 28 29 30 31 31 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31 32 34 34 34 34 34 34 35 35 36 37 38 38 38 39 40 41 42 43 43 43 The purpose of the current review is to continue the theme of the original, but to do so with minimal redundancy. The focus of the current presentation will be on necessary modifications to the perspective presented 10 years ago, and to the substantial additions to the general body of knowledge relating to the circadian visual system. The title of the 1994 review, “The Circadian Visual System,” remains of some import. We believe it is worth emphasizing that, taken as a whole, the research topic under consideration is “circadian,” is “visual” and certainly is a “system.” That is an aspect of the research topic of importance that should not be underestimated. We do not intend to cover all facets of circadian rhythm research and we also expect to have inadvertently overlooked research worthy of inclusion here. To the extent that has happened, we apologize. On a technical note, when specific cell types are mentioned as containing a particular neuromodulator, the comment refers to the fact that it has been demonstrated by immunohistochemical means. The work that is particularly ignored concerns the molecular biology of the SCN or other tissues. There will be no effort to review the burgeoning literature concerning the molecular mechanisms of the central circadian clock or of molecular oscillations in peripheral structures. Rather, we will touch on this literature when it can be used to amplify understanding of 4 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 the system properties governing circadian rhythm regulation. One issue at the forefront of the list of mysteries presently confronting that understanding is the spatial distribution of SCN neurons exhibiting clock-like behavior. The general question is, “Are all SCN cells pacemakers?” While some experimental work suggests that the answer is no, the topic is an area of intense research and is discussed in some detail below. A second question is, “Do all pacemaker cells in the SCN oscillate with the same phase?” Evaluation of a variety of molecular oscillations in SCN cells has given the impression that the molecular behavior of a whole SCN reflects the behavior of the individual cells. While this may be true on average, many individual SCN neurons do not express rhythmicity in phase with the average of the whole (Quintero et al., 2000). This development in the analytical approach to pacemaker activity among groups of cells emphasizes the importance of molecular activity within individual cells and points to the need to evaluate spatial relationships and connectivity among oscillatory neurons within the SCN. When microelectrode arrays are used for long-term recordings, the action potential firing rhythm periods of individual of SCN neurons show a greater diversity in dispersed cultures compared to organotypic cultures. These data suggest that interneuronal communication is required to fine tune the period of the clock (Nakamura et al., 2002; Herzog et al., 1998; Liu et al., 1997a; Albus et al., 2002). The final behavioral period is an average of these individual neuronal periods (Liu et al., 1997a). The demonstration of phase differences among SCN neurons is a variant on the larger theme indicating differences in phase between the two SCN in a single animal (De la Iglesia et al., 2000). Thus, from the point of view of this review, utility of the molecular analysis of cellular clocks serves to highlight the need to understand the system properties intrinsic to the SCN, as well as its regulation of efferent response to afferent information, whether that information be visual or some other sensory modality. 2. Psychophysics of the circadian rhythm system The Pickard et al. (1987) IGL lesion paper was one of the first to employ nonsaturating light stimuli to elicit circadian rhythm phase responses. The method eliminated ceiling effects caused by saturating light, providing a more accurate view of psychophysical sensitivity to light, rather than a simple statement that animals respond (or not) to the stimulus. Subsequently, the psychophysics of circadian rhythm phase response to light presented at CT19 has been explored in greater detail. The hamster (Nelson and Takahashi, 1991, 1999; Zhang et al., 1996), mouse (van den Pol et al., 1998) and gerbil (Dkhissi-Benyahya et al., 2000) circadian systems estimate the importance of an acute photic stimulus by effectively “counting” photons. It may be more appropriate to refer to this activity as “temporal integration” which is a broader term. The process is demonstrated by the fact that there is a clear relationship between the stimulus duration and irradiance (photons/cm2) with respect to the magnitude of the phase shift. That is, dim, long duration stimuli have the same effect as brief but bright stimuli, if the irradiances (i.e., total photons or Joules/cm2) are equivalent. Most impressively, the photic intensity information in a series of light pulses appears to be summed across the series (Nelson and Takahashi, 1991), as long as the individual pulses are of sufficient duration (i.e., minutes). The integrative capacity of the circadian system appears to be time-limited to the extent that, under test conditions explored to date, if a photic stimulus is sufficient to produce a maximal phase shift, more photons at the same time or up to 2 h later have no additional effect on phase shift magnitude (Nelson and Takahashi, 1999). In such cases, the circadian system is deemed to be “saturated” with respect to its ability to show a further phase response to light. An unexpected result (van den Pol et al., 1998), demonstrated the important point that a form of “temporal integration” may occur even when the light stimulus is subthreshold. For example, in the mouse or hamster, a brief (2–3 ms), bright (1013 photons/cm2/s) flash will not elicit a phase shift (Nelson and Takahashi, 1991; van den Pol et al., 1998), but a series of flashes totaling 120 ms will yield a normal phase response (van den Pol et al., 1998). Thus, some form of summation has occurred to elicit the phase shifts. It is not yet clear, however, that there is a systematic relationship between the number of flashes and the magnitude of the phase shift. Evidence from studies employing a series of 5 to 45 light flashes, each only 10 μs long, suggests otherwise (Arvanitogiannis and Amir, 1999). Each such series effectively induces FOS-IR in the SCN and yields 75 min phase shifts when delivered to rats at CT13. Thus, the fact of a light-induced phase shift may be separable from the process of “integration.” The entire visual system has been intact in all studies to date that involve “temporal integration.” Therefore, there is presently no information regarding the extent to which the integration mechanism includes or excludes the retina, SCN, IGL, visual midbrain, the serotonergic or other systems. Unilateral enucleation eliminates 50% of the retinal photoreceptors and their associated RHT projections, but had no effect on hamster re-entrainment rate (Stephan et al., 1982). On the other hand, the same publication showed that both advance and delay shifts of rats were retarded following unilateral enucleation. D. Earnest (unpublished work cited by Pickard and Turek, 1983) failed to find an effect of unilateral enucleation on hamster circadian period in LL. Similarly, unilateral enucleation appears to have no effect on lightinduced FOS in rat (Beaulé et al., 2001a) or hamster SCN (Muscat and Morin, unpublished data), or in SCN of hamster with one eye occluded, except in response to high irradiance or long duration light (Muscat and Morin, in press). In each of these investigations, saturating stimuli were employed and the experimental methods not sufficiently sensitive to determine whether there is an effect of unilateral enucleation on rate of re-entrainment or any other measure of circadian rhythm regulation (but see Tang et al., 2002 for a broad set of results suggesting a large effect of unilateral enucleation). Unilateral enucleation is also reported to shorten the circadian period of rats in LL, while a unilateral SCN lesion has no similar effect (Donaldson and Stephan, 1982). Given that the IGL regulates 50% of the hamster circadian period response to LL (Pickard et al., 1987; Morin and Pace, 2002) (no BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 equivalent studies have been done in the rat), the effect of enucleation could be attributable exclusively to loss of the retinal input to the IGL. Light induction of FOS-IR in the gerbil SCN (DkhissiBenyahya et al., 2000) has been interpreted as obeying the temporal integration rule found in the hamster for phase shifts and melatonin suppression. However, over the same irradiance range, the effect of light on FOS in ganglion cells of the gerbil retina appears to be all-or-none, rather than additive. Similar results have been found for light-induced FOS in hamster IGL cells (Muscat and Morin, unpublished data) and imply that neither the ganglion cell nor IGL neuron properties are altered by light in a manner consistent with “photon counting” responses of the circadian rhythm system. 3. Organization of the suprachiasmatic nucleus 3.1. Nomenclature An accepted anatomical nomenclature affords the opportunity to standardize the description of brain structures. This has not yet happened with respect to the SCN intrinsic anatomy. Historically, there have been two approaches to SCN organization, one based on the rat model and the other based on the hamster. The rat SCN has been commonly divided into dorsomedial and ventrolateral divisions. Although these are geographic designations, the conceptualization is based on the fact that, in this species, the vasoactive intestinal polypeptide (VIP) neurons plus most of the geniculohypothalamic tract (GHT) and retinal afferents occupy the latter, while the former is the location of most vasopressin (VP) neurons. The hamster SCN has a caudocentral region containing neurons identified by several different peptides and this has been referred to as the “central subnucleus” which partially overlaps with the region containing VIP neurons. In this species, there has been no anatomical attempt to designate a “ventrolateral” SCN division, although some investigators have applied rat-derived descriptors to the hamster. As indicated in Section 3.2, identification of a dorsomedial area based upon the location of VP-immunoreactive neurons is of dubious value, even in the rat. The nomenclature has become further confused with references to a “core and shell” SCN organization. For example, in a 1996 review, one of us (LPM) used the terms as an analogy to facilitate understanding of the relationship between the hamster central subnucleus and its surround (Miller et al., 1996). However, another contributor to that article employed the same terms in a different review, also published in 1996, as labels of regions more or less equivalent to the previously used dorsomedial and ventrolateral divisions in the rat SCN (Moore, 1996). Given the reasonable likelihood that a common SCN organizational plan will emerge and be applicable to rodents, and that apparent structural differences between rat and hamster SCN will be reconciled, we believe it is premature to adopt the “core and shell” terminology based upon their application to anatomical description in one species or another. More important, and as discussed below, we are of the opinion that the “core and shell” terminology (A) is not supported by the data in any 5 species; (B) is not consistent with the apparent anatomical differences between species; (C) has been employed with different meanings in different species, as well as within the same species; (D) unduly simplifies a structure which has a still-emerging level of organizational complexity and (E) if accepted in an unquestioning manner, will have a direct negative influence on experimental design, while augmenting the likelihood of erroneous and incomplete interpretation of new and available data. Another reason for caution in using the core/shell terminology is that they are based primarily on the localization of the neuronal cell body. SCN neurons have a soma that is approximately 10–15 μm in diameter and generally contain 1–3 dendrites. These dendrites extend for a distance of about 250 μm. Given the small size of the SCN (300–400 μm wide and 400–500 μm tall), a dendrite has the ability to project across much of the SCN (van den Pol, 1980, 1991; Silver and Brand, 1979; Jiang et al., 1997). This implies, for example, that a cell body in the dorsal part of the SCN could send a dendrite ventrally into the region where the densest retinal terminals are located. Indeed, individual dendrites from the dorsal SCN have been shown to extend into the ventral SCN and further into the optic chiasm (Silver and Brand, 1979). Rejection of “core and shell” nomenclature specifically does not mean that there is no specialized organization within the SCN. However, we believe the research goal should be to determine anatomical reality with a view toward consistent and constructive use of a nomenclature that is sufficiently fluid to permit revisions reflecting the evolution of knowledge about the SCN. 3.2. Distribution of cell phenotypes Essential to the understanding of circadian rhythm system function is knowledge about the intrinsic organization of the SCN, site of the predominant circadian clock in mammals. It has been commonly stated that all SCN neurons appear to contain GABA (Moore and Speh, 1993; Morin and Blanchard, 2001; Abrahamson and Moore, 2001), although an immunohistochemical, electron microscopic study of the rat SCN suggests that only 40–70% contain GABA (Castel and Morris, 2000). There is a dense GABAergic plexus throughout the nucleus, but a clear spatial organization of the SCN is evident if other cellular characteristics are evaluated. The two most obvious are the distribution of cell phenotypes and the distribution of cells projecting to non-SCN targets. The former can be ascertained by immunohistochemical means, while the latter relies largely upon retrograde tracer injections. A cogent argument has been made supporting the view that the SCN consists of two major divisions, a dorsomedial area related to the presence of VP neurons and a ventral area related to the presence of VIP cells, dense retinal afferents and neuropeptide Y-containing terminals of the GHT (Moore et al., 2002). However, the evidence seems to suggest the presence of more than two subdivisions within the SCN, with some more expressly related to cell phenotypes than others. For example, the rat SCN has VP cells distributed in a crescent extending from a ventral location, medially, dorsally and laterally, almost encircling a region that contains the VIP neurons. 6 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 These two areas are distinct from, but appear to overlap with, a predominantly dorsolateral region containing calretinin (CALR) and enkephalin (ENK) neurons (Moore et al., 2002). Encompassed by the region of VIP cells is a smaller domain of neurotensin neurons. In the mouse, the regional situation resembles that of the rat to the extent that there appear to be multiple SCN areas characterized by one or more particular cell phenotypes. VP cells are scattered across the rostral SCN, are located predominantly in the typical dorsomedial aspect of the central SCN and are again scattered through the entire caudal SCN. Some cells are also present in the ventral and ventrolateral SCN. ENK cells are grouped as a collection in the dorsal SCN, while VIP neurons are generally localized to the ventral part of the nucleus. GRP cells occupy a more central region, slightly dorsal to the VIP neurons and ventral to the ENK cells (Abrahamson and Moore, 2001; Silver et al., 1999). A green fluorescent protein reporter which minimizes interference from GRP immunoreactivity in cell processes, indicates that these neurons occupy a large percentage of the central mouse SCN and that the GRP region extends dorsolaterally to the perimeter of the nucleus (Karatsoreos et al., 2004). Interpretation of regional distinctiveness based on cell phenotype may be limited by the experimental methods. For example, recent data suggest that discrete VP and VIP regions may be more apparent than real. Immunohistochemistry for VP peptide generally reveals dense staining in the dorsomedial hamster SCN where there is a mix of cells and processes in which cells are often difficult to identify (e.g., Miller et al., 1996; Kalsbeek et al., 1993). In marked contrast, in situ hybridization methods detecting VP mRNA enable clear visualization of individual neurons. This procedure reveals that, at certain circadian times, the gene is active over a much wider portion of the SCN (Silver et al., 1999; Hamada et al., 2001), including areas that do not contain neurons clearly identifiable by VP peptide content. In fact, at certain circadian times, virtually no VP mRNA at all is detected in the SCN (CT20), whereas at CT8, the VP gene appears to be active in the majority of anterior SCN neurons (Hamada et al., 2004a). In situ hybridization methods also demonstrate a much larger distribution of VP mRNA in the rat SCN than expected from comparable results based on immunohistochemistry (Dardente et al., 2002). The method-related differences are also apparent when evaluating rat VIP. These results plus those for VP mRNA or peptide indicate clearly different, but extensively overlapping, distributions of VP and VIP cells in the rat SCN. The data have implications for all allegations of discreet VP or VIP regions and to any specific functions related to those cells or the areas in which they are purportedly found. Despite the foregoing caution, analysis of the hamster SCN indicates that it shares with other rodents the apparent separation of a VP region from a VIP region (Fig. 1) because there is no question that the most persistently evident dorsomedial part of the VP region does not overlap with the VIP region (Hamada et al., 2004a; Card and Moore, 1984). The hamster SCN also has a dorsolateral region generally devoid of a presently known specific cell phenotype. The hamster SCN also contains a feature initially thought to be unique to this species, namely a small, fairly central region identifiable by its distinct sets of neurons. The region was originally identified as Fig. 1 – Schematic organization of the hamster SCN at a level through the central subnucleus identified by SP, CALB, CALR and GRP immunoreactive cells. (A) Approximate locations of cell types. VP, SS and CCK are found in a generally dorsomedial location, although this may vary according to time of day (indicated by shading). CCK cells are also evident ventrolaterally (black dots); (B) terminal fields of the three major afferent pathways. PACAP and glutamate from retinal projections occupy the entire SCN, to a varying degree (see Part C of this figure). 5-HT terminals from the median raphe nucleus fill essentially the entire nucleus, but only sparsely in the central and dorsolateral regions. NT, NPY, ENK and GABA arrive from the IGL, although the GABA terminals from the IGL have not been explicitly identified (see text) and (C) identifiable zones of retinal projections. Black = region of dense retinal innervation, predominantly from the contralateral retina. Cross-hatched = region of modest innervation predominantly from the ipsilateral retina. Shaded gray = region of lesser, approximately equal, innervation from each retina. This diagram is a guide, not a rule. The lines do not represent absolute boundaries. BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 the location of substance P (SP) cells (Morin et al., 1992) visualized with the use of colchicine. However, the same region contains numerous cells synthesizing calbindin (CALB) (Silver et al., 1996a) which is more easily visualized. The region also contains neurons immunoreactive to gastrin releasing peptide (GRP) (Miller et al., 1996) and calretinin (CalR; Silver et al., 1996a; Blanchard and Morin, unpublished data). Furthermore, this caudocentral SCN subnucleus overlaps to some degree with the VIP region (Aioun et al., 1998). The complexity of the caudocentral region of the hamster SCN is borne out by colocalization studies. About 8% (Silver et al., 1996a) or 65% (LeSauter et al., 2002), 37 and 33% of CalB cells have colocalized SP, GRP and VIP, respectively. About 80–90% of SP cells and 43% of GRP cells also contain CalB and 60% of VIP neurons located within the region of the CalB neurons actually containing CalB (LeSauter et al., 2002). GRP has also been observed colocalized with VIP (Aioun et al., 1998). Colocalization of CalB and cholecystokinin (CCK) does not occur (LeSauter et al., 2002). CalR has not been examined for the possibility of colocalization in the SCN, nor have most other combinations of antigens. The region containing VP neurons is matched reasonably well by the locations of CCK cells. Nevertheless, to the limited extent that the issue has been examined, there is no colocalization of VP and CCK in the hamster (Blanchard and Morin, unpublished data). In the most ventrolateral part of the nucleus, CCK cells are evident at a location containing VP fibers (LeSauter et al., 2002). The caudocentral SCN subnucleus may be special with respect to circadian rhythm regulation. This issue is considered separately below. From the comparative anatomical perspective, it is useful to question the extent to which other species have a clearly defined region of likely homology. The answer is not clear. The only other species known to date with SP cells at a similar location is the Djungarian hamster (Reuss and Bürger, 1994). The grass rat has CALB-IR neurons clustered in the SCN (Mahoney et al., 2000), apparently more centrally located than those in the hamster in which the subnucleus is located in the caudal half of the SCN. In rats, CALB-IR neurons are more prevalent in the ventral SCN, but are not as tightly clustered as in the hamster (Mahoney et al., 2000; Arvanitogiannis et al., 2000). In mice, CALB neurons are present within the general central SCN area, the surrounding region and generally throughout the entire nucleus (Abrahamson and Moore, 2001). However, the mouse SCN does have a clearly defined central region notable because of the absence of a specific set of identifiable neurons (LeSauter et al., 2003). This region corresponds most closely to the location of neurotensin and GRP neuron clusters (Abrahamson and Moore, 2001). A region with reasonably clear homology to the hamster central subnucleus has been described in the golden mantled squirrel, but is recognized by a distinct cluster of ENK, rather than SP, cells (Smale et al., 1991). The rat SCN is more compressed along the dorsoventral axis than it is in the mouse or hamster. A cell grouping obviously homologous to those in the hamster caudocentral subnucleus has not been described in the rat. However, an argument can be made that cells containing GRP occupy a similar position. The GRP cells, in particular, meet the criteria of being tightly distributed, more or less centrally located 7 within the SCN, with a distribution not identical to another known phenotype (Dardente et al., 2002 but see Moore et al., 2002 for a different perspective). The group of rat GRP cells appears to be smaller than the group of VIP cells and positioned within the distribution of those cells. A small number of NT neurons has been described in the rat SCN in a distribution that overlaps with the lateral collection of VIP cells (Moore et al., 2002). Another candidate rat SCN homolog of the caudocentral hamster SCN subnucleus consists of the region occupied by CalR cells. These are present in a location distinctly different from the other cell phenotypes in this species, overlapping the dorsomedial VP cells to some extent, but largely occupying a separate region in the dorsal part of the nucleus (Moore et al., 2002). As has been noted previously (Morin, 1994), there are substantial species differences in SCN structure. However, there are at least two organizational characteristics which many species seemtoshare.ThesearetherespectivegenerallocationsoftheVP and VIP cell groups. The former tends to occupy a dorsomedial SCN position, while the latter group sits centrally in the ventral part of the nucleus (Fig. 1). This spatial characteristic has been observedintheSCNofrat(Moore,1983),hamster(CardandMoore, 1984), grass rat (Smale and Boverhof, 1999), golden mantled groundsquirrel(Smaleetal.,1991),Europeangroundsquirrel(Hut et al., 2002), Octodon degus (Goel et al., 1999), blind mole rat (Negroni et al., 1997), monkey (Moore, 1993) and human (Moore, 1993).However,theminkandmuskshrewarestrikingexceptions to this rule as these species have no VP neurons present at the location normally identified as the SCN (Martinet et al., 1995; Tokunaga et al., 1992). Cassone et al. (1988) evaluated comparative SCN organization by analyzing retinal projections to the SCN and the relative distribution of various peptidergic neurons. They distinguish “visual” and “nonvisual” regions of the SCN, with the latter receiving a much reduced density of retinal projections compared to the former. The “visual” region usually contains VIP cells, although it is noteworthy that the specific location of this region varies. In the eutherian mammals examined (house mouse, guinea pig, cat, pig), the “visual” region resides in the ventral or ventrolateral SCN. Among marsupials, there are no VIP cells evident in the SCN of the kowari or stripe-faced dunnart, while in the bandicoot, VIP cells are codistributed medially and dorsomedially with the VP neurons. The retinal projection of the Virginia opossum and gray short-tail opossum distributes ventrally and medially in the caudal half of the SCN (Cassone et al., 1988). In these two species, VIP and VP neurons are codistributed through the retinorecipient region and medially in more rostral SCN. Thus, in general, VIP neurons tend to be associated with the densely retinorecipient region, whereas the VP neurons are also located there in some species, but not in others, including most of the eutherian mammals. Whether these differences in peptidergic cell distribution reflect the functional organization of the SCN remains to be demonstrated. It is also worth noting that the laterodorsal SCN of most mammals is a “specialized” region remarkable because of the general absence of non-GABA cell phenotypes (Abrahamson and Moore, 2001; Moore et al., 2002; Silver et al., 1999; Card and Moore, 1984; Smale and Boverhof, 1999; Smale et al., 1991; Goel et al., 1999; Cassone et al., 1988). 8 3.3. BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 Intra-SCN connections Studies of intra-SCN connections fall into two basic categories. One involves filling individual cells and mapping processes within the nucleus. The second involves the use of tract tracers applied iontophoretically or by small volume injection. The first procedure is limited by the fact that relatively few cells can be examined, usually only one per SCN. A successful application of the cell filling technique has demonstrated that dendritic processes of CALB neurons in the hamster SCN have a predominantly dorsomedial orientation (Jobst et al., 2004). This suggests that CALB neurons receive input from dorsomedially located neurons, many (but not all) of which contain VP or CCK (LeSauter et al., 2002). The tract tracer application method has the advantage of labeling more cells, but the disadvantage of usually spilling label into regions adjacent to the intended target. With that caveat in mind, small iontophoretic injections of biotinylated dextran amine (BDA) into the SCN have provided a good initial appraisal of connectivity within the nucleus. Confocal microscopic analysis of the injected brains shows that most, but not all, of the cell phenotypes are connected to CALB neurons (Fig. 2), the focus of this particular study (LeSauter et al., 2002). Particularly noteworthy is the absence of efferent or afferent connections between CALB and VP neurons. Interconnections among other pairs of neuron phenotypes have not been examined. Use of the Bartha pseudorabies virus as a tract tracer has provided a novel perspective on intrinsic SCN connectivity. For example, an injection into the dorsomedial hypothalamus retrogradely labels, by 48 h postinjection, many neurons in the dorsal third of the ipsilateral rat SCN (Leak et al., 1999). About 20–24 h later, labeled cells are present throughout much, if not all, of the SCN. An injection of virus into the subparaventricular zone initially labels cells largely in the ventral two thirds of the rat SCN, but by 54 h, labeled cells densely populate the whole nucleus. The data suggest that cells in the dorsal SCN receive projections from cells in the ventral SCN and vice versa (Leak et al., 1999). It has not been determined whether all SCN cells become labeled with virus after injection into a target of SCN efferents. Comparable studies do not exist for other species. 4. In nonmammalian species, the existence of photoreceptors not specialized for classical visual function has long been recognized (Gaston and Menaker, 1968; Menaker, 1968; Menaker and Keatts, 1968; Menaker and Underwood, 1976; Enaker et al., 1970; Underwood and Menaker, 1970). These photoreceptors regulate photoperiodism and entrainment of circadian rhythms in nonmammals. For the same purposes, mammals require photoreceptors in the eye (Foster et al., 2003; Yamazaki et al., 1999). A critical study by Foster et al. (1991) demonstrated that rd/rd mice, sustaining the near-total loss of rods and cones along with their respective photopigments, retain substantially normal phase responses to light. In addition, the rd/rd mice perform similarly to controls even after 767 days, although there are no electroretinogram responses after 210 days of age (Provencio et al., 1994). The implication of these studies was that a novel retinal photoreceptor was sufficient for lightinduced phase shifts in mammals. In 1998, Provencio et al. (1998a) identified the molecule, melanopsin, and described its presence in Xenopus brain, eye and photosensitive skin melanophores. The human and mouse melanopsin genes were then cloned and demonstrated in cells of the mouse and monkey retinal ganglion cell layer (Provencio et al., 2000). 4.1. Fig. 2 – Connectivity of cell types within the hamster SCN. CCK = cholecystokinin; GRP = gastrin releasing peptide; VIP = vasoactive intestinal polypeptide; VP = vasopressin. Arrows indicate the direction the cell types project. Numbers indicate the percentage of cells with afferent contacts. Dashed arrows indicate SCN-afferent projections containing serotonin (5-HT) and neuropeptide Y (NPY) from the median raphe nucleus (Mn) and intergeniculate leaflet (IGL), respectively. Data drawn from Table 1 in LeSauter et al. (2002). Retinal photoreceptors and projections Melanopsin ganglion cell characteristics The results of Provencio and colleagues were rapidly adapted to specifically identify photoreceptive retinal ganglion cells (Berson et al., 2002) with additional work demonstrating that melanopsin behaves as a photopigment capable of activating a G-protein (Newman et al., 2003; Melyan et al., 2005; Qiu et al., 2005; Panda et al., 2005; Isoldi et al., 2005). Although there is debate concerning the shape of the action spectrum, the best indications are that the melanopsin action spectrum peaks at about 480 nm. Several studies have been designed to evaluate light sensitivity of photoreceptive cells contributing to the retinohypothalamic tract (RHT). The results clearly demonstrated that a special class of ganglion cells projecting to the suprachiasmatic nucleus (SCN) are both photoreceptive in the absence of known synaptic input (Berson et al., 2002) and contain melanopsin (Hattar et al., 2002; Gooley et al., 2001). In the hamster retina, there are approximately 1600 melanopsincontaining cells (Morin et al., 2003), comprising an estimated 1.5% of all ganglion cells (Tiao and Blakemore, 1976; Hsiao et al., 1984; Rhoades et al., 1979). In the rat, the estimate is 2.5% (about 2455 melanopsin cells) and, in the mouse, 1% (about 730 cells) (Hattar et al., 2002). These cells provide the retina with BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 what has been described as a “photoreceptive net” provided by overlapping, large arbors of beaded dendrites (Berson et al., 2002; Provencio et al., 2002). Apparently, all portions of the melanopsin-containing cells are photoreceptive (Berson et al., 2002). Melanopsin of retinal origin has been described in the SCN of rats (Beaulé et al., 2003b), although it has not been observed in the SCN of mouse or hamster (Provencio, unpublished data; Blanchard and Morin, unpublished data). In the hamster, the melanopsin cells are spread homogeneously throughout the retina, as they also are in the cat (Semo et al., 2005). The mouse may have a higher density of melanopsin in the superior retina (Hattar et al., 2002), a feature that has been reported in the rat (Hattar et al., 2002; Hannibal et al., 2002) and the diurnal species, the Nile grass rat (Blanchard, Novak and Morin, unpublished data). Nearly all the melanopsin-containing cells are present in the ganglion cell layer of the retina. However, there is a small percentage of the cells found as “displaced” to the inner nuclear layer (Berson et al., 2002). In either case, the dendritic arbor is most extensive along the border between the inner nuclear and inner plexiform layers. The predominant location of the dendrites is in the OFF sublayer of the inner plexiform layer. The frequency histogram of soma diameters of mouse melanopsin ganglion shows a mean of about 16.3 μm (Hattar et al., 2002). In the cat, SCN-projecting ganglion cell diameter is similar to the rat (17.2 μm) with dendrites ramifying primarily in the inner plexiform layer (Pu, 1999). The size distribution is not symmetrical, but skewed toward larger diameters for both species. Photoreceptive ganglion cells were first described following intra-SCN injection of a retrograde tracer. This permitted identification of ganglion cells contributing to the RHT and could be combined with recording from those cells in the absence of input from other retinal neurons (Berson et al., 2002). With or without the presence of drugs blocking synaptic input, the cells are slow to respond to light stimuli with saturating light pulses requiring hundreds of milliseconds, and near threshold stimuli requiring about 60 s, to response onset. Latency to maximal depolarization is reduced as stimulus irradiance increases. Subsequently, the depolarization slowly decays to a plateau that more or less endures for the duration of the stimulus, with the plateau proportional to the stimulus energy. The cells are wavelength-sensitive with a peak response at about 484 nm, similar to the results from action spectra for rodent circadian rhythm phase response (Takahashi et al., 1984; Provencio and Foster, 1995). For a fixed wavelength stimulus, peak depolarization increases linearly until reaching a saturating irradiance. Spike frequency appears related to the extent of depolarization. Subsequent to stimulus offset, repolarization is very slow, taking minutes after stimuli of high irradiance. Spike discharges are maintained until polarity returns near baseline. The membrane depolarization is produced by an inward current that is activated by the light pulse (Warren et al., 2003). The current activates slowly reaching a peak several seconds after the application of a light pulse. The time course of the membrane depolarization and the inward current are similar. The signal transduction pathways coupling melanopsin to the activation of the inward current are not known. The current was unaffected by substitution of extracellular Na+ and shows both inward and 9 outward rectification. These data are consistent with the current being mediated by channels of the trp family (transient receptor potential) of channels (Warren et al., 2003). Properties of SCN-projecting ganglion cells (identified by retrograde tracer) recorded from a cat eyecup preparation in which there was no elimination of rod/cone contribution indicate that they exhibit sustained responses of the “on” or “on–off” center variety; have a spectral sensitivity peak at about 500 nm and response is optimal with motionless or slow-moving stimuli (Pu, 2000). 4.2. Retinal ganglion cell projections and bifurcation Retinal projections to the SCN were definitively described in 1972 (Moore and Lenn, 1972; Hendrickson et al., 1972) and focused scientific attention squarely on that nucleus as the probable site of the neural circadian clock. Evaluation of the RHT has continued as methods have improved and more species are evaluated. The extent to which the retina projects to each SCN is highly variable across species. The diurnal grass rat has bilateral retinal innervation of the SCN, although a preponderance arrives from the contralateral retina (Smale and Boverhof, 1999). This differs from what is seen in the diurnal golden mantle ground squirrel in which RHT innervation is exclusively from the contralateral retina (Smale et al., 1991). A recent report shows modest ipsilateral SCN innervation by the retina in the diurnal California ground squirrel (Major et al., 2003). In contrast, following analysis of darkfield images, the hamster RHT was described as bilaterality symmetrical (Johnson et al., 1988a). The issue of laterality and pervasiveness of the retinal projection in the hamster SCN has now been evaluated with confocal microscopy (Muscat et al., 2003). The data provide several novel observations. First, nearly the entire SCN is innervated by the RHT, consistent with earlier HRP results (Pickard and Silverman, 1981). Second, despite the extent of innervation, it is not homogenous within the SCN. And third, the axons from one retina project to most or all of the SCN bilaterally. The central and dorsal SCN receive dense innervation predominantly from the contralateral retina, while the ventromedial SCN receives moderate innervation largely from the ipsilateral retina. Thus, innervation of the SCN by one retina is generally pervasive, but with clear regional specificity. Of particular interest is the presence of the densest retinal innervation both to the region of the CALB-containing central subnucleus of the SCN and to a region slightly more dorsal. The heavily innervated dorsal area corresponds to a zone in which phosphorylated extracellular signal-regulated kinase (pERK) is found (Lee et al., 2003), a characteristic that is apparently important to pERK activity. Other species also appear to have overlapping, but preferred SCN regions of innervation by retinal projections. This is apparent in the rat, but not the mouse (Shivers and Muscat, 2004). One apparent difference between the RHT terminal fields of these species and that of the hamster is the substantially absent innervation of the dorsomedial SCN that largely corresponds to the area in which most VP cells are present. The retina projects to the SCN via the RHT, a major direct projection, and through a secondary, indirect pathway 10 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 through the IGL and the GHT. In addition, there are numerous retinal projections to disparate brain regions many of which have potential, reciprocal connections to the SCN through the IGL (Morin and Blanchard, 1998). One such is a direct retinal projection to the dorsal raphe nucleus, with an initial description in cats given in 1978 (Foote et al., 1978). Interest was further heightened with the demonstration, based on both anterograde and retrograde tracing data, of a similar pathway in rat (Shen and Semba, 1994). This pathway has also been observed in gerbil (Fite et al., 1999, 2003) and O. degus (Fite and Janusonis, 2001). The pathway has not been found in hamster, although it has been sought several times (Morin and Blanchard, unpublished data; Fite, unpublished data). Bifurcation of ganglion cell axons and subsequent projection of these cells to the SCN and IGL (Pickard, 1985) appears to be a common characteristic of the circadian visual system. The presence of ganglion cells that bifurcate to innervate both the SCN and IGL has been recently confirmed, with the additional information that similar bifurcation is present for individual cells projecting to both the SCN and superior colliculus or OPT (Morin et al., 2003). Injection of two retrograde tracers, one into each SCN, also demonstrates that at least some ganglion cells bifurcate and send at least one axon branch to each SCN. Further, at least some of these cells contain melanopsin, as do some that project to the OPT. In contrast, individual ganglion cells apparently do not bifurcate and project bilaterally to the IGL, OPT or the SC. Other retinorecipient regions have not been simultaneously examined for both bifurcation and melanopsin cell afferents. Nor has the question of individual ganglion cells projecting to more than two visual targets been examined. The prospect is likely that individual ganglion cells have multiple axonal processes (beyond two) that would allow a rather small number of photoreceptors to commonly influence a broad range of visual functions. A novel contribution to the analysis of retinal projections to the SCN comes from two studies by Abe and Rusak (1992). In the initial paper, hamsters were electrically stimulated in the IGL with the result that FOS protein synthesis was induced in the centrodorsal SCN. The preliminary interpretation was that GHT activation was causal to FOS expression (Abe et al., 1992). However, a subsequent study (Treep et al., 1995) showed that FOS expression could not be stimulated in bilaterally enucleated animals. This result suggested an alternate interpretation, namely, electrical stimulation may have elicited antidromically activated retinal ganglion cells which produced orthodromic activation of the SCN via the collaterals from bifurcating axons in the RHT (Morin et al., 2003; Pickard, 1985). The resulting pattern of FOS protein expression would uniquely represent the distribution of retinal projections that bifurcate and project to both the IGL and SCN. The extent to which such bifurcating projections originate from ganglion cells that do or do not contain melanopsin is unknown. Photoreceptive ganglion cells contributing to the RHT contain melanopsin (Hattar et al., 2002; Gooley et al., 2001; Morin et al., 2003; Hannibal et al., 2002; Gooley and Saper, 2003). To date, however, there has not been a comprehensive description of melanopsin ganglion cell projections to other brain areas. The initial report of melanopsin cell targets showed X-gal staining for β-galactosidase activity in a transgenic mouse strain with tau-lacZ targeted into the melanopsin gene locus (Hattar et al., 2002). This allowed visualization of β-galactosidase fused to a protein anterogradely transportable to axon terminals. The blue reaction product emphatically revealed projections to the SCN, IGL and olivary pretectal nucleus (OPT). Subsequently, retrograde tracing studies have confirmed melanopsin-containing ganglion cells projecting to these retinorecipient targets, as well as to the ventral lateral preoptic area, and the subparaventricular zone of the hypothalamus (Morin et al., 2003; Gooley and Saper, 2003; Sollars et al., 2003). Melanopsin cells in the hamster (Morin et al., 2003), but not the rat (Gooley and Saper, 2003), have been reported to project to the superior colliculus. The retrograde studies confirm the projections from melanopsin cells to initially defined major visual targets, but suggest that not all the ganglion cells projecting to these areas contain melanopsin. Estimates are that 10– 20% of ganglion cells projecting to the SCN do not contain melanopsin (Morin et al., 2003; Gooley and Saper, 2003; Sollars et al., 2003). The suggestion that melanopsin cells and their projections create a “nonimage forming” visual system has been recently put under pressure with the observation that at least some of these cells project to the monkey dorsal lateral geniculate nucleus (Peterson et al., 2003; Dacey et al., 2005). These cells appear to provide both irradiance and color information to the dorsal lateral geniculate nucleus, a site involving in the processing of feature-related information, i.e., images. Melanopsin cell projections to dorsal lateral geniculate nucleus have not been found in the rat (Gooley and Saper, 2003). As indicated above, the melanopsin cells are known to project to several other retinorecipient nuclei and bifurcation may be a common feature of axons projecting through the RHT to the SCN. Therefore, it is likely that the melanopsin cells projecting to the dorsal lateral geniculate nucleus also bifurcate and innervate many, if not all, other subcortical visual regions. An unexpected development has arisen from a thorough evaluation of an engineered version of the Bartha strain pseudorabies virus (PRV152), as a retrograde transsynaptic tracer (Pickard et al., 2002). Previously, viral tracing unequivocally demonstrated a subset of retinal ganglion cells following injection into the contralateral eye. These cells presumably gave rise to the RHT (Moore et al., 1995; Provencio et al., 1998b; Hannibal et al., 2001a). However, a new interpretation of these results is based on the fact that PRV152 is exclusively a retrograde tracer that makes its way to the SCN multisynaptically through the nucleus of Edinger–Westphal in the midbrain oculomotor complex (Pickard et al., 2002). Cells in the SCN, IGL and OPT are infected, as are several ganglion cell types in the contralateral retina. Only a subset of these contribute to the RHT. Viral transsynaptic tracing is a powerful method and has the added advantage of revealing second order retinal neurons, bipolar and amacrine cells, that synapse with the primary ganglion cells (Belenky et al., 2003). This observation has significant implications for the manner in which light regulates circadian rhythm phase. BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 4.3. Classical and ganglion cell photoreceptor contribution to regulation of circadian rhythms, masking, the pupillary light reflex and melatonin suppression 4.3.1. Circadian rhythms Three classes of photoreceptive cell types reside in the mammalian retina. The current view is that while all effects of light on the circadian visual system are accounted for by the three types, no single photoreceptor is necessary for entrainment. The rd/rd mouse strain suffers postnatal degeneration of rods and cones. These individuals have essentially normal phase response to light (Foster et al., 1991; Provencio et al., 1994; Freedman et al., 1999) and have an action spectrum for phase shifts that peaks at 480 nm (Yoshimura and Ebihara, 1996; Hattar et al., 2003) (but see Provencio and Foster, 1995). Recently, entrainment stability at threshold levels of illumination was assessed (Mrosovsky, 2003) and, with this simple procedure, only 10% of rd/rd mice were able to remain entrained to a 12:12 LD photoperiod when the daytime light intensity was 15 stops (b2 lx), compared to about 80% of wild-type mice. This result supports the view (Yoshimura et al., 1994) that there is a circadian system response to light that is exclusively rod and/or cone-sensitive. Rods may regulate hamster entrainment under very dim lighting conditions (Gorman et al., 2005). Neurophysiological methods also support the view that both rods and cones (primarily 510 nm green and secondarily 375 nm near-ultraviolet) provide photic information to the SCN (Aggelopoulos and Meissl, 2000). A flurry of investigations followed the discovery of melanopsin photoreceptive retinal ganglion cells. Melanopsin gene knockout (KO) mice were made by two different methods, yet the effects on light-induced rhythm responses were very similar (Panda et al., 2002; Ruby et al., 2002a). The animals entrain and free-run properly in normal light–dark photoperiod and constant dark conditions, respectively. However, phase response to light is attenuated in the KO animals. With a 480 nm stimulus, phase shift magnitude was only about half that of wild-type mice at each of three, nonsaturating irradiance levels (Panda et al., 2002). A saturating, white light pulse also yielded a diminished phase shift from the KO animals, although it was about two thirds normal (Ruby et al., 2002a). A second defect in response to light by the melanopsin KO animals was the failure of constant light (LL) to elicit a properly lengthened circadian period. In both strains, LL (white light) effectively increased the period, but in melanopsin KO animals, the change was only about 55–65% of controls (Panda et al., 2002; Ruby et al., 2002a). It is worth noting that similar effects on light-induced circadian period lengthening can be achieved by IGL lesions (Morin and Pace, 2002), possibly because such lesions would destroy the functional effectiveness of melanopsin cell projections to the IGL (Hattar et al., 2002). Light administered at CT16 was able to induce c-fos mRNA in SCN cells of melanopsin KO animals, although there has been no test of the normality of this expression (Ruby et al., 2002a). An important outcome of the melanopsin KO studies is the implication that both classical photoreceptors and intrinsically photoreceptive, melanopsin-containing retinal ganglion 11 cells contribute, approximately equally, to circadian rhythm regulation by light. Subsequently, two sets of investigators, again using somewhat different methods, addressed the issue of photoreceptor types and circadian rhythm regulation using animals defective in both melanopsin production and classical photoreceptor function. Again, the results are substantially the same: animals lacking melanopsin but bearing the rd/rd genotype (Panda et al., 2003) or undergoing rod and cone degeneration plus having defective phototransduction in cones (Hattar et al., 2003) free-run under normal light–dark photoperiod conditions. Lack of entrainment was not altered by light intensity nor was circadian period under LL different from the circadian period under DD. In addition, light was unable to inhibit nocturnal melatonin synthesis (Panda et al., 2003). It should be noted that the defects in rhythm response to light are not the result of loss of ganglion cells that normally synthesize melanopsin or their projections (Hattar et al., 2003). 4.3.2. Masking, pupillary light reflex and melatonin suppression Other accessory visual functions are also modified by the absence of melanopsin. Although light-induced negative masking is reduced, particularly at lower light intensities, in rd/rd mice, the melanopsin KO alone yields masking that is about 30% greater than in the rd/rd mice (Panda et al., 2003). When the melanopsin KO and rd/rd traits are combined, the mice do not exhibit negative masking to light (Hattar et al., 2003; Panda et al., 2003). Melanopsin does not seem to be involved in the positive masking that occurs in response to dim light exposure (Mrosovsky and Hattar, 2003), but apparently contributes to negative masking in the presence of light ≥10 lx (∼6.7 μW/cm2). Pupillary constriction in response to photic stimuli is much reduced in rd/rd mice and the response has a longer latency. At high irradiances, pupillary response equals that in wild-type mice, but the response of rd/rd mice is markedly attenuated at intermediate and lower irradiances (Hattar et al., 2003; Panda et al., 2003; Lucas et al., 2001). Unlike masking and phase response to light, the pupillary light reflex is relatively unmodified in the absence of melanopsin. Only at irradiances N10 × 1012 photons/cm2/s is there a modest sensitivity deficit. The full effect of light on the pupillary light reflex can be accounted for by the additivity of rod/cone and melanopsin photoreceptor contributions (Panda et al., 2003; Lucas et al., 2003), an observation supported by the absence of any pupillary light reflex in melanopsin KO animals lacking rod and cone function (Hattar et al., 2003; Panda et al., 2003). A single opsin/vitamin A-based photopigment with a peak sensitivity around 480 nm appears to drive the pupillary light reflex in rodless, coneless mice (Hattar et al., 2003; Lucas et al., 2001). A contribution of the pupil to circadian rhythm regulation has not been studied and is seldom considered as a factor regulating circadian rhythm response to light. However, it is useful to note (a) that both classical and melanopsin photoreceptors contribute to both responses (Hattar et al., 2003; Panda et al., 2003; (b) that, within limits, phase response magnitude is proportional to the total number of photons assessed by the circadian visual system across the duration of light exposure (Nelson and Takahashi, 1991; (c) that the pupil 12 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 area can vary by a factor of 16 (Hattar et al., 2003; Lucas et al., 2003) and (d) that the pupil acts to gate exposure of the circadian visual system to the very stimulus type to which it optimally detects and responds. Mice which cannot synthesize melanopsin or which bear the rd/rd mutation show, to the extent tested, normal lightinduced suppression of pineal melatonin (Lucas and Foster, 1999; Lucas et al., 1999). The two photoreceptor classes appear redundant to the extent that loss of both eliminates melatonin suppression by light (Panda et al., 2003). 5. tract Neuromodulators of the retinohypothalamic 5.1. Glutamate It is generally believed, based on abundant indirect evidence, that glutamate is the primary neurotransmitter of the retinohypothalamic tract. This topic (up to 1996) has been extensively reviewed (Ebling, 1996; Hannibal, 2002a) and will not be considered in depth here. However, despite the numerous studies of function involving use of glutamate receptor agonists and antagonists, there is much less certainty regarding its actual presence in the RHT. Most convincing in this regard has been the electron microscopic demonstration that glutamate immunoreactivity occurs in cholera-HRP identified terminals of retinal projections to the mouse and rat SCN (De Vries et al., 1993; Castel et al., 1993). Electrical stimulation of the optic nerve induces SCN release of [3H]glutamate or [3H]-aspartate (but not [3H]-GABA) loaded onto rat slice preparations (Liou et al., 1986). However, direct stimulation of the SCN yields even greater release of the two amino acids, suggesting their presence in nonretinal terminals and SCN cells, in addition to RHT terminals. Pharmacological studies suggest that aspartate may be the most potent excitatory neurotransmitter in the rat RHT (Shibata et al., 1986). Two laboratories have evaluated the uptake and retrograde transport of [3H]-aspartate injected into the rat SCN. While the two investigations agreed with respect to nonretinal projections to the SCN, one identified retinal ganglion cells contributing to the RHT (Devries et al., 1995) and the other did not (Moga and Moore, 1996). More recently, glutamate has been immunohistochemically identified in the RHT colocalized with PACAP which may identify most, if not all, retinal projections to the SCN (Hannibal et al., 2000). Vesicular glutamate transporters, VGluT1 and VGluT2, account for nearly all the glutamate transporters in the brain and are generally abundant in retinorecipient nuclei (Fujiyama et al., 2003; Land et al., 2004), for example, in the dorsal lateral and ventral lateral geniculate nuclei. However, they are not abundant in either the IGL (where their presence is weak, at best) or the SCN. The two transporters are weakly evident in the SCN, but not at the identical locations (Fujiyama et al., 2003). Unilateral enucleation has little, if any, effect on transporter visibility in the SCN, although bilateral enucleation may do so. One emergent difficulty resulting from the studies of glutamate function has been the general inability to obtain a light-type phase response curve (PRC) to direct SCN applica- tion of excitatory amino acid. The initial study using glutamate generated a PRC more of the NPY-type (Meijer et al., 1988), while aspartate had no effect (De Vries and Meijer, 1991). Subsequently, NMDA was shown to elicit phase delays when administered at CT13.5 and advances at CT19, consistent with a light-type PRC (Mintz and Albers, 1997) and a full, light-type PRC (although the subjective night is longer and maximal delays equal maximal advances) to direct SCN application of NMDA, have been demonstrated (Mintz et al., 1999). The effects of NMDA on circadian rhythm phase were blocked by NMDA receptor antagonists. In vivo glutamate yields a PRC that differs from that for NMDA for reasons that are not clear. Injection of NMDA onto the SCN during the early subjective night elicits light-like phase delays which cannot be blocked by simultaneous tetrodotoxin (TTX) application (Gamble et al., 2003). NMDA treatment during the subjective day has no direct effect on rhythm phase (Mintz et al., 1999), but it can inhibit phase advances induced by the GABAA agonist, muscimol (Gamble et al., 2003; Mintz et al., 2002). In this respect, NMDA during the subjective day mimics the effect of light (Mintz et al., 2002). However, the action of NMDA during the day is blocked by TTX treatment suggesting that sodium-dependent action potentials are necessary for effects of light on rhythm phase during the subjective day, but not during the subjective night. A corollary to the issue of glutamate in the RHT concerns the biochemical pathway that is likely necessary for processing the neurotransmitter in the SCN. This pathway involves the de novo synthesis of glutamate in neurons and glia, plus the recycling of extracellular glutamate through glia back to neurons (Hertz, 2004). Extracellular glutamate concentration in the peri-SCN region is greatest during the hours of darkness (Rea et al., 1993a; Glass et al., 1993). Glial fibrillary acidic acid concentration in SCN astrocytes is lowest after lights off (Glass and Chen, 1999). 5.2. N-Acetylaspartyl glutamate A significant question that has not been resolved is whether glutamate is the sole amino acid transmitter in the RHT contributing to circadian rhythm regulation. N-Acetylaspartyl glutamate (NAAG) is also present in the RHT (Moffett et al., 1990, 1991; Tieman et al., 1991), although at the synaptic cleft, NAAG is converted to its constituent amino acids and each is available for postsynaptic activity (Baslow, 2000; Neale et al., 2000; Coyle, 1997). However, unlike NAAG, neither aspartate nor glutamate is released from retinal ganglion cell terminals in a calcium-dependent manner (the RHT has not been specifically tested; see Tieman and Tieman, 1996 for a review). Whether this constitutes the source of photically induced glutamate release or the transmitter is independently released by RHT terminals remains to be discovered. The most recently available information (Moffett, 2003) suggests the presence of NAAG in a portion, but not all, of the rat RHT (ventrolateral part of the SCN, i.e., not the whole retinorecipient region). Analysis of prospective NAAG function is complicated by the presence of numerous NAAG-IR neurons in the SCN with particularly dense expression in dorsomedial cells (Moffett, 2003). BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 5.3. Glutamate receptors One presumption, given that the RHT is likely to use glutamate as a transmitter and that glutamate agonists or antagonists alter circadian clock function, is that there are glutamate receptors in the SCN. The most comprehensive study of glutamate receptor localization in the SCN has been performed with hamsters (Stamp et al., 1997) and demonstrates that the GluR1 receptor is most widely distributed in the SCN with greatest density in a region corresponding approximately to that which receives dense innervation from the contralateral retina (Muscat et al., 2003). Both the GluR2/3 and GluR4 receptors are distributed primarily in the dorsomedial SCN, although there are also substantial GluR4 receptors in the central and ventral part of the nucleus. One important consideration regarding glutamate receptors is the fact that they can also be abundant on astrocytes (Porter and McCarthy, 1997), and may indirectly modulate neuronal activity by altering glial response to neurotransmitters (Haak et al., 1997). Astrocytes may also be a source of glutamate that can activate neuronal glutamate receptors (Liu et al., 2004). Astrocytes may be part of a route yielding glutamate receptor-activated release of astrocytic endothelial nitric oxide synthase that can act in parallel with glutamateactivated neuronal nitric oxide synthase through which light can induce NO release in the SCN thereby altering circadian rhythm phase response (Caillol et al., 2000). 5.4. Nitric oxide and RHT signal transduction Early articulation of the prospect that nitric oxide might contribute to circadian rhythm regulation was provided by Amir (1992) who tested whether the SCN mediated the increased heart rate response to acute photic stimulation. Infusion of L-NG-nitro-arginine-methyl ester (L-NAME), a competitive inhibitor of nitric oxide synthase, over the SCN greatly attenuated the heart rate increase. This attenuation could be overcome by simultaneous infusion of excess Larginine, the natural substrate for the enzyme. Similar effects on the heart rate response were also obtained with a glutamate NMDA-type receptor antagonist and it was suggested (Amir, 1992) that nitric oxide mediates the effects of light on the SCN. Subsequently, Ding et al. (1994) published an extensive series of experiments probing the contribution of nitric oxide to photic signal transduction in the SCN. As a precursor to the work, the investigators evaluated phase response of the SCN neuronal firing rhythm to acute perfusion with glutamate applied to a rat brain slice preparation. This yielded a very tidy glutamate-induced PRC (Ding et al., 1994) that closely mimicked the normal rat light PRC (Summer et al., 1984), with glutamate yielding concentration-dependent phase shifts across a range of 10−6 to 10−2 M glutamate. The phasic effects of glutamate were mimicked by in vitro and in vivo application of NMDA (Ding et al., 1994; Colwell and Menaker, 1992; Colwell et al., 1990, 1991; Takeuchi et al., 1991), a specific agonist for one type of glutamate receptor. Using in vitro activation of the NMDA glutamate receptor sequelae as the indicator of a functional photic signaling pathway, the photic effects were mimicked by administering 13 nitric oxide generators to the slice (Ding et al., 1994). Sodium nitroprusside, S-nitroso-N-acetyl-penicillamine and hydroxylamine application yielded equivalent, glutamate-like phase advances and delays during the subjective night, but no phase change during the subjective day. Further, the glutamateinduced phase shifts could be blocked by any of several types of competitive substrates for nitric oxide synthase. The procedures were extended to the in vivo situation in which L-NAME was able to completely block hamster phase advances induced by light. This blockade was reversed by increasing the availability of L-arginine substrate (Ding et al., 1994). L-NAME was also able to attenuate hamster phase delays (Watanabe et al., 1995). The data suggest that light acting through the RHT releases glutamate in the SCN, activating the NMDA receptors on SCN neurons. This increases intracellular calcium concentration, followed by nitric oxide synthase stimulation and nitric oxide production (Ding et al., 1994). Nitric oxide may act as a short distance neuromodulator in the photic signal transduction pathway. The effects of melatonin and serotonin on rat circadian rhythm phase may also require nitric oxide synthesis activation in the SCN (Starkey, 1996). The foregoing basic principles have been linked to lightinduced phosphorylation of the cAMP response element binding protein (CREB) in SCN neurons (Ginty et al., 1993). The appearance of phosphorylated CREB (pCREB) is the earliest known postsynaptic biochemical marker of photic signal transduction in the SCN. In vivo light-induced pCREB immunoreactivity in rat SCN cells attained maximal expression within 10 min following a 10 min 150 lx light pulse at CT19 and this could be mimicked by glutamate application to a slice preparation (Ding et al., 1997). The effects of glutamate on pCREB immunoreactivity were blocked by an NMDA receptor blocker (APV) or a nitric oxide synthase inhibitor (L-NAME). As yet, the steps between postsynaptic glutamate action and induction of CREB phosphorylation or nitric oxide synthesis/ release have not been determined. It is important to note that light- or glutamate-induced pCREB and nitric oxide activity are dependent on circadian clock phase indicating that the responsiveness of postsynaptic targets of the RHT is limited at certain times by feedback from the circadian clock. Whether this means the postsynaptic cells are intrinsically “clock” cells or they receive rhythmic inhibitory feedback from elsewhere is not known. Nitric oxide synthase activity in hamsters is rhythmically related to the presence of an alternating light–dark cycle. Light administered at any time of day elevated enzyme activity and the day–night enzyme rhythm did not persist in DD (Ferreyra et al., 1998; Agostino et al., 2004; Chen et al., 1997). This is consistent with the view that the consequences of nitric oxide release, but not the release itself, are regulated by the circadian clock. There is no complete consistency in the literature concerning nitric oxide function in circadian rhythm regulation. Light-induced FOS protein, thought to be an event downstream of CREB phosphorylation (Kornhauser et al., 1990), has been blocked in rat SCN, but not IGL, by peripheral injection of L-NAME (Amir and Edelstein, 1997). In contrast, the same procedure in hamsters has blocked light-induced activity rhythm shifts, but not light-induced FOS in the SCN (Weber et al., 1995). This may indicate a species difference. 14 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 However, in the hamster, it is further recognition of the fact that FOS protein expression is not a necessary component of phase response to light (Colwell et al., 1993a,b). The anatomical evidence in support of the SCN as a site for nitric oxide modulation of circadian rhythmicity is not particularly encouraging. The earliest descriptions of potential nitric oxide synthesizing substrate based on NADPH-diaphorase staining in the circadian rhythm system revealed no cells in the rat SCN (Vincent and Kimura, 1992) and sparse cells in the Siberian hamster SCN (Decker and Reuss, 1994). Occasional neurons immunoreactive for the neuronal form of nitric oxide synthase have also been observed in rat SCN, and some of these contain VIP (Reuss et al., 1995). More recently, a dense plexus of processes immunoreactive for the neuronal form of nitric oxide synthase has been identified surrounding a few immunoreactive cells in ventral rat SCN (Chen et al., 1997), but this has not been seen by other investigators (Caillol et al., 2000; Wang and Morris, 1996). The most convincing anatomical study indicates no cells in the SCN of rat or mouse identifiable by NADPH-diaphorase staining, but in both species, immunoreactivity for neuronal nitric oxide synthase revealed distinct cell populations within the SCN. In the mouse, the labeled neurons are evenly distributed through the SCN with about one tenth of the SCN neurons labeled. In contrast, all labeled rat neurons are grouped laterally in the ventral SCN among immunoreactive neuronal processes. Neither labeled cells nor processes were evident in the dorsomedial SCN (Wang and Morris, 1996). However, under special slice conditions, no nitric oxide synthase immunoreactivity was observed in the rat SCN although the ventral lateral part of the nucleus is densely labeled with cyclic GMP immunoreactivity. NADPH-diaphorase is not always a useful indicator of nitric oxide synthase (see Wang and Morris, 1996 for discussion). A subsequent report using similar methods, but different antisera, identified no cells containing neuronal nitric oxide synthase in the hamster SCN and a few in the ventral rat SCN. Nonneuronal cells (probably astrocytes) and processes immunoreactive for endothelial nitric oxide synthase were observed in the SCN of both species (Caillol et al., 2000). Despite the biochemical manipulations indicating that nitric oxide synthase activity is necessary for phase response to light or glutamate, mutant mice lacking the gene coding for endothelial (Kriegsfeld et al., 2001) or neuronal (Kriegsfeld et al., 1999) nitric oxide synthase apparently entrain normally to the light–dark cycle and light induces normal FOS expression in the SCN. As suggested by Caillol et al. (2000), it is possible that there are parallel neuronal and glial pathways by which photic information gains access to the circadian clock. If this is the case, then the only effective knockout might require deleting the genes coding for both endothelial and neuronal nitric oxide synthase. 5.5. PACAP Pituitary adenylate cyclase activating polypeptide (PACAP) did not become a molecule of interest to the circadian rhythm field until 1997 when Hannibal et al. (1997) described its presence in retinal ganglion cells plus a projection of these cells to retinorecipient regions of the SCN and IGL. The same paper demonstrated that, in a concentration-dependent fashion, PACAP administered at CT6 onto a rat SCN slice preparation could induce 3.5 h phase advances (tests at CT14 or 19 yielded no phase change). VIP, which can act on receptors similar to those for PACAP, also yielded phase advances, but the concentration sensitivity and resulting phase response were much lower (Hannibal et al., 1997). The argument was offered that PACAP constitutes a neuromodulator in the RHT which, along with glutamate, normally acts to modify circadian rhythm phase in response to retinal photoreception. Subsequently, large, concentration-dependent, PACAP-induced (CT6) phase advances were observed in hamster SCN slices (Harrington et al., 1999). However, substantial phase delays were also observed if 1 nM PACAP was administered at CT14, but lesser or greater concentrations had progressively diminished effects. In addition, small phase delays or advances were obtained in vivo following infusion of 1 nM PACAP onto the hamster SCN at CT14 and CT18, respectively (Harrington et al., 1999). Thus, PACAP in vivo mimicked the effect of light in terms of the direction of the elicited phase shift (tests during the subjective day were not performed). Replication of the foregoing PACAP effects has proven elusive. Additional in vivo hamster tests have usually, but not always, yielded phase delays following administration of various PACAP concentrations at CT14. Only phase delays were obtained at CT18, and small phase delays (no advances) were observed with one PACAP concentration given at CT6 (Piggins et al., 2001a). Recently, a third investigation of hamster in vivo effects obtained small phase delays following a moderately high concentration of PACAP administered at CT14 (Bergstrom et al., 2003). As is obvious, there are numerous inconsistencies in the available data which require reconciliation before a function of PACAP can be accepted. The Hannibal lab has developed a monoclonal PACAP antibody (Hannibal et al., 1995) that has enabled production of a large amount of information regarding the anatomy of structures containing this peptide (see Hannibal, 2002a for a review). PACAP is present in the RHT (Hannibal and Fahrenkrug, 2004; Hannibal et al., 1997, 2002; Bergstrom et al., 2003) where it colocalizes with glutamate (Hannibal et al., 2000). PACAP-ir neurons in the rat SCN have also been reported (Piggins et al., 1996), although there may have been crossreactivity with VIP. In addition, PACAP appears to be found in axons and terminals in most of the retinorecipient regions of the rat brain (Hannibal and Fahrenkrug, 2004) including the IGL, ventral lateral preoptic area and subparaventricular hypothalamus. Two exceptions may be the commissural pretectal area and the dorsal lateral geniculate nucleus, although that information is difficult to discern. PACAP is widespread in brain (Hannibal, 2002b) and it is not always clear whether the PACAP observed is actually present in retinal projections or is from other sources. PACAP immunoreactivity, in addition to identifying projections to the SCN, identifies a small population of retinal ganglion cells in the rat most of which are found in the superior retina (Hannibal et al., 2002). Most, if not all, of these cells also contain melanopsin and glutamate. Hannibal et al. (2002) has suggested that all ganglion cells projecting to the SCN contain both PACAP and melanopsin. However, the issue BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 has not been directly evaluated using retrograde tracers injected into the SCN. The retrograde tracer procedure has shown that 10–20% of RHT neurons do not contain melanopsin (Morin et al., 2003; Gooley and Saper, 2003; Sollars et al., 2003). The apparently close relationship between PACAP and melanopsin raises the possibility that PACAP released by melanopsin cell electrical activity may generate prolonged postsynaptic activity in the SCN that endures beyond the rapid release effects of glutamate. The suggestion that all melanopsin-containing ganglion cells contain PACAP also implies a major role for that peptide in normal photic regulation of circadian rhythms, assuming it acts as a neuromodulator. In the context of light and glutamate effects on circadian rhythm phase, in vivo application of PACAP antibody or receptor antagonists to the SCN attenuates the phase delaying or advancing effects of light (Bergstrom et al., 2003; Chen et al., 1999). Ordinarily, glutamate applied to an SCN slice preparation mimics the effect of light on the phase of the neuron firing rate rhythm with phase delays and advances induced during the early and late subjective night, respectively (Ding et al., 1994; Chen et al., 1999). When glutamate and PACAP are coinfused onto the slice preparation, phase delays are enhanced and phase advances are abolished. In contrast, the PAC1 receptor antagonist, PACAP6-38, enhanced glutamate-induced phase advances while blocking phase delays (also blocked by PACAP antibody infusion). The implication is that glutamate cannot induce phase delays without cofunctioning PACAP, whereas glutamate is better able to induce phase advances in the absence of functional PACAP (Chen et al., 1999). The role of PACAP in circadian rhythm regulation has been recently addressed through the use of PACAP KO mice (Colwell et al., 2004; Kawaguchi et al., 2003). The results are mixed, both within and between studies. Absence of the gene coding for PACAP does not modify rate to re-entrain to an advanced or delayed LD12:12 photoperiod, phase response to dark pulses at CT6, the circadian period in LL or the negative masking effect of light on wheel running (Colwell et al., 2004). Nevertheless, the same study showed that, in the PACAP KO mice, both advance and delay phase shifts to normally saturating or nonsaturating light stimuli administered at CT16 or 23 are greatly reduced, the circadian period is shorter in DD and the phase angle of entrainment is earlier. The second study (Kawaguchi et al., 2003) failed to find a difference in circadian period during DD or an effect of the KO on phase delays to light at CT15, although the phase advances to light at CT21 were about half of the expectation. To make matters more confusing, wild-type mice had greater light-induced FOS protein in the SCN at CT15 than did PACAP KO mice, but not at the time at which light yielded reduced phase advances (CT21). At the present time, it is difficult to reconcile the observation (Colwell et al., 2004) of (a) a large effect of the gene KO on phase response to light pulses with the absence of an effect on rate of re-entrainment; (b) the presence of reduced FOS at a time at which the same stimulus yields a normal rhythm phase shift; (c) the presence of a reduced phase shift at time at which the same stimulus yields normal FOS expression and (d) a failure of the two studies (Colwell et al., 2004; Kawaguchi et al., 2003) to find equally deficient phase delays in PACAP KO mice. 5.6. 15 Substance P Confusion also abounds with respect to the presence of SP in the RHT. In contrast to PACAP, there is more positive evidence regarding function of SP than there is for its anatomical presence in the RHT. SP has been suggested as a peptide native to retinal ganglion cells projecting to the SCN (Takatsuji et al., 1991; Mikkelsen and Larsen, 1993) and the distribution of SP fibers and terminals in the rat SCN is similar to that for retinal input (Piggins et al., 2001b). Other attempts to confirm a SP projection have not succeeded (Otori et al., 1993; Hartwich et al., 1994), and the presence of a SP terminal plexus in the SCN varies substantially across species (Piggins et al., 2001b). A fairly comprehensive analysis of the rat RHT has demonstrated PACAP and anterograde tracer in the SCN terminal plexus of the RHT, but apparently there is no colocalization with SP (Hannibal and Fahrenkrug, 2002). Similarly, SP does not colocalize with PACAP in the rat retina. According to one report, most retinal SP cells are present in the inner nuclear layer and outer part of the inner plexiform layer. The few SP cells in the rat ganglion cell layer differ in size from the PACAP neurons and are considered to be “displaced amacrine cells” (Hannibal and Fahrenkrug, 2002). This contradicts an earlier report demonstrating SP-containing ganglion cells identified by retrograde label as projecting to the SCN (Takatsuji et al., 1991). However, the results of Hannibal and Fahrenkrug (2002) (Negroni et al., 1997) are consistent with the presence of SP in the SCN surviving bilateral enucleation (Otori et al., 1993). A similar conclusion has been reached by other investigators following bilateral enucleation of either rat or hamster (Hartwich et al., 1994). The SP receptor, neurokinin 1 (NK1), is generally absent from the ventral SCN of both rat and hamster (Mick et al., 1994, 1995), with low levels of NK1 receptor present in neurons which are found along the dorsolateral margin of the rat SCN. Another report (Takatsuji et al., 1995) also indicates a general absence of the receptor from the ventral rat SCN, but demonstrates its presence on a few cells in the most ventral part of the nucleus and in the underlying optic tract. Most evident receptor is on neurons and dendrites scattered through the dorsal and lateral part of the nucleus. Dendrites of the labeled neurons are described as extending ventrally and, in enucleated animals, NK1 is found in dendrites contacting degenerating retinal projections via asymmetric axo-dendritic synapses (Takatsuji et al., 1995). This suggests that SP-containing retinal fibers terminate on cellular elements in the SCN with NK1 as a receptor. A detailed evaluation of NK1 receptor distribution in the rat, mouse, golden and Siberian hamsters generally confirms the above observations as holding across species (Piggins et al., 2001b). The major point of difference is the absence of receptor from the ventral SCN in all species except the rat. In all species, the SCN is surrounded by a clear margin of dense NK1 receptor and there is a distinct cluster of cells identifiable by NK1 immunoreactivity just inside extending to just outside the dorsolateral border of the SCN. SP is present in fibers and terminals of the rat, mouse, golden and Siberian hamster IGL (Morin et al., 1992; Piggins et al., 2001b), although the origin of this input is not known. The NK1 receptor is densely distributed throughout the entire IGL of the same species (Piggins et al., 2001b). 16 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 With respect to function, SP yielded increased 2-deoxyglucose uptake in the rat SCN during the subjective night, but not during the day. It also produced a light-like PRC in the action potential rhythm (Shibata et al., 1992; Hamada et al., 1999; Kim et al., 2001). Both effects were blocked by the SP receptor antagonists. Cycloheximide also blocked SP-induced phase shifts in the slice preparation (Shibata et al., 1994). About 43% of SCN neurons were excited by application of SP to rat brain slices (11% inhibited), but there was no night/day difference (Shirakawa and Moore, 1994). Similar results have been found in the hamster SCN (Piggins et al., 1995a). In both species, SP modulated neuronal response to glutamate (Shirakawa and Moore, 1994; Piggins et al., 1995a). SP also induced glutamate release in rat SCN slices (Hamada et al., 1999). In optic nerve stimulated slices, an SP antagonist reduced excitatory postsynaptic currents in rat SCN neurons. The effect on NMDA and non-NMDA receptor-mediated responses was similar (Kim et al., 1999). However, SP-induced phase delays could be blocked by NMDA or non-NMDA receptor antagonists, whereas SP antagonists were unable to block glutamate-induced phase delays (Kim et al., 2001). The latter results are interpreted as indicating that SP works upstream of glutamate. Intracranial SP application to the hamster SCN in the early subjective night elicited small phase delays. The effects were not dose-dependent in the pico/nanomolar range and were not seen at other circadian times (Piggins and Rusak, 1997). The use of SP receptor agonists or antagonists has yielded more consistent and sometimes larger effects. Light-induced FOS was significantly reduced by pretreatment with the nonspecific SP antagonist, spantide. The reduction was concentration-specific and varied according to SCN region. Greater reduction occurred in the dorsocaudal SCN than in the ventrocaudal SCN and in the rostral SCN than in the caudal SCN (Abe et al., 1996). The authors compare the effects of spantide to those of NMDA and non-NMDA antagonists with respect to the distribution of light-induced FOS and concluded that the glutamate receptor antagonists were involved in light responsiveness of the ventrocaudal SCN, whereas SP BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 contributed to light responsiveness of dorsocaudal SCN neurons. Intraperitoneal injection of an NK1 receptor antagonist had no effect on hamster rhythms in DD, but in LL, phase advances were obtained following treatment at CT8 (Challet et al., 1998a, 2001). These were not associated with increased wheel running at the time of injection. NK1 antagonists had no effect on light-induced phase delays at any of three intensities (Challet et al., 1998a, 2001). However, at CT19, they attenuated light-induced phase advances by about 30–45%, if administered prior to the light pulse. The antagonists were unable to block the effect of light on phase response if administered after the light pulse (Challet et al., 2001). The effect of spantide on rhythm regulation in hamsters has apparently not been investigated. 6. SCN structure–function relationships The structure–function relationship that appears to be emerging is one of both time and space. That is to say, the phases of intrinsic oscillatory events vary according to SCN region. Attempts to refine the designation, “region,” according to cell phenotype are progressing, but must be considered as fluid. As is discussed below, the actual number of “regions” in the SCN is highly debatable (2, 3 or even 4 for the rat; Figs. 3A–E), even within a single species, as is the correspondence of a nominal region to a particular cell type or organizational attribute. Perhaps the dominant question pertaining to function of SCN neurons concerns the extent to which each is an oscillator. The prevailing view, until fairly recently, has been that the SCN is composed of many intrinsically oscillatory neurons which interactively synchronize to yield a monophasic rhythm (Herzog et al., 1998; Liu et al., 1997a). Gillette 17 et al. (1993) provided evidence, using extracellular recording methods, that separated dorsomedial and ventrolateral parts of the rat SCN oscillated more or less equally in vitro, although amplitude of the rhythm from the ventrolateral region was reduced. This early attempt to distinguish regions of functional significance in which one SCN area differs from another with respect to oscillatory spike discharge provided a negative result, but presaged a topic that has evolved along with the availability of molecular biological tools. More recently, more refined methods have allowed greater detail to be gleaned from individual SCN slices (Schaap et al., 2001, 2003). Subtle differences in phase and waveform are apparent both between the bilateral SCN and across regions within a single rat SCN. Whereas most attempts to evaluate rhythmicity of SCN cells have been done with reference to location or phenotype, Saeb-Parsy and Dyball (2003) evaluated cells based on their targets of projection. Cells projecting to the arcuate and/or the supraoptic nucleus had two peaks in firing rate, one in the early night and the other in the late night, in contrast with cells projecting elsewhere. As described below (Section 6.1.3), there is accumulating evidence that at least some cells may not oscillate. However, attempts to identify individual SCN pacemaker cells, while successful in doing so, have not been able to provide information regarding the percentage of SCN cells that are intrinsically oscillatory or their particular cell characteristics (Herzog et al., 1997, 1998; Liu and Reppert, 2000, Liu et al., 1997a; Welsh et al., 1995; Aujard et al., 2001). Three generalities arise from these studies (Liu and Reppert, 2000). One is that the behavior of oscillatory cells evinces a wider range of intrinsic circadian periods if the cells are isolated from one another than if they are coupled. The second is that GABA is able to Fig. 3 – Suggested organization of the SCN varies according to species, rostrocaudal location within the SCN, time of day, laboratory, criteria for region designation and the evolution of ideas. RAT: (A1 is rostral to A2) Structure using the “core” and “shell” nomenclature (Leak and Moore, 2001). Note fairly substantial differences in position that depend on the level within the SCN. (B) Geographical structure using “dorsomedial” (DM) and “ventrolateral” (VL) designations. Drawn from histology in Nagano et al. (2003). The area VL′ set off by the dashed line has been added to indicate a portion of the SCN that behaved differently from either the DM or VL, but was not acknowledged by the authors. (C1, C2) Divisional structure as suggested from the variation in density of FOS-IR cells (dots). Drawn from the histology in (Beaulé et al., 2001b). Note that the smaller number of FOS-IR cells within the middle of the 3 divisions of (C2) results from light exposure. The dashed line in panels C1 has been added to demarcate a lateral region that did not appear to vary with respect to FOS induction. (D) Three divisions (dorsomedial periventricular, dorsomedial central, ventrolateral) derived from the expression of Per1 (Yan and Okamura, 2002). Dashed line has been added to set off a region that varies minimally with respect to Per1 expression. Compare to panels C1 and C2. (E) Three divisions (dorsomedial, ventrolateral main, ventrolateral medial) derived from the expression of VIP-IR and Per1. The VLmed receives sparse or no retinal innervation (Kawamoto et al., 2003). MOUSE: (F) Expression of Cry1-IR (dots; F1—ZT23, F2—ZT14-light induced) and Per1 (dots; F3—ZT23, F4—ZT14-light induced). Drawn from histology in Field et al. (2000). The circular central area was drawn to encompass most of the Cry1-IR cells and held constant in panels F2–F4. Note that light-induced Per1 tends to be absent from the region of rhythmic Cry1 expression (F1—ZT23), but has substantial overlap with the area showing rhythmic Per1 expression (F3—ZT23). (G) Regions indicated by antiphase rhythmic expression of Per2. Drawn from histology in (King et al., 2003). The central area in panel G1 was drawn to encompass most of the Per2-IR cell nuclei (dots; CT0) and retained in panels G2 which shows the widespread expression of Per2 in nuclei (dots) at CT12. (H) Compartments indicated by the location of VP-IR cells and fibers or GRP cells as indicated by a green fluorescent protein signal. Drawn from Karatsoreos et al. (2004). (I) Solid lines diagram the mouse SCN “core” and “shell” according to one formulation (Yan and Silver, 2004). The dashed line indicates the border of the “core” as presented in another formulation (Abrahamson and Moore, 2001). HAMSTER: (J) SCN and CALB-identifiable central subnucleus (drawn from Hamada et al., 2001). (K) Cells expressing VP mRNA (dots) in the rostral SCN at (K1) CT20 and (K2) CT4 or in the caudal SCN at (K3) CT20 and (K4) CT4 (drawn from histology of Hamada et al., 2004b), outlines included, with the enclosed ventrolateral area designating the region containing CALB neurons in this study). 18 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 shift the phase of individual, noninteracting SCN neurons in a phase-dependent manner. Third, the phases of oscillations among a collection of independent cells are synchronized by repeated bath application of GABA at the same time every day. There is no information concerning the cell phenotype whether from hamsters (Herzog et al., 1998), rats (Welsh et al., 1995) or mice (Liu and Reppert, 2000; Liu et al., 1997a). The ability to evaluate the simultaneous rhythmic behavior of multiple individual cells has provided a new perspective on the oscillatory organization of the SCN. In mouse SCN with a green fluorescent protein (GFP) reporter of Per1 gene activity (Kuhlman et al., 2000), most neurons oscillate in synchronized fashion, but with numerous distinct phases (Quintero et al., 2003). An estimated 11% (LD) and 26% (DD) of the cells evaluated were judged as nonrhythmic. Unexpectedly, cells tend to be grouped, having one of three preferred phases that differ if the animals have been entrained or freerunning. In SCN obtained from mice in LD, 78% of neurons had peak GFP expression at ZT5, ZT8 or ZT11 (most cells peaked at ZT8). With a history of DD, most cells peak at CT12 and 92% had peaks in the CT12, CT15 or CT18 phase groups. The half-maximum phase plot indicates CT12 for the DDhoused animals and ZT8 for the LD housed animals. Several valuable observations are evident in this study (Quintero et al., 2003): (1) not all SCN neurons are rhythmic; (2) different rhythmic neurons have different phases; (3) there are three preferred phases of rhythmic SCN neurons, a characteristic previously observed using multiunit in vivo electrophysiological methods (Meijer et al., 1997); (4) the preferred phase of rhythmic neurons varies according to photoperiod history. The results support the conclusion that, assuming clock genes actually determine the pacemaker properties of SCN neurons, other factors control the phase relationship between the locomotor rhythm and circadian clock phase. This is demonstrated by the fact that the preferred phase of gene expression is about 4 h earlier in LD housed mice compared to DD housed mice when assessed relative to activity onset (Quintero et al., 2003). An exception to the above evidence concerning the timing of oscillations in SCN neuronal activity has been observed in the hamster. Whereas there is a neuronal activity rhythm peak recorded approximately once every 24 h if the SCN tissue is cut in the coronal plane, there are two distinct peaks if the SCN is cut in the horizontal plane (Jagota et al., 2000). The phases of the two peaks are modulated by photoperiod history (Mrugala et al., 2000). This phenomenon appears to apply only to hamsters, and has not been seen in mouse or rat (Burgoon et al., 2004). These results support the view that there are functionally distinct regions within the SCN; different regions oscillate differently; and the “standard” perspective (here, the coronal view) is not always the fully correct and adequate perspective. 6.1. Function of cellular neuromodulators in the SCN 6.1.1. GABA As indicated above, GABA is the predominant neurotransmitter in cells of the rat, mouse and hamster SCN. It is, therefore, not a surprise that manipulation of GABA neurotransmission in the SCN alters circadian rhythm function. One potential complication to the interpretation of the functional analyses of GABA in the SCN is the presence of a GABAergic afferent projection from the IGL (Morin and Blanchard, 2001). The basic observation is that peri-SCN administration of the GABAA agonist, muscimol, substantially reduces the magnitude of light-induced phase shifts, while similar application of the GABAA antagonist, bicuculline, substantially increases phase shift magnitude (Gillespie et al., 1996). These results are inconsistent with prior data obtained using systemic drug injections (Ralph and Menaker, 1987, 1989). Nevertheless, the effects of intracranial (i.e., SCN) drug application appear to be repeatable and consistent, although the effect of bicuculline may depend on circadian time (Gamble et al., 2003; Mintz et al., 2002; Gillespie et al., 1997). Similarly, bicuculline augments and muscimol suppresses light-induced Fos protein in the hamster SCN (Gillespie et al., 1999). The GABAB receptor may also be involved in modulation of light-induced phase shifts. Peri-SCN injections of a GABAB receptor agonist, baclofen, block light-induced phase delays and advances, but the antagonist, CGP-35348, has no effect (Gillespie et al., 1997). Baclofen also blocks glutamate-induced phase delays and this action is not affected by TTX (Mintz et al., 2002). The GABAB agonist also effectively blocks lightinduced Fos at CT13.5 and CT19 (Gillespie et al., 1999), although an antagonist has no effect. The effect of GABAB receptor activation is the result of an inhibition of glutamate release from the RHT terminals (Jiang et al., 1995). The GABAA agonist, muscimol, is also able to induce phase advances when administered to DD-housed hamsters during the middle subjective day (Novak et al., 2004; Smith et al., 1989) and this effect is inhibited by a concomitant light pulse (Mintz et al., 2002). 6.1.2. VIP and VIP/PACAP receptors VIP neurons are clustered in the ventral SCN of most mammalian species and use VIP as a neuromodulator to communicate with other neurons in the SCN through activity at the PAC1 or VPAC2 receptor, both of which are found in the SCN (Cagampang et al., 1998a,b). A complicating factor in the analysis of receptor function in the context of the circadian system is the fact that both receptors bind VIP and PACAP with approximately equal affinity. PACAP-containing terminals are found in projections from the retina, as well as from other, unidentified brain regions (Hannibal and Fahrenkrug, 2004; Hannibal et al., 1997). VIP peptide has been implicated in the regulation of circadian rhythm phase and period, although conflicting observations have been reported (Gillespie et al., 1996; Piggins et al., 1995b; Albers et al., 1991). More recent work has focused on function of the PAC1 and VPAC2 receptors with respect to circadian rhythm regulation and the results have been quite provocative. Calcium imaging methods suggest that the effects of PACAP on SCN neurons are elicited through the PAC1 receptor because of the absence of response to equimolar VIP application (Kopp et al., 1999). The PAC1 knockout mouse tends to have a shorter circadian period in DD than wild-type and light-induced phase shifts are less after stimulation at certain circadian times compared to the wild type (Hannibal et al., 2001b). Fos protein and Per1/2 gene induction were also BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 much less in the knockout animals following a light pulse during the early subjective night, although they were not different during the late subjective night. The effects of PAC1 receptor knockout are small compared to the consequences of losing a functional VPAC2 receptor. The latter appears to be an essential component of the circadian rhythm system, at least in mice. Its absence in the Vipr2 KO mouse is associated with nearly complete loss of the circadian locomotor rhythm assessed in constant dim red light (Cutler et al., 2003). Similarly, rhythms in SCN gene expression (Per1, Per2, Cry1, VP were lost, although a vastly attenuated, but statistically significant, rhythm in BMAL1 expression was evident (Harmar et al., 2002; Cutler et al., 2002). In the Vipr2 KO mice, there was also very little light-induced FOS protein compared to what is normal in the wild type. In the SCN slice preparation, the ensemble circadian rhythm in neuronal firing rate is lost in the Vipr2 KO mouse (Cutler et al., 2003). An additional feature of this study was the demonstration that a specific VPAC2 receptor antagonist had the same effect on the ensemble neuronal firing rhythm of wild-type animals as the gene defect does on the Vipr2 KO animal. In contrast to the gene knockout model, a transgenic mouse overexpressing the VPAC2 receptor has a much longer circadian period and appears to be more sensitive to light as indicated by faster re-entrainment to an advance of the light– dark photoperiod (Shen et al., 2000). At the level of individual SCN cells, there is also an absence of rhythmic gene expression in Vipr2 KO mice (Harmar et al., 2002). An explanation for the function of the VPAC2 receptor within the context of circadian clock structure and intercellular pacemaker regulation has not been determined, although the suggestion has been made that rhythmic VIP serves to maintain coupling between oscillating neurons in the SCN (Harmar et al., 2002). This explanation does not account for the fact that about 15% of the congenitally anophthalmic ZRDCT-An mouse strain are arrhythmic despite the presence of VIP neurons in the SCN (Laemle and Ottenweller, 2001). Thus far, there is a demonstrable requirement for the presence of the VPAC2 receptor in order for mice to display circadian rhythmicity, but no demonstration that VIP itself is explicitly necessary or greatly involved in rhythm generation or regulation. Nearly half the mouse SCN neurons immunoreactive for VP also have the VPAC2 receptor as do about 31% of the VIP and CalR neurons (Kallo et al., 2004). In general, there is a high degree of regional correspondence between the distribution of mouse neurophysin (marker for VP) and for PHI-containing (marker for VIP) neurons with the distribution of cells containing VPAC2 receptor (King et al., 2003), although the degree of colocalization was not established in this paper. The regional correspondence is not perfect as a central zone in the mouse SCN is devoid of VIP or VP neurons, but contains a few cells with VPAC2 receptors. The specific importance of VIP for circadian rhythms has been emphasized in mice (Colwell et al., 2003; Aton et al., 2005). VIP neurons project widely from the SCN to various targets (Kalsbeek et al., 1993), but also appear to have intraSCN connections (LeSauter et al., 2002; Daikoku et al., 1992; van den Pol and Gorcs, 1986). Two thirds of cells in the mouse SCN have spontaneous inhibitory postsynaptic 19 potentials that are enhanced by VIP. These cells are distributed throughout the SCN with no discernible regionrelated pattern (Itri and Colwell, 2003). VIP infused into the hamster peri-SCN region or onto the rat SCN slice during the early subjective night induces phase delays, while infusions during the late subjective night yield phase advances, with a PRC similar to that for light (Piggins et al., 1995b; Albers et al., 1991; Reed et al., 2002) (although there is controversy concerning whether or not PHI and GRP are necessary for obtaining phase shifts). In mice lacking genes coding for PHI and VIP, a variety of rhythm-related measures were altered. In particular, the circadian period was shorter by about an hour, phase response to light was greatly reduced or eliminated, there was more variability in activity onset under LD and cohesive rhythms were generally not maintained under DD (Colwell et al., 2003). Mice lacking only the gene coding for VIP had similar characteristics. Most notably, about two thirds of the animals had running rhythms in DD that expressed multiple noncircadian components. Importantly, the behavior of VIP KO mice was strikingly similar to mice lacking the gene (Vipr2) coding for the VPAC2 receptor (Aton et al., 2005). The importance of VIP to normal rhythmicity in the VIP KO mice has been established by reinstating SCN rhythmicity and synchrony among SCN neurons by periodic application of VIP agonist to a tissue culture preparation, but this has no effect on VPAC2 KO mice (Aton et al., 2005). 6.1.3. SCN Calbindin and the central subnucleus of the hamster There has been emphasis on the central subnucleus of the SCN and the role its constituent CALB cells may play in circadian rhythm regulation. A subset of SCN neurons expresses the Ca2+ binding protein CALB (Arvanitogiannis et al., 2000; LeSauter and Silver, 1999). CALB contains six highaffinity Ca2+ binding domains and contributes to intracellular Ca2+ signaling and buffering (Leathers et al., 1990; Roberts, 1994). As described above, the CALB neurons are clustered within the caudocentral SCN. This region contains about 250 CALB neurons and is approximately 300 μm long, 180 μm high and 130 μm wide (Silver et al., 1996a). Partial lesions of the SCN that completely destroyed the CALB region yielded animals with arrhythmic locomotor activity. In contrast, if the lesions spared a portion of one or both CALB regions, behavioral rhythmicity was maintained (LeSauter et al., 1996). The circadian locomotor rhythm is restored after implantation of fetal SCN tissue into the third ventricle of previously SCN-lesioned adult hamsters (Ralph et al., 1990). Restoration of rhythmicity requires the presence of CALB neurons within the transplanted grafts and the strength of the restored locomotor rhythm is correlated with the number of surviving CALB neurons (LeSauter and Silver, 1999). Small lesions of the SCN that damage the CALB region bilaterally eliminate a number of behavioral and physiological rhythms. If the CALB region on either side was partially or completely spared, the rhythms remained intact (Kriegsfeld et al., 2004). These data suggest that the region of the SCN containing these CALB positive neurons is important in the generation of circadian rhythms. An important question to be answered is whether it is the CALB neurons (or other neurons 20 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 in this region) or whether it is the network connections between the cells in this region that are critical for the generation of circadian rhythms. The central subnucleus receives dense input from the retina via the retinohypothalamic tract and the CALB neurons are directly contacted by retinal projections (Muscat et al., 2003; Bryant et al., 2000). A light pulse during the subjective night increased FOS expression in the majority of CALB neurons of the hamster SCN. These data led to the development of a model in which the CALB neurons serve as a “gate” for photic input to the SCN (Antle et al., 2003a). This model must be reconciled with anatomical data demonstrating that the RHT projects to all regions of the hamster SCN and the fact that the densest retinal terminal field extends well beyond the CALB subnucleus (Muscat et al., 2003). In contrast to results in the hamster, CALB and FOS colocalization are rare in the grass rat (Mahoney et al., 2000). Destruction of CALB neurons in the rat SCN does not alter light-induced FOS expression, freerunning rhythms or photic entrainment in that species (Beaulé and Amir, 2002). Neurons in the hamster central subnucleus do not show a rhythmic expression of messenger RNAs for Per1 and Per2 (Hamada et al., 2001, 2004a). However, light can induce the expression of both these clock genes in the central subnucleus. This has led to the conclusion that the central subnucleus is a nonrhythmic region of the SCN, while the dorsomedial region corresponding to the general location of VP neurons is rhythmic because the cells in this area express Per1 and Per2 in a rhythmic manner. However, it may not be possible to determine the rhythmicity of individual neurons with in situ hybridization techniques if only a portion of the neurons in a region are rhythmic. Further, electrophysiological evidence suggests that at least some neurons in the central subnucleus fire action potentials in circadian manner. The mean spontaneous firing rate (SFR) of non-CALB neurons in the central subnucleus has a peak at ZT6 and a nadir at ZT19 (Jobst and Allen, 2002). Whether the rhythmicity of these nonCALB neurons is driven by endogenous clocks located in neurons of the central subnucleus or whether it is due a network phenomena of interconnections between rhythmic and nonrhythmic neurons is an important area for future research. In contrast, CALB neurons had a mean SFR of less than 1 Hz and showed no circadian pattern of action potential firing (Jobst and Allen, 2002). It is noteworthy, but of unknown significance, that some CALB neurons contact a neighboring CALB or non-CALB cell via gap junctions (Jobst et al., 2004). In the mouse, increased firing frequency is correlated with increased expression of Per1 (Kuhlman et al., 2003). This suggests that CALB neurons are a functionally distinct subpopulation within the SCN that do not contain a rhythmic circadian clock. The data raise the intriguing possibility that the interactions between rhythmic and nonrhythmic neurons are required to generate circadian locomotor rhythms. There is also the untested possibility that the GRP, SP and CALR neurons that occupy the central subnucleus along with the CALB cells are likewise nonrhythmic. CALB expression is dynamic depending on the genotype, developmental age and the lighting conditions. Tau hamsters have significantly more CALB expressing neurons than wildtype hamsters (LeSauter et al., 1999). In mice, there is significantly more CALB expression in young animals that gradually decrease as the animals approach adulthood (Ikeda et al., 2003). CALB expression is inversely correlated with the duration of the light cycle (Menet et al., 2003). Hamsters maintained on short duration photoperiods had significantly more CALB expression than those maintained on long photoperiods (Menet et al., 2003). Hamsters reared in DD or short photoperiod conditions had more CALB than controls (Tournier et al., 2003). Similarly, rats maintained in LL conditions had fewer CALB-containing neurons then in rats maintained in DD (Arvanitogiannis et al., 2000). Neonatal monosodium glutamate (MSG) exposure increased the number of CALB-expressing SCN neurons and prevented the disruption of circadian rhythms produced by LL in rats (Arvanitogiannis et al., 2000). The parallel effects of MSG on CALB and LL changes on rhythms suggest that CALB may be important in the regulation of response to LL. CALB appears to play a key role in the photic responses in hamsters. CALB is present in the cell nucleus and cytoplasm of central subnucleus SCN neurons during the day, but is absent from the cell nucleus during the night (Hamada et al., 2003). Reduction of CALB expression by antisense oligonucleotides blocked light-induced phase shifts during the night, but light could produce shifts of both behavioral and Per rhythms during the day (Hamada et al., 2003). Interestingly, these effects occurred despite the fact that the CALB mRNA was only reduced 38% and the CALB protein only reduced 28% (Hamada et al., 2003). Also interesting was the rapidity of the effect, which occurred only 4 h after the antisense injections. Therefore, while the CALB neurons do not have rhythmic clock gene expression or show rhythms in action potentials, CALB expression in the nucleus may be controlled in a circadian manner. Thus, these cells appear to be rhythmic according to at least one criterion, but not by others. The mechanisms controlling this cellular regulation are unknown. They may involve rhythmic feedback from the dorsomedial “rhythmic” SCN or may result from the interaction between rhythmic and nonrhythmic neurons in the central subnucleus. The central subnucleus is heterogeneous and CALB cells constitute a minority of the neurons (LeSauter et al., 2002; Jobst and Allen, 2002). A better understanding of mechanisms underlying intercellular communication between non-CALB and CALB neurons and how they respond to synaptic input will be important to understand how the SCN generates a functional circadian output. Double-label methods have demonstrated that 94% of CALB neurons also contained GABA (Jobst et al., 2004). Of note, most of the cellular profiles in the central subnucleus contain GABA, although many GABA neurons in the central subnucleus did not contain CALB. In addition to CALB and GABA colocalization in the central subnucleus, glutamate decarboxylase 67 (GAD67), one of two related isoforms of the synthetic enzyme for GABA, was detected in CALB cell bodies. Immunoreactivity for the GABAA receptor α2 and β2/3 subunits was prominent in the SCN (Jobst et al., 2004). Confocal microscopic analysis demonstrated that β2/3 and α2 did not colocalize with CALB. Labeling for the β2/3 subunits was diffusely distributed around CALB neurons, whereas labeling for the α2 subunit appeared as discrete BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 puncta. The data indicate that α2 and β2/3 subunits contribute to the composition of GABAA receptors in the CALB region. A limitation of this study is that the GABAA receptor subunits were not localized to either postsynaptic or presynaptic regions. GAD65, an isoform of GAD exclusively located within synaptic terminals (Erlander et al., 1991), is expressed in a punctate pattern throughout the SCN, including the central subnucleus (Jobst et al., 2004). 6.1.4. Bombesin and gastrin releasing peptide Bombesin contains 14 amino acids, GRP has 27 and they share a 7 amino acid sequence homology. There is a very small literature concerning the effects of bombesin on SCN function. Immunoreactive cells are abundant in the Djungarian hamster (Reuss, 1991), although cross-reactivity with GRP is a potential problem (Panula et al., 1984). Bombesin increased the firing rate of about 50–60% of golden hamster neurons studied, while inhibiting the rate in half of the remainder (Piggins and Rusak, 1993; Piggins et al., 1994). Neuromedin B, another in the class of bombesin-like peptides, activated about 40% of SCN neurons and inhibited very few (Piggins et al., 1994). The responses of individual cells to application of several different bombesin-like peptides were similar in more than 80% of the tests. Responses to the peptides were attenuated by pretreatment with antagonists to the GRP-preferring receptor which is present in the SCN (Ladenheim et al., 1992, 1993). More cells were activated by GRP and neuromedin B at ZT12-16 than at ZT4-8 (Piggins et al., 1994). Bombesin has not been similarly tested. GRP was originally reported to induce light-like phase shifts in hamster only if infused onto the SCN along with VIP and PHI (Albers et al., 1991), but may be equivalently functional if applied alone (Piggins et al., 1995b). The action potential rhythm in rat or hamster slice preparations exhibited large, light-like phase responses to GRP and these effects could be blocked by bombesin 2 receptor antagonists. Bombesin 2 and bombesin 1 receptors were identified in SCN, although there is no evidence that bombesin 1 contributes to rhythm regulation (McArthur et al., 2000). GRP injected into the hamster third ventricle at CT 13 induces sizable phase delays in association with induction of Per1, Per2 and FOS (Antle et al., 2005). Interestingly, the gene expression occurs almost exclusively in the densely retinorecipient region in the dorsal SCN, just above the central subnucleus identified by CALB cell presence (Muscat et al., 2003). This is the same area in which pERK is expressed, dependent upon an intact RHT, during the subjective night (Lee et al., 2003). And, phosphorylation of ERK is apparently necessary for phase response to GRP because if phosphorylation is pharmacologically blocked, the phase shifts are also blocked (Antle et al., 2005). Although spontaneously rhythmic ERK phosphorylation does not occur in the absence of the RHT, the effect of enucleation on phase response to GRP has not been examined. In the mouse, GRP infused into the peri-SCN region at CT16 elicited small phase delays and induced Per and Fos gene expression (Aida et al., 2002). GRP receptor was also found throughout most of the SCN, although density was clearly greatest in the dorsocaudal part of the nucleus. Mice lacking the GRP receptor had normal phase delays in 21 response to a 15 min 30 lx light pulse at CT16, but unlike wild-type animals, the delays were not greater if the light was 300 lx. Small (approximately 20%) decrements were also seen in Per2 (but not Per1) and Fos response to the 300 lx light (Aida et al., 2002). In ovariectomized, estrogen-primed rats, pituitary prolactin is released in a daily surge timed by the SCN (Mai and Pan, 1993, 1995; Freeman et al., 2000). Properly timed bombesin infusion into the peri-SCN region blocks the prolactin surge (Mai and Pan, 1995). The implication is that bombesin neurons participate in the efferent control of prolactin release timing. This raises the general issue of whether individual SCN cell phenotypes are concerned with the control of single or multiple efferent systems. 6.1.5. Neuromedin U and neuromedin S Neuromedin U (NMU) is a 24 amino acid peptide that shares a 7 amino acid sequence homology with the somewhat larger neuromedin S (NMS) (Mori et al., 2005). NMU and its receptors, NMU-R1 and NMU-R2, are both present in the rat and mouse SCN (Mori et al., 2005; Graham et al., 2003, 2005; Nakahara et al., 2004). NMU and NMU-R1 have high amplitude rhythmic gene expression that persists in DD, whereas rhythmic expression of the NMU-R2 gene is lower amplitude (Graham et al., 2003; Nakahara et al., 2004). NMU is present in some, but not all, VP neurons and some, but not all, NMU-containing neurons also have VP; it apparently does not colocalize in VIP neurons (Graham et al., 2003). The distribution of NMU in rat is most similar to the distribution of VIP neurons, but is present throughout much of the SCN. NMU-R1 and R2 receptor distributions are similar to that for the peptide. Phase advances of about an hour followed ventricular injection of NMU at CT0 into rats; injections at CT6 yielded phase delays of about 0.6 h; and from CT8 to CT23, there was no phase response. FOS protein expression was widely induced by intraventricular NMU (Graham et al., 2003). Mice lacking the gene coding for NMU have rhythmic feeding behavior, but the phase angle of entrainment may be altered (Hanada et al., 2004). NMS is distributed in the rat SCN in a pattern similar to VIP and there was rhythmic expression peaking at ZT11 (Mori et al., 2005). The rhythm was lost in DD and a light pulse acutely reduced expression. Intraventricular injection of NMS at CT23 induced 1 h phase delays and, at CT6, about 2 h phase advances with a dead zone between CT9 and CT22. FOS expression in dorsal SCN cells was also induced by NMS. The PRC for NMS was similar to that for NPY (Albers and Ferris, 1984; Albers et al., 1984), whereas that for NMU appeared to be somewhat different. The general shape of the PRC for NMS was very much light-like, but was shifted such that phase delays began at CT24 with a cross-over point at CT3 and the phase advance region ended at approximately CT8-9 (Nakahara et al., 2004). 6.1.6. Vasopressin The Brattleboro rat is deficient in VP, yet has circadian rhythms (Ingram et al., 1998). It has been concluded that VP is not necessary for circadian rhythm expression. In fact, transplanted SCN from Brattleboro rats will restore normal circadian rhythmicity in arrhythmic hosts (Boer et al., 1999). 22 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 Although VP is not a necessity for rhythm expression in Brattleboro rats, transplanted SCN from normal animals can restore rhythmicity in paraventricular hypothalamic neurons and this rhythmicity is likely to be the result of VP stimulation (Tousson and Meissl, 2004). The issue is rendered more complicated by the demonstration that rhythmicity of VP cells evaluated in vitro shows advances and delays according to circadian time of VIP stimulation. Interestingly, the same study failed to observe a phase shifting effect of glutamate which would be expected to act either directly on the VP cells or on the VIP cells projecting to the VP cells (Watanabe et al., 2000). 6.1.7. Diurnality–nocturnality In the grass rat, a diurnal rodent, the above effect of GABA agonists administered during the subjective day may occur at phases opposite to those effective in the nocturnal hamster (Novak and Albers, 2004a). In particular, muscimol injected in the peri-SCN region at this time induces phase delays rather than phase advances and these effects are not blocked by TTX. Opposite to the effect in nocturnal species, phase shifts caused by peri-SCN muscimol are not inhibited by simultaneous light exposure (Novak and Albers, 2004b). However, nocturnal and diurnal species appear to respond similarly with respect to the ability of GABAA and GABAB agonists to inhibit phase response to light (Novak and Albers, 2004b; Novak et al., 2004). Assuming that the action of injected GABA agonists is occurring within the SCN, then the antiphase GABA activity in diurnal vs. nocturnal animals is the first indication that the SCN contributes to this species-specific behavioral trait. In a subparaventricular region contiguous to the dorsal SCN, the lower subparaventricular zone, the grass rat has higher expression of FOS during the night than during the day, an effect not observed in rats (Smale et al., 2001; Schwartz et al., 2004; Nunez et al., 1999). Moreover, the day– night pattern of expression is roughly opposite that which occurs in the SCN of either the Norway lab rat or grass rat. The suggestion has been made that the neuron firing rate rhythm can become inverted in the peri-SCN region for nocturnal (Inouye and Kawamura, 1979), but not for diurnal (Sato and Kawamura, 1984), species and may contribute to the manifestation of nocturnal or diurnal activity traits (Smale et al., 2003). 6.2. Acetylcholine Cholinergic innervation of the SCN has been of interest for many years and there are numerous reports of cholinergic receptors on SCN cells and modification of circadian rhythm function by cholinergic agonists or antagonists (see Morin, 1994 for a review). Several anatomical reports have described choline acetyltransferase (ChAT) immunoreactive processes in the SCN, although the histology has not been robust and the demonstrable fibers have been located in somewhat different places, depending upon the particular study (van den Pol and Tsujimoto, 1985; Bina et al., 1993; Ichikawa and Hirata, 1986; Kiss and Halasz, 1996). Cholinergic fibers have also been shown to make contacts directly on SCN neuron (Kiss and Halasz, 1996). Perhaps the most convincing evidence of cholinergic input to the SCN is the demonstration that, following an intra-SCN injection of retrograde tracer, labeled cells containing ChAT are found in several brain nuclei including the lateral dorsal and pedunculopontine tegmentum, areas concerned with sleep regulation (Bina et al., 1993). Retrogradely labeled cholinergic cells were also found in the basal forebrain. Excitotoxic lesions of the nucleus basalis magnocellularis destroyed about 75% of cholinergic cells in this location yielding a decrease in cholinergic processes in the SCN (Madeira et al., 2004). Bilateral nucleus basalis lesions also diminished the numbers of countable VP and VIP cells in the SCN by about 30% and reduced volume and mRNA levels of VP and VIP cells by about 34%. The immunotoxin, 192 IgG-saporin, destroys cells bearing the low affinity nerve growth factor receptor, p75NTR, which are generally cholinergic (Leanza et al., 1995). Intraventricular infusion of the immunotoxin abolished p75NTR in the rat SCN without affecting VIP neurons (Moga, 1998). However, intrahypothalamic immunotoxin injection into the rat greatly reduced numbers of CALB neurons (Beaulé and Amir, 2002). The circadian period length was related to the extent of cell loss. Typically, the period was much shorter in lesioned rats and about a third of them became arrhythmic. Animals sustaining immunotoxin lesions of cholinergic cells also had about a 50% reduction in light-induced FOS cell counts. In another study (Erhardt et al., 2004), 192 IgGsaporin was injected into the ventricle or directly into the SCN, but neither procedure yielded deficits in CALB or VIP neurons and changes in circadian period or general rhythmicity were not observed. Nevertheless, the immunotoxin generally reduced the ability of light to produce phase delays or advances. The rhythm-related effect of 192 IgG-saporin treatment may be related to disruption of effective retinal projections to the SCN. Unilateral enucleation reduces optical density of p75NTR in the contralateral SCN by about 50% (Bina et al., 1997). As reviewed (Morin, 1994; O'Hara et al., 1998), the cholinergic agonist, carbachol, has generally yielded light-like phase responses. However, the topic is not without controversy. For example, peri-SCN carbachol induced robust phase delays when injected at CT14 and advances at CT22, but also produced large phase advances in about half the animals receiving the drug at CT6 (Bina and Rusak, 1996). The phase shifts during the subjective night are consistent with a lightlike action, but the shifts during the day are not. The responses were blocked by pretreatment with atropine, but not by mecamylamine, suggesting that a muscarinic receptor mediates the cholinergic effects. The functional presence of muscarinic receptors in the SCN region is supported by ample additional data, particularly from SCN slice preparation studies (Liu and Gillette, 1996; Liu et al., 1997b; Artinian et al., 2001) and there is evidence supporting a rhythm function specifically for the M1 receptor (Gillette et al., 2001). Despite the foregoing, the nicotinic receptor may also mediate the effects of acetylcholine on circadian rhythmicity. The nicotinic antagonist, mecamylamine, blocked the phase shifting effects of light at CT19 and greatly attenuated lightinduced FOS in the dorsomedial hamster SCN with little inhibition more ventrally (Zhang et al., 1993). In the rat slice preparation, the PRC to nicotine was phase-independent with BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 advances occurring throughout the circadian day and the phase shifts could be blocked by mecamylamine (Trachsel et al., 1995). In vivo, nicotine administered subcutaneously at CT16 elicited significant phase delays and induced FOS in the SCN and mecamylamine greatly attenuated FOS expression (Ferguson et al., 1999). A preliminary description of rat response to nicotine indicated that phase shifts were unreliable following systemic administration, but were light-like following nicotine intraventricular infusion (O'Hara et al., 1998). There are also reports that a strain of rats bred for cholinergic supersensitivity has a shorter circadian period than controls, an altered phase angle of entrainment and reduced FOS induction in SCN neurons (Shiromani et al., 1988; Ferguson and Kennaway, 1999). 6.3. Neurophysiology of the SCN action potential rhythm Action potential firing by SCN neurons is regulated as a circadian rhythm. Higher frequencies are observed during the subjective day than during the subjective night with the mean population firing frequency peaking near CT6 (Gillette, 1991). During the subjective day, action potential firing frequencies range from less than 1.0 Hz to 14 Hz, compared to the subjective night when action potentials fire with frequencies of 0.5 to 5.0 Hz. The presence of fast firing neurons during the day is responsible for the increased mean population firing frequency observed in SCN slices (Gillette, 1991). This circadian rhythm of action potential firing is a property of individual neurons because the firing difference is maintained when SCN neurons are grown in dispersed cell cultures (Welsh et al., 1995; Herzog et al., 1997; Abe et al., 2000; Honma et al., 1998a; Shirakawa et al., 2000). A current hypothesis proposes that the molecular circadian clock sends signals to regulate the activity of ion channels responsible for setting the membrane potential and action potential firing rate. This hypothesis predicts that disruption of clock gene function would alter the action potential firing of SCN neurons. Mutations of clock genes alter the period of action potential firing rhythms. Microelectrode array recordings of slice cultures from Clock homozygous mice demonstrated neurons with a period near 27 h that was significantly longer than neurons of wild-type or Clock heterozygous mice (period near 24 h) (Nakamura et al., 2002). In a similar study, the period of Clock heterozygous mice was longer than that of the wild type mice (24.5 vs. 23.3 h), while the Clock homozygous mice were arrhythmic (Herzog et al., 1998). In the tau mutant hamster, which has a shortened circadian period, the circadian rhythm of action potential firing is also shortened to approximately 20 h (Liu et al., 1997a). SCN neurons from mice with mutations of the cryptochrome 1 or cryptochrome 2 genes are arrhythmic (Albus et al., 2002). Also consistent with this model are the observations of McMahon and colleagues who used a transgenic mouse expressing a green fluorescent protein driven by the Per1 promoter to demonstrate that the frequency of action potential firing was linearly correlated with Per1 transcription (Quintero et al., 2003). SCN neurons express a wide repertoire of ion channels and regulation of the activity of these ion channels by signals from the molecular clock would lead to regulation of action 23 potential firing. The presence of a diffusible messenger is supported by the observation that spontaneous firing of SCN neurons “washes out” during the dialysis of the intracellular milieu that occurs during whole-cell recording (Schaap et al., 1999). Currently, the signaling pathways connecting the molecular clock in the nucleus to changes in membrane potential are unknown. However, significant progress has been made toward describing the ion channels responsible for the regulation of spontaneous firing. Many membrane properties of SCN neurons show a diurnal or circadian variation. SCN neurons have input resistances in the range of 1–3 GΩ when measured with whole cell recording techniques. The input resistance of SCN neurons is higher during the day compared to the night in slices maintained in either LD or DD (De Jeu et al., 1998; Kuhlman and McMahon, 2004). The resting membrane potential of SCN neurons has been reported to be more depolarized during the day than during the night (De Jeu et al., 1998; Kuhlman and McMahon, 2004; Pennartz et al., 2002). These differences in membrane potential are maintained in rats maintained in DD consistent with their regulation by the circadian clock (Kuhlman and McMahon, 2004). However, a significant day–night difference in the membrane potential has not always been observed (Schaap et al., 1999; Kim and Dudek, 1993; Teshima et al., 2003). Difficulties in estimating the “resting” membrane potential of spontaneously firing neurons and dialysis of intracellular contents may contribute to these different results. The resting membrane potential has been proposed to be set, in part, by a Ba2+-sensitive, fast-activating, outwardly rectifying K+n current (De Jeu et al., 2002). Interestingly, this current did not show a circadian rhythm in activity, although given the high input resistance of SCN neurons, a small change in this current could significantly change the resting membrane potential. The membrane potential of some SCN neurons has a low frequency (2–7 Hz) oscillation that is mediated by L-type Ca2+ channels and is observed when action potentials are blocked by TTX (Jiang et al., 1997; De Jeu et al., 1998; Pennartz et al., 2002; Jackson et al., 2004). Action potentials in SCN neurons are characterized by a rapid upstroke of membrane depolarization (∼+25 mV) followed by membrane potential repolarization. An afterhyperpolarization (AHP; ∼−80 mV), a membrane potential that hyperpolarizes below the resting membrane potential and which is dependent on extracellular Ca2+, follows the action potential upstroke (Cloues and Sather, 2003; Thomson, 1984; Thomson and West, 1990). The rapid upstroke of the action potential is driven by current flowing through voltage-gated Na+ channels and is completely blocked by TTX (Thomson and West, 1990; Huang, 1993; Wheal and Thomson, 1984). During the interval between individual action potentials (interspike interval), the membrane potential shows a steady depolarization that reaches spike threshold (Kononenko et al., 2004; Pennartz et al., 1997). This interspike interval can be modulated by synaptic input, particularly inhibitory postsynaptic potentials generated by GABAergic input (Pennartz et al., 1998; Kononenko and Dudek, 2004). These “depolarizing ramps” are sensitive to the Na+ channel blocker, TTX, and to riluzole, a compound used to treat amyotrophic lateral sclerosis, that blocks a persistent Na+ current. Voltage-gated Na+ channels rapidly activate and inactivate during a 24 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 membrane depolarization. However, many neurons have a small Na+ current that shows very slow or no inactivation (for review see Crill, 1996). This “slowly-inactivating” or “persistent” Na+ current (INa,S or INa,P) is TTX-sensitive and may represent current flowing through a noninactivating class of voltage-gated Na+ channels or a modal variation of the normally transient Na+ current. In SCN neurons, a slowly inactivating Na+ current (in I = 50–250 ms at − 45 mV) is blocked by TTX, and riluzole similar to the INa,P. The INa,S may represent a third modal state of voltage-gated Na+ channels (Jackson et al., 2004). It has been hypothesized that the INa,S is primarily responsible for driving the membrane depolarization during the interspike interval (Kononenko and Dudek, 2004; De Jeu and Pennartz, 2002). Jackson et al. (2004) recorded from acutely isolated dorsomedial SCN neurons and used action potential waveforms as voltage signals under voltage clamp to determine the currents flowing during the interspike interval. The ionic currents flowing during the action potential waveform are the same as those that flow during the action potential firing. The majority of the current during the interspike interval was carried by TTX-sensitive Na+ current. A nimodipine-sensitive Ca2+ current was also present during the during the interspike interval. However, the amplitude and charge carried by the Ca2+ current are only 26% of that carried by the Na+ current (Jackson et al., 2004). Further, the Ca2+ current turns on later in the interspike interval than the Na+ current and is not significant until the membrane potential approaches threshold. The authors conclude that the slowly inactivating Na+ current is primarily responsible for setting the action potential frequency while contribution of voltage-gated Ca2+ currents to the action potential firing frequency is small (Jackson et al., 2004). Other candidate ion currents have been proposed to contribute to setting the action potential frequency (Kim and Dudek, 1993; Pennartz et al., 1997). The H-current (IH), a nonselective cation channel activated by membrane depolarization has been hypothesized as contributing a depolarizing current, but a direct test of this hypothesis did not yield any evidence for an IH contribution (Akasu et al., 1993; De Jeu and Pennartz, 1997). The A-current (IA), a fast-activating, rapidly inactivating K+ current present in all SCN neurons, may contribute to setting the duration of the interspike interval (Huang, 1993; Bouskila and Dudek, 1995). The low-threshold Ttype Ca2+ current has also been proposed as a contributor to the setting of action potential frequency (Akasu et al., 1993). No direct evidence for the IH or IT has been presented. The AHP that follows membrane potential repolarization also contributes to setting action potential firing frequency. The AHP duration correlates with the spontaneous firing rate, the slower the AHP decay, the slower the firing rate (Cloues and Sather, 2003). If the AHP is blocked, then the rate of firing of SCN neurons increases. In SCN neurons, the AHP is dependent on Ca2+ entering the cell via predominately L- and R-type voltagegated Ca2+ channels that activate both large-conductance (BKCa) and small-conductance (SKCa) Ca2+-activated-K+ channels. Blockade of apamin-sensitive SKCa channels (only SK2 subunits in SCN) has been reported to produce an increase or no effect on spontaneous action potential frequency. The difference probably reflects a diversity of SKCa expression in SCN neurons. Block of iberiotoxin-sensitive BKCa did not alter firing frequency. An apamin- and iberiotoxin-insensitive KCa current was diurnally modulated and contributes to the spike frequency (Cloues and Sather, 2003). The AHP is dependent on both small conductance (SKCa) and large conductance (BKCa) Ca2+-activated-K+ channels. Abundant experimental evidence demonstrates that Ca2+ plays an important role in the activity of SCN neurons. First, removal of extracellular Ca2+ abolishes the circadian pattern of action potential firing (Shibata et al., 1984). Second, some SCN neurons have a regular pattern of action potential firing characterized by small deviations in the interspike interval (Thomson, 1984). Reduction of the extracellular Ca2+ concentration disrupts this regular firing pattern, whereas an increase potentiates the action potential spike amplitude and the rate of membrane repolarization (Thomson and West, 1990). Removal of Ca2+ or block of Ca2+ channels with high concentrations of extracellular Mg2+ reduces the spike amplitude, rate of membrane potential repolarization and the amplitude of the AHP (Thomson and West, 1990). Third, there is a circadian rhythm of intracellular Ca2+ levels with higher levels during the subjective day than during the subjective night (Ikeda et al., 2003; Colwell, 2000). The Ca2+ rhythm is not blocked by TTX although action potentials are completely eliminated. Ryanodine, which depletes intracellular Ca2+ stores, significantly blocked both the intracellular Ca2+ rhythm and disrupted the action potential firing (Ikeda et al., 2003). GABA is the most prominent neurotransmitter in the SCN (Moore and Speh, 1993; Morin and Blanchard, 2001) and there are abundant inhibitory postsynaptic potentials that can be recorded in SCN neurons (Jiang et al., 1997; Kim and Dudek, 1992). Activation of GABAA receptors is traditionally thought to produce cellular inhibition due to activation of a chloride conductance and membrane hyperpolarization or the shunting of excitatory currents. However, in the SCN, descriptions of the actions of GABA on the SCN neuronal activity have been complicated and controversial. The majority of recordings had shown GABA to be an inhibitory neurotransmitter during the day (Bos and Mirmiran, 1993; Gribkoff et al., 1999; Liou and Albers, 1991; Liou et al., 1990). However, other investigators described the activation of GABAA receptors to be primarily excitatory, producing an increase in firing activity or membrane depolarization during the day reducing action potential firing and producing a membrane hyperpolarization during the night (Wagner et al., 1997, 2001). This diurnal rhythmicity was proposed to be caused by a daily change in the intracellular chloride concentration (Wagner et al., 1997, 2001). A high daytime intracellular chloride concentration was proposed to account for the observations. An excitatory GABA response would be observed when the equilibrium potential of Cl− (ECl) is less negative than the resting membrane potential. Contrary to those results, a different group of investigators reported that GABAA receptor activation produces only inhibitory effects during the day and excitatory effects in a subpopulation of SCN neurons during the night (De Jeu and Pennartz, 2002). The reversal potential during the night was more depolarized than during the day consistent with an increased intracellular chloride concentration. The specific reasons for these dramatically different observations remain unknown. Technical issues such as style of whole cell BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 recording, duration of GABA application and the GABA concentration may all contribute to the different observations. 6.4. Inter-SCN differences in gene expression One of the more intriguing developments during the last 10 years has been the observation that the two SCN can oscillate out of phase (De la Iglesia et al., 2000). In male hamsters with split rhythms caused by LL exposure, the putative circadian clock gene, Per1, is maximally expressed in one SCN while another clock gene, BMAL1, is simultaneously expressed maximally in the other SCN (normally, Per1 and BMAL1 are expressed about 180° out of phase and maximal expression cannot be seen simultaneously; Honma et al., 1998b; Shearman et al., 2000). High Per1 expression normally occurs during the middle subjective day, a characteristic that is also true in SCN from hamsters with split rhythms. The genes coding for VP and FOS proteins are also expressed rhythmically in normal animals at the same phase as Per1. In the animals with split rhythms, activity of these genes remains temporally synchronized with that for Per1. Despite the continuous presence of light, photically induced Per or FOS expression does not appear to occur during the subjective night (De la Iglesia et al., 2000). This observation is curious because it implies that a photic action normally occurring in DD-housed animals in is being blocked, at least in the case of animals with split rhythms. Apparently, LL-housed animals with unsplit rhythms have not been tested to determine if there is light-induced gene expression during the subjective night. It would also seem possible, although perhaps unlikely, that the rhythmic gene expression observed in SCN of animals with split rhythms is induced by a temporally gated action of light rather than being an expression of intrinsic cellular rhythmicity. The observation that the two SCN may oscillate in antiphase has raised the possibility that each SCN may have specific control over the timing of individual physiological events. This has been examined by evaluating the control of the gonadotropin, luteinizing hormone (LH), in animals with split running rhythms. Ordinarily, estrogentreated, ovariectomized female hamsters have a single daily LH peak timed by a circadian clock (Alleva et al., 1971; Bittman and Goldman, 1979), but in animals with split rhythms, there are two such LH peaks in antiphase (Swann and Turek, 1985). The circadian clock action occurs through regulation of pituitary LH release by septal-preoptic neurons synthesizing gonadotropin releasing hormone (GNRH) (De la Iglesia et al., 1995). Activation of GNRH neurons, as indicated by FOS expression, is maximal at the time of LH release (Urbanski et al., 1991; Doan and Urbanski, 1994). When estrogen-treated, ovariectomized hamsters have split rhythms in LL, there is daily FOS expression in GNRH cells predominantly (about 5:1) localized to the side of the forebrain in which SCN FOS expression is also dominant (about 5.5:1) (De la Iglesia et al., 2003). The implications of this form of lateralized circadian organization for regulatory physiology are far-reaching and there are preliminary data supporting the view that each SCN controls the ipsilateral expression of rhythmicity in peripheral organs (Yamazaki et al., 2000). 6.5. Regional expression of Fos 6.5.1. Hamster 25 An early observation (Rea, 1992) was that light did not homogeneously induce FOS expression throughout the hamster SCN. Rather, the protein was heavily induced in the ventral half of the nucleus both at CT13 and CT18, but there was also large induction of FOS in the dorsal half at CT18. This study lacked no-light controls, but the result has been corroborated (Chambille et al., 1993; Guido et al., 1999). FOS induction by light is similar in the dorsal and middle thirds of the hamster SCN at both ZT15 and ZT22 (no response and large response, respectively) and in the ventral third (very large responses at both times) (Guido et al., 1999). Also, the FOS response to light is greater in the ventral third of the SCN than in the other two thirds at both ZT15 and ZT22. Much of the ventral third of the SCN, in which dense, light-induced FOS occurs, corresponds to the central subnucleus that can be identified by the presence of CALB neurons and dense retinal innervation. One point of the Guido et al. (1999) results is that FOS induction by light occurs throughout the SCN, not just in the central subnucleus. The effects of GABA agonists on facilitation or inhibition of light-induced FOS are proportionally equivalent in rostral, middle and caudal thirds of the SCN (Gillespie et al., 1999). When FOS counts were made through an entire SCN section at the midcaudal level, there was no response at all to a 1 min light exposure at ZT15, whereas the same stimulus at ZT18, 21 or 24 yielded a near maximal result. A longer exposure to light at ZT15 greatly increased the FOS response, but never to the level seen at the other times. A complementary analysis (Chambille et al., 1993) has additionally demonstrated that while light at CT14 induces a small increase in FOS in the rostral 3/4 of the SCN, there is substantial FOS induction in this area when light is administered at CT19. The above-cited papers demonstrate a region by time interaction with respect to gene induction by light stimulation, with region being defined by rostrocaudal or dorsoventral level. Further, the Guido et al. (1999) work indicates that the sensitivity of SCN neurons to photic input varies according to time during the subjective night. Implicit, but not studied, is the likelihood of region by time interaction with respect to sensitivity to light. The observations of region by time interaction with respect to light-induced FOS expression have not had an impact on more recent developments focused on photic regulation of putative clock genes. 6.5.2. Rat Fos gene expression spontaneously oscillates in the SCN with most of the amplitude accounted for by the expression in the dorsomedial region (Schwartz et al., 1994; Koibuchi et al., 1992). However, a low amplitude oscillation is present in the ventrolateral region (Sumova et al., 1998). Regional FOS oscillation persists in vitro (Prosser et al., 1994; Geusz et al., 1997). Multiple Fos-family genes are light-induced in the ventrolateral region of the rat SCN. These include c-fos, fra-2 and fosB. It has been suggested that each gene is a candidate mediator of light effects on SCN neurons and regulation of circadian rhythm phase that may enable reasonably normal rhythm function in the absence of normal gene activity 26 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 (Schwartz et al., 2000; Honrado et al., 1996). A double label study has shown that only a small percentage of VP cells contain spontaneously expressed FOS and that a similarly small percentage of cells expressing FOS also contain VP (Sumova et al., 2000). The daily profile of spontaneously expressed FOS is altered according to the length of the entraining photoperiod, a phenomenon with implications for photoperiodism (see Sumova et al., 2004 for a review). Analysis of light-induced FOS expression in various SCN regions has been rendered more complex by the observation that light can inhibit it (Beaulé et al., 2001b). In the rat, light induces large increases in FOS expression in neurons throughout the ventral SCN. As indicated above, the dorsomedial region does not show light-induced gene expression, but does have constitutive FOS that varies according to circadian time. A particularly novel result in this study is the demonstration of an actual drop in the number of cells expressing FOS in an apparently specialized sector of the dorsomedial SCN (Fig. 3C1, 2) following light administered at CT2 or CT14, or in entrained animals, at ZT1 or ZT2 (Beaulé et al., 2001b). 6.6. Regional clock gene expression 6.6.1. Mouse The advent of digoxigenin procedures for in situ hybridization has permitted analysis of light-induced clock genes at the cellular level. As indicated by these procedures, light applied at CT16 induced Per1 mRNA in the ventral, but not the dorsomedial, mouse SCN (Shigeyoshi et al., 1997). In contrast, the dorsomedial region exhibited a circadian oscillation of Per1 expression with a peak near CT4 and similar rhythmicity in Per1 expression was present throughout the mouse SCN. A two component model of SCN operation was proposed in which the SCN is organized into dorsomedial “type A” cells and ventral “type B” cells (Shigeyoshi et al., 1997). The latter are strongly influenced by light, while the former are light-independent. Similar results have been obtained in the rat (Yan et al., 1999). In the Per1-GFP mouse, the extent of regionality appears to depend upon the lighting history. In LD housed animals, approximately equal numbers of cells rhythmic for Per1 were found in the medial and lateral halves of the SCN (Quintero et al., 2003). In contrast, in DD housed animals, about 66% of the medial neurons were rhythmic compared to 34% in the lateral SCN. There were also differences in the dorsoventral distributions of rhythmic cells, with twice as many found in the ventral SCN of LD housed animals compared to similar percentages in the two halves of DD housed mice. As described above, the Per1-GFP mice have rhythmic neurons with peak activity at one of three preferred phases. In DD housed animals, the vast majority of cells peaking at CT18 are present in the medial SCN, whereas the medial–lateral difference becomes progressively less at CT15 and CT12, respectively. A similar relationship between peak phase and laterality is evident in LD housed mice, but is not as pronounced (Quintero et al., 2003). In organotypic slice cultures of SCN from mice carrying the Per1promoter-driven luciferase reporter gene, most cells identified by the reporter show oscillatory gene expression and these cells are widely distributed throughout the SCN (Yamaguchi et al., 2003). In the organotypic slice preparation, luminescence rhythm peaks of Per1 expression in cells of the dorsal mouse SCN have a broad distribution of phases, but most are maximal in advance of those in middle and ventral thirds of the nucleus (Quintero et al., 2003). The cells rhythmically expressing Per1 in the dorsal third are unable to collectively sustain a cohesive rhythm, unlike those more ventrally located. It is noteworthy, however, that each dorsal cell continues a seemingly normal oscillation uncoupled from the others in the same dorsal region. This suggests that the more ventral oscillatory cells are imparting a signal to those in the dorsal SCN, thereby controlling phase of rhythmic cells in that region. In addition, the investigators note that results from the horizontal slice preparation indicate the dorsal neurons are not necessary to maintain either rhythmicity of the ventral cells or their oscillatory synchrony (Yamaguchi et al., 2003). The presence of preferred phases by regionally specific collections of oscillatory neurons (Quintero et al., 2003; Yamaguchi et al., 2003) is consistent with the view described for hamsters (Hamada et al., 2004a) (discussed below), although the interpretation may be substantially different. On the one hand, discussion of mouse SCN tends to emphasize the presence of many oscillatory neurons collectively synchronized, but with different phases of peak Per1 gene expression. On the other, the hamster work describes the “slow spread of gene expression” from one area to another. The appearance of “spread” may be the consequence of what has been described in mice (Yamaguchi et al., 2003), namely, the presence of oscillatory neurons with grouped or a gradient of phases. The mouse data have an implication for the direction of causation. The dorsal oscillatory neurons cannot maintain synchrony when separated from those more ventrally located. At least in the mouse, this strongly suggests that the direction of causality with respect to phase angle and synchrony among oscillators is from the more ventral rhythmic cells to the dorsal rhythmic cells. Other studies of the mouse SCN note a central region (Fig. 3F3) with greatly reduced rhythmic Per1 and Per2 expression (Karatsoreos et al., 2004; LeSauter et al., 2003; King et al., 2003; Reddy et al., 2002). This area corresponds closely to the distribution of GRP neurons (note: the GRP cells were identified in a CALB-GFP reporter mouse in which, for some unknown reason, about 90% of the GFP cells in the SCN also contained GRP, and for an equally unknown reason, no cells in the SCN known to contain CALB were identified by GFP (Karatsoreos et al., 2004). However, the most central part of the region containing putative GRP cells has distinct expression of both Per1 and Per2 at ZT2 or CT2, times when rhythmic expression is minimal elsewhere in the SCN (King et al., 2003). Previously, a similar sized, fairly discrete collection of neurons in the centrodorsal (not dorsomedial) mouse SCN was described as expressing Per1 at ZT0 or CT0 (Hastings et al., 1999). With respect to light-induced clock gene expression in the mouse, a light pulse at CT13-14 has been reported to cause Per1 expression throughout the central and dorsolateral SCN in a region complementary to the medial area in which rhythmic Per1 is expressed (Karatsoreos et al., 2004). There is a high degree of correspondence between light-induced Per1 and the GRP cell distribution. The same appears true for FOS expression. The region of light-induced Per1 and GRP cells is BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 completely filled with retinal projections which tend to be much sparser in the medial part of the SCN in which rhythmic Per1 or Per2 expression predominate. However, another report indicates that light-induced Per1 is found in the ventral third of the SCN corresponding largely to the area containing PHI neurons (King et al., 2003). This is consistent with observations that VIP neurons containing Per1 jump from 31% to 59% following a light pulse at ZT21 (Kuhlman et al., 2003). In contrast, Per1 is expressed in about 35% of VP neurons at this time, but is not elevated in response to light. VIP and VP cells expressing Per1 comprise about 51% of the total neurons spontaneously expressing that gene at ZT10, indicating that there is no specific correspondence between cell phenotype and rhythmic clock gene activity. The information also indicates that rhythmic gene expression is not solely the province of dorsomedial neurons, as the VIP cells are consistently present in the ventral part of the SCN. Light-induced Per2 protein in the SCN is described (King et al., 2003) as being expressed with breadth and magnitude equivalent to that which occurs at its maximal rhythmic expression. One specific characteristic of the rhythmic expression is its relative absence from the GRP cell region (Karatsoreos et al., 2004; LeSauter et al., 2003; King et al., 2003) (Figs. 3G1, 2; H). Cells identifiable as containing the VPAC2 receptor had both rhythmic and light-induced expression of Per2 (King et al., 2003). There appear to be some differences in the results of two comparable mouse studies (Karatsoreos et al., 2004; King et al., 2003), but it is difficult to determine whether or not they are real or simply the result of substantially different methods. 6.6.2. Rat A comprehensive analysis of Per1 and Per2 gene expression in the entire rat SCN has produced a modification of the dorsomedial/ventrolateral SCN divisional model to include three functionally distinct regions (Yan and Okamura, 2002). The single dorsomedial region has been divided into periventricular and central parts (Fig. 3D). The former overlaps a fairly thin, dorsomedial portion of the SCN identifiable by VP neurons. The central portion overlaps the ventrolateral part of the VP cell group as well as the dorsal part of the VIP cell distribution. The third, ventrolateral, portion of the SCN generally corresponds to the bottom 80% of the region containing VIP cells. The three regions are reported to be distinct with respect to their expression of Per1. For example, the peak expression in the periventricular division is at CT4, but at CT8 in the central division. Overall, the profiles of Per1 expression are different in the three regions. A second distinguishing feature is the Per1 response to light which, for the periventricular and central divisions, is nonexistent at CT16, while extremely robust for the ventrolateral division. In contrast to Per1, rhythmic expression of Per2 is similar in the periventricular and the central divisions, thereby indicating a different SCN divisional structure. An analysis of Per2 protein has shown rhythmic expression in dorsomedial and ventrolateral rat SCN, but no induction in either location after a light pulse at CT13 (Beaule et al., 2003a). It has been conceptually convenient to consider the rat SCN as having two parts, the dorsomedial and ventrolateral “divisions.” Moreover, it has also been convenient to view 27 the ventrolateral division as the sole SCN region with retinal innervation and VIP neurons, while the dorsomedial division has no retinal innervation and contains VP neurons. However, convenience must give way to a more complex reality as suggested by the presence of two functionally distinct parts of the dorsomedial rat SCN (Yan and Okamura, 2002). Similarly, when the ventrolateral region containing VIP neurons has been examined closely, it also can be subdivided into differently functioning units. VIP cells identify an elliptical area occupying the ventral half of the rat SCN. However, cells in the medial quarter of this region do not express lightinduced Per1 mRNA nor, as demonstrated in adjacent sections, does this area receive much, if any, retinal innervation (Kawamoto et al., 2003). Thus, the nominal ventrolateral rat SCN containing VIP cells is divisible into a lateral part in which Per1 is induced by light and a medial part in which the cells do not have this response (Fig. 3E). The foregoing results are of significance because they demonstrate, at least for the rat, that (a) cells throughout the entire SCN oscillate, although the magnitude is greater in some regions than in others; (b) there are regional distinctions between the rhythmic expression of Per1 and Per2; (c) there are at least four functionally distinct regions within the SCN; (d) the SCN appears to have a compartment in which cells rapidly express Per genes in response to light, but this does not occur in the other three compartments and (e) there is a large, but clearly imperfect, anatomical correspondence between known cell phenotypes and the three regions. In addition, it is noteworthy that the ventrolateral division, unlike the others, does not extend the entire length of the SCN (Yan and Okamura, 2002). One obvious burden imposed on investigators by the increased number of SCN subdivisions is the need to minimize confusion by using a common nomenclature characterized by fully understandable definitions. It is difficult to compare studies when, in one study, a region may be referred to as the “VP” area, a second study refers to the same area as the “dorsomedial region,” while a third study emphasizes a difference between the “medial dorsomedial” and the “lateral dorsomedial.” The difficulty is compounded by the fact that, because the information is so new, the laboratories making most of the anatomical observations have yet to achieve a standard nomenclature across publications. 6.6.3. Hamster A functional analysis of SCN divisions has also been made in the hamster, although without the same level of detail. A twocompartment description has been demonstrated, with one unresponsive to light and the other highly responsive to light (Hamada et al., 2001). The shape of the hamster SCN lends itself to a descriptive terminology more closely related to peptide content of cells than to the geographic descriptors of dorsomedial vs. ventrolateral. Thus, there is more emphasis on the central subnucleus and the fact that cells in this region behave differently than the more dorsomedial cells. As in the rat, there is a pronounced endogenous rhythm in Per1 and Per2 gene activity among neurons closely associated with the distribution of VP cells in the hamster SCN. As a generality, the distribution of these cells wraps around the medial half of the collection of CALB neurons in the central subnucleus. Cells in 28 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 the CALB-containing region express the Per1 and Per2 genes robustly in response to light. It is also the case that neurons in the CALB cell region do not have endogenous oscillations in Per1, Per2 or Fos gene expression unlike their more medial counterparts (Hamada et al., 2001). It is not known to what extent the Per1 or Per2 gene expression occurs rhythmically in VP neurons or is light-induced in CALB neurons. Further analysis of the hamster SCN has demonstrated that while the most robust Per gene response to light occurs in cells in and near the CALB cell region, a similar phenomenon occurs among cells in the dorsomedial SCN, the region of VP neurons (Hamada et al., 2004a). The time course of lightinduced Per1 gene expression in cells of this region is significantly longer than in the CALB region, suggesting an altogether different pathway for induction. Nevertheless, the result is consistent with the smaller number of retinal projections to the dorsomedial region than to the central CALB cell region in the hamster (Muscat et al., 2003). It is also the case that the VP gene itself not only has an intrinsic oscillatory expression in SCN neurons, it is also light-inducible in a slow onset manner similar to that for the Per1 gene in cells of the VP region (Hamada et al., 2004a). Rhythmic expression of VP mRNA in the hamster SCN appears to have at least two characteristics: there is always expression in the dorsomedial region (minimal at CT20), and is greatly increased in that region at CT8, while also appearing throughout a much larger part of the entire SCN (Hamada et al., 2004a). Per gene expression has similar rhythmic expression characteristics, but Per1 and Per2 mRNAs are virtually nonexistent at CT20. The investigators suggest that rhythmic gene expression is initiated among a small number of dorsomedial cells and spreads slowly through more caudal and ventrolateral parts of the SCN. There is clear intra-SCN organization to the temporal and spatial rhythmic expression of the Per and VP genes. However, in an oscillatory system where there is no beginning and no end, it is not clear that an origin of the gene expression rhythm can be defined. More important is the issue of whether cells in one part of the SCN transmit neural or humoral rhythmic information to other cells that is requisite for the expression or phasing of rhythmicity in those cells. Related to this issue is the question of cellular “gradients” in gene expression within the SCN (Hamada et al., 2004a; Yan and Okamura, 2002). A “gradient” implies gradual spatial change, a view contrary to observations of rhythmic gene expression occurring within specific sectors of the SCN (see Fig. 3 in Yan and Okamura, 2002). The observations of temporal and spatial rhythmic organization within the SCN are likely to be of great importance with respect to understanding the movement of information within the SCN itself as well as to the understanding of function attributable to the various sectors of the SCN. To our knowledge, there has not been an investigation of light-induced clock gene expression comparable to the comprehensive study of FOS by Guido et al. (1999). However, possibly unlike the situation in the rat (Yan and Okamura, 2002), a CT19 light pulse first induces Per1 expression in an area of the hamster SCN encompassing the central subnucleus, but this is followed, with time, by induction of gene expression in cells throughout most of the dorsomedial region as indicated by the location of VP neurons (Hamada et al., 2004a). Whether there is a “periventricular” part of the hamster SCN, as there is for the rat (Yan and Okamura, 2002), remains to be determined. 6.7. Stimulus induction of gene expression 6.7.1. Photic stimuli Rea (1989) showed photic induction of FOS protein in SCN neurons in 1989. During the past decade, there have been many other investigations directed at the expression of a variety of genes thought to be components of the circadian clock. Substantial information exists which shows rapid induction of Per1 and Per2, but not Cry1, Cry2 or Per3, gene expression following a light pulse (Shigeyoshi et al., 1997; Best et al., 1999; Field et al., 2000; Zylka et al., 1998; Shearman et al., 1997). Brief (15 min) light pulses at ZT14 yielded Per1 and Per2 gene expression increases that were 26 and 30%, respectively, greater than controls when measurements were made 1 h later. However, the changes in mRNA levels occurred without a noticeable increase in protein which is not evident until many hours later (Field et al., 2000). These results emphasize the investigators' point that there is not a reliable relationship between the phase shift magnitude and the level of light-induced Per gene expression. In a novel experiment, Paul et al. (2003) tested whether glutamate receptors mediate light-induced suppression of nocturnal pineal melatonin as well as light-induced expression of Per1 mRNA. As expected, both nocturnal environmental light and the glutamate receptor agonist, NMDA, applied to the SCN suppressed pineal melatonin and elevated Per1 mRNA. Joint application of AMPA or NMDA receptor antagonists with the NMDA blocked the effect of light on Per1 mRNA expression, but in the same animals, the antagonists were unable to prevent the light-induced reduction in melatonin. This study demonstrates the existence of two different pathways in the SCN. Both are light-responsive, with one presumably related to the regulation of circadian rhythm phase response and the other contributing to photic inhibition of nocturnal pineal melatonin. The study also raises an issue that should be of concern to all rhythm investigators. That is, whether the changes observed in the SCN are directly related to the phenomenon under investigation or simply correlates. This issue has been extended to evaluation of photic induction of the clock genes, Per1 and Per2 (Paul et al., 2004). The results indicate that both light and NMDA induced the expected gene expression in the SCN and also suppressed pineal melatonin. Blockade of sodium-dependent action potentials with TTX blocked both light-induced gene expression and melatonin suppression consequent to either light or NMDA. In contrast, induction of Per1 and Per2 by NMDA was not blocked by TTX (Paul et al., 2004). The results of the above studies are consistent with the view that there are two distinct mechanisms regulating light-induced gene expression and lightsuppression of pineal melatonin. 6.7.2. Nonphotic stimuli Whereas light during the subjective night induces Per gene activity in the SCN and causes rhythm phase shifts, it has little, if any, effect on these two measures during the subjective day. In contrast, phasically active nonphotic stimuli administered during the subjective day elicit rhythm BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 shifts when applied during the subjective day which is associated with decreased Per gene activity in the SCN. Several classes of nonphotic stimuli have this effect, including benzodiazepine treatment (Yokota et al., 2000), novel wheel activity (Maywood and Mrosovsky, 2001), injection-related arousal (Maywood et al., 1999), peri-SCN infusion of NPY (Maywood et al., 2002; Fukuhara et al., 2001) and serotonin receptor agonists (Horikawa et al., 2000). Antisense oligonucleotide to the Per1 gene administered directly into the peri-SCN region during the subjective day suppresses Per1 mRNA expression by about 60% (Hamada et al., 2004b). This treatment at CT6 induces a phase advance that averages about 60 min, a result consistent with the view that enhanced Per1 expression, in this case caused by a nonphotic stimulus, induces phase shifts. A proposed unifying hypothesis suggests that elevated Per gene expression and Per protein synthesis is causal to phase shifts induced either by light or by nonphotic stimuli (Maywood and Mrosovsky, 2001). Moreover, the hypothesis makes an unconventional specific prediction that light should be able to modify circadian clock function even during the “dead zone” of the PRC. The rationale is that, during the normal dead zone, light cannot alter phase because Per expression is already high and further expression cannot be elicited by light during the subjective daytime. However, light can be shown to alter SCN activity during the subjective day because light exposure at that time prevents the drop in Per gene activity normally induced by nonphotic stimuli (Maywood and Mrosovsky, 2001) or direct NPY infusion into the peri-SCN region (Maywood et al., 2002). The converse of this procedure is also demonstrable with nonphotic stimuli presented during the subjective night able to attenuate light-induced phase shifts (Mistlberger and Antle, 1998; Ralph and Mrosovsky, 1992). According to the Maywood and Mrosovsky (2001) unifying hypothesis, nonphotic stimulus should achieve its attenuating effects on light during the subjective night by reducing the extent of light-induced Per gene activity. However, direct tests of this proposition have yielded mixed results. In the first instance, benzodiazepine treatment produced an approximately 50% reduction in light-induced (5 lx, 15 min, CT20) phase advances, but had no effect on phase delays (Yokota et al., 2000). The effect on phase advances was associated with an approximately 40–50% drop in Per1 or Per2 mRNA (the corresponding test for phase delays was not performed). In contrast, access to a novel running wheel reduced lightinduced phase delays without altering light-induced phase advances, but the novel wheel activity had no effect on lightinduced Per1 mRNA (Christian and Harrington, 2002). In a similar investigation, access to a novel wheel for 1 h after a light pulse resulted in virtual abolition of phase advances in response to less intense illumination and substantial attenuation of response to more intense illumination. However, as in the above study, there was no reduction in either Per1 mRNA or FOS protein associated with the reduced phase response to light (Edelstein et al., 2003a). As noted by the authors, Per1 appears to be insufficient for phase shifts in locomotor rhythms and that, “The relationship between light and nonphotic stimuli is more complex than has been previously suggested (Maywood and Mrosovsky, 2001)” (see Edelstein et 29 al., 2003a for a discussion that considers a variety of issues related to the above inconsistencies). 6.7.3. Inconsistencies between phase response and gene expression Light-induced Fos gene activity is closely correlated with the ability of light to entrain circadian rhythms, but there is a general consensus that FOS is not an essential part of either the clock or the input pathway mediating entrainment (Colwell et al., 1993a,b,c). Moreover, FOS protein induction in response to light is usually, but not always, predictive of phase response. Quipazine injection at CT8 is significantly reduced FOS expression in hamster SCN, but there was no effect on phase shifts at this time and in mice, this drug had no effect on phase response, but increased FOS expression during the subjective day (Antle et al., 2003b). In mice entrained to a skeleton photoperiod of 1 h light pulses presented to create a 1:14:1:8 LDLD photoperiod, some animals ran during the long, and others during the short, interval of dark. The light manipulations revealed that only the light pulse occurring near activity onset (ZT12) appeared to influence locomotor rhythm phase. Irrespective of the effect on entrainment, among animals running during the long dark interval, light pulses at the beginning or the end of that interval equally induced Fos mRNA expression. Moreover, if animals ran during the short dark interval, Fos mRNA expression was induced by both pulses, but was much greater after the pulse at ZT21 (Schwartz et al., 1996). Thus, in this paradigm, there is a clear disparity between the entraining capability of light and the magnitude of Fos gene expression in response to light. Molecular absence of Fos does not prevent entrainment of mice (Honrado et al., 1996). In vivo blockade of Fos availability is associated with an inability of light to induce phase shifts in rats (Wollnik et al., 1995). However, JunB gene activity was simultaneously blocked in this study and the negative effects on light-regulated phase control may have been the consequence of the suppression of the activity of both genes, rather than only Fos (see Schwartz et al., 2000 for a discussion). Edelstein et al. (2003a) suggest that their data from nonphotic stimulus conditions are consistent with the view that several different mechanisms contribute to the regulation of circadian phase depending on the nature of the stimulus involved. Most importantly, they conclude that phase shifts can occur without necessarily modulating Per gene expression. This is contrary to the general view that phase shifts are dependent upon Per gene activity, although not at all inconsistent with the available literature. It is also the case that Per1 and Per2 expression is not the same in the SCN of animals showing rhythm splitting in LL as it is in animals showing a form of induced splitting in DD referred to as “behavioral decoupling” (De la Iglesia et al., 2000; Edelstein et al., 2003b). In the latter case, “splitting” consisting of a daily bout of induced locomotion is followed by a resting interval during which Per gene expression is increased throughout most of the SCN. However, when the elevated gene expression occurred during the rest phase following bouts of spontaneous locomotion, Per gene expression was also elevated, but only in the dorsomedial part of the nucleus (Edelstein et al., 2003b). Also, despite having several characteristics similar to 30 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 spontaneous splitting in LL conditions, the DD-housed behaviorally decoupled animals had no indications of bilateral asymmetry in Per gene expression in the two SCN, unlike the situation in animals showing LL-induced splitting (cf., De la Iglesia et al., 2000). There are several forms of dissociation between the regulation of gene expression and the phasic effects of light on circadian rhythms. Perhaps the simplest is the temporary loss of synchrony between gene expression rhythms during clock resynchronization to a shifted photoperiod. The re-entrainment rate of certain gene rhythms in mouse is faster than that of others and depends upon the direction of the phase shift. (Reddy et al., 2002). The rhythm of mPer1/2 expression in the mouse phase advances rapidly in response to light, while re-entrainment lags for mCry1. In contrast, the rhythms of mCry1 and mPer1 or mPer2 reentrain at the same rate during a phase delay. These gene expression results match corresponding behavioral data showing slower advances and faster delays (Reddy et al., 2002). A variation on the theme of temporary desynchronization among gene expression rhythms is the demonstration that they (Per1, Per2, Cry1) shift rapidly in the ventrolateral rat SCN, but more slowly in the dorsomedial region (Nagano et al., 2003). A second form of dissociation has been demonstrated in mice lacking the PAC1 receptor (Hannibal et al., 2001b). A light pulse that induces a 100 min phase delay in wild-type mice elicits a larger phase delay in the mutant mice. However, the mutant mice have a vastly attenuated Fos or Per gene induction response to light. A substantially different experiment has yielded the observation that imposition of a 6 h photoperiod advance rapidly induces a large phase advance in the Per1-luc bioluminescence rhythm relative to unshifted controls. This phase differential persists for at least 6 days in DD. In contrast, such advances did not occur in in vivo SCN electrophysiological or behavioral rhythms (Vansteensel et al., 2003a). A third form of dissociation addresses the question of whether particular genes, or change in their expression, are always necessary for rhythm expression. One example is the gene, Clock. Mice homozygous for a mutant form of Clock rapidly which when absent yields mice that rapidly become arrhythmic in DD (Vitaterna et al., 1994). More recently, however, a similar test procedure has been applied to Clock mutant mice, but with an LL background to the rhythm expression. In this circumstance, many of the mice exhibit clear circadian rhythms despite the absence of the Clock gene (Spoelstra et al., 2002). Other investigators have suggested that running may be an inadequate variable to assess in order to determine whether or not the Clock mouse in DD is rhythmic, and present data supporting the view that such mice are indeed fundamentally rhythmic and entrainable, but with a much longer period than normal (Kennaway et al., 2003). In quite a different situation with the mixed in vivo/in vitro preparation, novel wheel access for 3 hduring the subjective day rather quickly reduced Per1 expression in the SCN as might be expected during the midsubjective daytime. However, under the conditions of the experiment, there was no associated shift in the phase of the SCN firing rate rhythm in animals so treated (Yannielli et al., 2002). On the other hand, if an additional 2 h of wheel access was permitted (with or without additional running), a the firing rate rhythm advanced about 2.4 h. Brains taken 5 h, rather than 3 h, after the initial access to wheels had reduced Per2 expression, but Per1 had returned to normal. One conclusion is that clock resetting in response to activity-related stimulation may require a 3–5 h poststimulus latency (Yannielli et al., 2002; Antle and Mistlberger, 2000), whereas other nonphotic stimuli or light may not require the same amount of time (Mead et al., 1992; Sumova and Illnerova, 1996). 6.8. Polysialic acid and SCN function There are a variety of possible neurobiological roles that may be played by the molecule, polysialic acid (PSA) and its carrier, the neural cell adhesion molecule (NCAM), which form PSA-NCAM (particularly during development and in the context of neural plasticity; see Rutishauser and Landmesser, 1996). PSA-NCAM is distributed along plasma membranes of appositions between neurons, at synapses and between glia (Shen et al., 1997, 1999). It is of interest that the SCN of Siberian (Glass et al., 1994) and golden hamsters (Fedorkova et al., 2002), mice (Shen et al., 1997) and rats (Prosser et al., 2003) contains concentrations of PSA that fluctuates rhythmically. In LD, PSA concentration drops precipitously and by more than 90% during the dark hours. Although the shape of the curve describing PSA content in the SCN is substantially different during DD, clear rhythmicity persists (Glass et al., 2003a). Moreover, light greatly boosts transient PSA expression in the SCN (but not in hippocampus). NCAM rhythmicity is similar in the SCN, although its rhythm in DD is much less robust than that for PSA (Glass et al., 2003a). Mice lacking the NCAM-180 isoform that carries PSA have a shorter than normal circadian period, a longer activity phase and become arrhythmic within 3 weeks of DD (Shen et al., 1997). The mutant mice also show light-induced phase delays smaller than control mice (Glass et al., 2000a). The role of PSA itself has been investigated using an enzyme, endoneuraminidase (endoN), to remove PSA. Animals treated with endoN have shorter circadian periods in DD, although they do not appear to become arrhythmic (Shen et al., 1997). Similar to the mutant animals, the endoNtreated mice have diminished phase delays in response to light; they also have reduced light-induced FOS expression (Glass et al., 2000a). In contrast to the diminished effects of light in mice sustaining endoN removal of PSA, hamsters so treated had augmented phase response to nonphotic stimuli (61% increase to 3 h arousal; 120% increase to 8-OH-DPAT injection) applied at ZT6. In the PSA-depleted rat SCN slice preparation, direct application of 8-OH-DPAT elicited a 41% greater phase advance (Fedorkova et al., 2002). Also in the slice preparation (Prosser et al., 2003), PSA levels remain rhythmic. Glutamate treatment of the slice induced a 100% increase of PSA in the SCN, but this was blocked by endoN pretreatment. Treatment with endoN also blocked the phase shifting effects of glutamate or electrical stimulation of the slice. The strong implication of this BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 research is that retinal input to the SCN requires PSA to be functional. A further analysis of the molecular requirements form normal mouse rhythmicity was performed using three strains deficient, to varying degrees, in PSA and NCAM. Animals lacking both PSA and all forms of NCAM become arrhythmic in DD and tended to run so much when lights are on that entrained rhythms are often difficult to discern (Shen et al., 2001). A similar strain, but not lacking NCAM isoform 120 has normal entrainment under LD, but is arrhythmic in DD, suggesting that NCAM-180 and/or NCAM-140 are required to avoid arrhythmicity in PSA deficient mice. The conclusion from these studies is that some combination of PSA and NCAM is necessary for normal rhythmicity and entrainment. The mechanisms through which this action is achieved are presently unknown. 7. SCN connectivity 7.1. Humoral factors One of the more surprising results during the last decade of research on SCN function has been the demonstration that rhythmic events can be controlled, in all likelihood, by nonneural linkage to the circadian clock. The availability of perinatal SCN transplant methods enabled this conclusion. The procedure developed with hamsters by Ralph et al. (1990) used embryonic SCN to restore locomotor rhythms in lesioned, arrhythmic hosts, but SCN efferent connections were restored to an uncertain degree (Canbeyli et al., 1991; Lehman et al., 1987). Subsequently, transplantation of viable, membrane encapsulated SCN restored locomotor rhythmicity without any apparent neural connections to adjacent hypothalamus (Silver et al., 1996b). In contrast, rat to hamster or mouse to hamster SCN transplants restore rhythmicity in association with substantial growth of donor SCN projections into host hypothalamus, preoptic area and septum (Sollars and Pickard, 1993; Sollars et al., 1995; Lehman et al., 1998). It appears to be the case that behavioral rhythms, but not endocrine rhythms, are restored by transplantation of embryonic SCN (MeyerBernstein et al., 1999; LeSauter and Silver, 1998). Despite declarations that the SCN contains the timing apparatus governing transplanted circadian periodicity, there are other possible explanations of the phenomenon and there may be species differences in the extent to which period is the exclusive property of the SCN (Sollars et al., 1995). It is also the case that the specific location of the transplant within the host brain is relatively unimportant as long as the site is in or near the third ventricle (such as adjacent lateral ventricle) (LeSauter et al., 1996; Lehman et al., 1987; Aguilar-Roblero et al., 1994). In addition, transplants consisting of dissociated SCN cells in the anterior hypothalamic parenchyma or medial hypothalamus also restore rhythmicity (Silver et al., 1990). Precision of activity onset reinstated is related to the transplant distance caudal or dorsal (but not rostral) to the SCN. Based on these data, the suggestion has been made that the target of a neurohumor conveying period information from the SCN is located near or slightly rostral to the SCN (LeSauter et al., 1997). 7.2. 31 Efferent anatomy Watts et al. (1987) identified six general regions to which the rat SCN projects. Subsequent studies using the anterograde tracer, Phaseolus vulgaris leucoagglutinin, yielded generally similar pathways in the hamster brain for which there is (1) an anterior projection to the ventral lateral septum, bed nucleus of the stria terminalis and anterior paraventricular thalamus; (2) a periventricular hypothalamic projection to the medial preoptic region, subparaventricular zone, the paraventricular, ventromedial and dorsomedial nuclei and the premammillary area and (3) a posterior projection to the posterior paraventricular thalamus, precommissural nucleus and olivary pretectal nucleus (Kalsbeek et al., 1993; Morin et al., 1994). In the hamster, it is likely that the cells projecting to the olivary pretectal nucleus are actually located in the peri-SCN, as is indicated by retrograde tracer injections into the pretectum (Morin and Blanchard, 1998). Such cells are also seen following retrograde tracer injections into the IGL, ventrolateral geniculate, posterior limitans nucleus, commissural pretectal nucleus and medial pretectal nucleus (Morin and Blanchard, 1998; Morin et al., 1992; Vidal and Morin, 2005). In particular, cells projecting to the IGL from the SCN region do not reside within the SCN proper, but rather around its dorsal perimeter with a substantial probability that their dendrites extend ventrally into the nucleus. Watts identified an IGL-efferent projection in the SCN of rats and the cells of origin can be seen within the SCN in this species (Watts and Swanson, 1987). The hamster also differs from the rat in that it has a distinct caudal projection that ascends to innervate posterior paraventricular thalamus (a separate projection innervates the anterior paraventricular thalamus in both rats and hamsters) (Morin et al., 1994). Given that retrograde tracer studies identify cells around, but not in, the SCN for some of the putative SCN targets (IGL, VLG, pretectum), it is distinctly possible that others (such as the posterior amygdala or posterior paraventricular thalamus) might also receive afferents from peri-SCN cells. Lesion methods have been used in conjunction with immunohistochemistry to identify SCN projections having specific peptide content (Kalsbeek et al., 1993). In the hamster, VP cells project to the medial preoptic, parvicellular paraventricular and dorsomedial hypothalamic nuclei and the anterior paraventricular thalamus. Cells containing VIP project to the anterior paraventricular thalamus, parvicellular paraventricular hypothalamus, subparaventricular area and the dorsomedial hypothalamus. SCN efferent projections containing GRP were identified in the subparaventricular area, dorsomedial hypothalamus and supraoptic nucleus. The topography of projection neurons in mouse SCN has been inferred (Abrahamson and Moore, 2001) from the presence of VP or VIP immunoreactive fibers in many of the areas identified as receiving SCN projections in other species. Analysis of SCN projection neurons by retrograde tracer methods provides at least a preliminary indication of a structure–function relationship. Successful retrograde procedures indicate both targets of SCN efferent projections and the location of neurons providing those efferents. Retrograde 32 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 tracing methods have provided much of the recent information regarding the putative “core” and “shell” organization of the SCN (Moore et al., 2002). In this regard, there are two references that need to be compared. The 1987 study of Watts and Swanson (1987) reached conclusions different from those of Leak and Moore (2001), although the two investigations were equally broad and used basically similar methods. As a result, it is not possible to determine which interpretation is correct. On the one hand, Watts and Swanson (1987) emphasized the result that, “...cells in dorsal as well as ventral parts of the SCh project to each of the terminal fields examined.” (p. 230, abstract). Three cases showing labeled cells following retrograde tracer injection into the paraventricular hypothalamus were illustrated. In Case E46, the labeled cells were predominant in the dorsomedial SCN with few in the ventral part of the nucleus. In Case E47, there were many more cells labeled and the great preponderance was found in the ventral half of the SCN. In Case PVH33, fewer labeled cells were present, and the greatest density of such cells was in the ventrolateral quadrant of the SCN. On the other hand, a predominance of retrogradely labeled neurons in the dorsomedial SCN compared to the ventral part following paraventricular nucleus tracer injection has been reported (Leak and Moore, 2001; Vrang et al., 1995a). The observation of injection-related variability by Watts and Swanson is consistent with an equivalent observation by Leak and Moore who noted opposite patterns of dorsomedial:ventrolateral SCN cell labeling that depended upon the precise location of tracer injection in the subparaventricular zone (medial injection = 5.6:1; lateral injection 1:5.6). In the hamster, tracer injection into the paraventricular hypothalamus yields a “pattern of retrogradely labeled cells in the SCN […] uniform across cases […] scattered throughout the subdivisions of the SCN, and no tendency for backfilled cells to be located in the dorsomedial SCN was observed” (Munch et al., 2002; p. 53), i.e., there is an absence of topographic organization in the species in which it might be most expected. The Watts and Swanson study remains the most substantial examination of the extent to which individual SCN cells containing a particular neuropeptide project to various targets. In general, tracer injection into a target nucleus retrogradely labels SCN neurons containing VP and VIP, although the great majority do not contain either peptide. In the mouse, SCN cells projecting to the subparaventricular area have been shown to express FOS protein in response to night time light exposure (De la Iglesia and Schwartz, 2002). In the hamster, SCN neurons projecting to the paraventricular hypothalamus have also been shown to express FOS in response to light. The percentage of the cells expressing FOS is much greater at ZT19 than at ZT14 (Munch et al., 2002). It was also determined that some of the same cells contained VP, VIP or GRP. Of these, cells with the combined traits of projecting to the paraventricular nucleus, expressing FOS in response to light and containing VIP were most common and the combination in which the cells contained VP was rare. Direct SCN projections to gonadotrophin releasing hormone cells of the basal forebrain (De la Iglesia et al., 1995; Van der Beek et al., 1997) and to the rat paraventricular hypothalamic nucleus and corticotropin releasing hormone cells, in particular, have been reported (Vrang et al., 1995a,b). SCN neurons have also been reported to project to, and make likely contact with, both hypocretin (orexin) and melanin concentrating hormone neurons in the rat lateral hypothalamus (Abrahamson et al., 2001). Transneuronal viral tracers have been utilized to determine polysynaptic routes from the SCN. Following virus injection into the rat locus coeruleus, cells are retrogradely labeled in the dorsomedial hypothalamic nucleus, a known target of SCN neurons. The transneuronal tracer also labels SCN cells, but this does not occur following lesions of the dorsomedial hypothalamus. Furthermore, the circadian rhythm in firing rate of locus coeruleus cells is abolished after by the same lesions, suggesting that the SCN control of rhythmic neuronal activity in the locus coeruleus is via a two synapse circuit with a first order synapse in the dorsomedial hypothalamic nucleus (Aston-Jones et al., 2001). Injection of virus into the hamster medial vestibular nucleus also labels a few neurons in the SCN. This is a polysynaptic pathway that probably includes the locus coeruleus which projects to the medial vestibular nucleus (Horowitz et al., 2005). Viral tracers injected into fat pads (brown or white adipose tissue) identified neurons in Siberian hamster and rat SCN (Bamshad et al., 1998, 1999). The actual number of retrogradely labeled SCN neurons is small, and an estimated 2% of these also contain VP (Shi and Bartness, 2001). Injections placed in the adrenal medulla (Ueyama et al., 1999), pancreas (Buijs et al., 2001) and other structures (see Ueyama et al., 1999; Bartness et al., 2001 for reviews) label cells in the SCN. The general theme of these studies is that the SCN regulates sympathetic outflow, a feature evident in the viral tract tracing of pineal innervation (Larsen, 1999; Larsen et al., 1998; Teclemariam-Mesbah et al., 1999). However, it appears that there is also SCN control of parasympathetic output (Ueyama et al., 1999; Kalsbeek et al., 2000). The combination of the two types of peripheral innervation is consistent with the presence of a circuitous polysynaptic route from the SCN through the midbrain to the eye identified by virus injection into that organ (Pickard et al., 2002; Smeraski et al., 2004). There is a large amount of convergence by SCN-efferent projections onto brain regions receiving IGL input (Fig. 4). In addition, many of the same areas are also innervated by retinal and olfactory system projections (Johnson et al., 1988a; Morin and Blanchard, 1999; Morin et al., 1994; Cooper et al., 1994; Ling et al., 1998). 7.3. Afferent anatomy The SCN-afferent projections in the hamster were initially described in a fairly comprehensive fashion by Pickard (1982) in the early 1980s. There have been two additional such investigations, and in another species, the rat (Moga and Moore, 1997). In this study, a combination of retro- and anterograde tracing studies demonstrated additional projections to the SCN arising from infralimbic cortex, lateral septum, substantia innominata, ventral subiculum, subfornical organ, ventromedial hypothalamus, arcuate nucleus, posterior hypothalamus, pretectal nuclei, median raphe and IGL, but not in several of the brainstem regions identified by BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 33 Fig. 4 – Convergence of SCN (solid lines) and IGL (dashed lines) projections onto diencephalic and basal forebrain regions (black areas) in the hamster. Gray areas receive innervation only from the IGL. Only the premammillary area (white) receives input solely from the SCN. Thickness of the lines indicates approximate density of the projection. Note that the hamster SCN does not project to the IGL, although peri-SCN cells do so. See text regarding further convergence of visual and olfactory input to many of these areas. Drawn after Morin et al. (1994) and data from Morin and Blanchard (1999). Bina et al. (1993), as containing cells projecting to the SCN. The observations of Moga and Moore (1997) in the rat are substantially similar, but broader than, those of the older, more limited hamster study (Pickard, 1982). As part of a larger analysis, Krout et al. (2002) evaluated monosynaptic inputs to the rat SCN identified by CTB labeling. These investigators observed about 40 regions sending direct projections to the SCN, largely in agreement with the results of Moga and Moore (1997). However, Krout et al. (2002) also observed a more widespread distribution of labeled cells in areas such as the locus coeruleus and divisions of the parabrachial complex, among others, but did not see such cells in the substantia innominata and only a few or none in the olivary pretectal nucleus. In all studies involving tracer injection into the SCN, the most likely cause of label disparity across injections is the extent of label spread outside the nucleus, uptake by processes in close proximity to the nucleus or uptake by cell processes extending significant distances to enter the nucleus (Morin and Blanchard, 1998; Morin et al., 1992, 1994; Gerfen and Sawchenko, 1984). The above-mentioned report (Krout et al., 2002) described about 40 brain regions with cells monosynaptically projecting to the SCN. Injection of an exclusively retrograde (Pickard et al., 2002), transneuronal virus label into the SCN expanded the number of brain nuclei with cells projecting to the rat SCN to about 100–140 regions (variation may relate to label spread outside the SCN) (Krout et al., 2002). The regions with likely polysynaptic projections to the SCN include numerous additional olfactory structures, divisions of the cortex, hippocampus, nuclei of the amygdala, thalamus, hypothalamus, as well as nuclei in the midbrain and medulla from which there are very few reported direct SCN projections. While this study is important, it should be noted that any uptake by terminals in the peri-SCN region is likely to identify regions not connected to the SCN. A similar thorough examination of polysynaptic projections to the SCN has not been systematically completed in other species. However, some information is available for the hamster and shows both similarities and significant dissimilarities with the rat data. In particular, there appears to be little or nothing in the way of a polysynaptic projection from the hamster tectum and pretectum to the SCN, yet more oculomotor midbrain structures contain labeled cells than in the rat SCN (Horowitz et al., 2004). A polysynaptic projection from the medial vestibular nucleus to the SCN has been reported in the hamster. Focused studies have documented SCN-afferent projections from the pretectal complex, with cells identified in the olivary, medial and posterior pretectal nuclei (rat) (Mikkelsen and Vrang, 1994) and from the median raphe nucleus (MeyerBernstein and Morin, 1996; Hay-Schmidt et al., 2003). Cholinergic cells in the substantia innominata, preoptic nucleus, diagonal band of Broca, medial septum, pedunculopontine, laterodorsal tegmental and parabigeminal nuclei have also been reported to project to the SCN in the rat (Bina et al., 1993). As noted above, there is disagreement concerning which, if any, these nuclei send projections to the SCN (Moga and Moore, 1997; Krout et al., 2002). 34 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 8. Intergeniculate leaflet 8.1. Nomenclature The IGL has been, and continues to be, confused with the ventral lateral geniculate nucleus. Focus on the term, “leaflet,” as a descriptor of the IGL has drawn attention away from the actual boundaries of the nucleus and, until fairly recently, has not had a clearly identifiable homolog in nonrodent species. It is now established that the medial division of the ventral lateral geniculate nucleus is the cat homolog of the IGL (Nakamura and Itoh, 2004; Van der Gucht et al., 2003; Pu and Pickard, 1996). In the monkey, the homologous structure is known as the pregeniculate nucleus (Van der Gucht et al., 2003; Moore, 1989). However, there is uncertainty regarding the neuromodulator content of the GHT across primate species (Chevassus and Cooper, 1998). In the hamster, the definition of the IGL has gradually evolved to include all regions of the lateral geniculate complex in which NPY neurons and cells projecting to the SCN may be found. As indicated from the developmental data described below, NPY cells found in medial areas near the acoustic radiation and ventral to the leaflet portion of the IGL have not completed full migration (Botchkina and Morin, 1995). The region of the laggard migratory NPY cells also contains neurons projecting to the SCN which are not found elsewhere in the lateral geniculate complex outside of the IGL (Morin and Blanchard, 1995; Morin et al., 1992). There is also a corresponding group of enkephalin-containing cells, especially in the medioventral part of the IGL (Morin and Blanchard, 1995). This description of the hamster IGL is generally consistent with that for the rat (Moore and Card, 1994), although there are slight species differences with respect to its cross-sectional appearance at various rostrocaudal levels. 8.2. Development IGL development has been described for the hamster (Botchkina and Morin, 1995). The IGL is probably best considered as part of the so-called ventral thalamus with a developmental origin different from the dorsal lateral geniculate nucleus (Altman and Bayer, 1979a,b). The hamster IGL develops out of the reticular neuroepithelium with the first NPY cells evident on embryonic day 9–10. These are evident against a background of radial glial cells extending dorsolaterally. NPY neurons apparently migrate along a specialized set of radial glial processes reaching their final destination in what will become the IGL on E14. The radial glial cell bodies dislocate from the ventricular germinal zone on E15, and transform into the astrocytes that become evident in the IGL across postnatal days 5–10. Some NPY neurons do not achieve complete migration and remain relatively displaced in what is now considered ventromedial IGL. Fiber outgrowth from NPYcontaining IGL neurons is evident by P0, and by P3, they penetrate the SCN. The adult-like NPY terminal plexus in the SCN is achieved by approximately P10. The P3 arrival in the SCN of the NPY component of the GHT approximately coincides with the end of SCN synaptogenesis (Speh and Moore, 1990), arrival of serotonergic innervation (Botchkina and Morin, 1993) and precedes the initial retinal innervation of the SCN by about 2 days (Speh and Moore, 1993). The arrival of retinal projections in the lateral geniculate region occurs on E13, but there is a delay in terminal arborization until about E15.5 (Jhaveri et al., 1991). The timing of retinal innervation of the IGL is not known. 8.3. Anatomy 8.3.1. Geniculohypothalamic tract The NPY neurons were the first IGL cell phenotype shown to project to the SCN (Card and Moore, 1982; Moore et al., 1984). The initial description of IGL projections to the contralateral nucleus in the rat indicated that many of the cells contained enkephalin, but none of this phenotype projected to the SCN (Card and Moore, 1989). There are substantial species differences with respect to IGL organization and in the hamster, only a very small percentage of NPY or enkephalin neurons project to the contralateral IGL (Morin and Blanchard, 1995). In both species, GABA neurons are present in the IGL and project to the SCN (Morin and Blanchard, 2001; Moore and Card, 1994). In the rat, there is a fourth, unidentified, cell type (Moore and Card, 1994). In the hamster, a fourth class consists of neurotensin neurons (Morin and Blanchard, 2001). It is useful to recognize, as is further indicated below, that while ample numbers of the IGL neurons also project to the pretectum, only a small percentage contain any of the various neuromodulators thus far evaluated, implying existence of at least one other cell type in hamster. NPY cells can be used to identify the IGL as they occur nowhere else in the lateral geniculate complex (Morin and Blanchard, 2001; Moore and Card, 1994). In the hamster, neurotensin, which is almost completely colocalized with NPY, can likewise identify the nucleus, albeit with somewhat greater difficulty because it is present in only about half the NPY neurons. There is colocalization ranging from 7% (percent of GABA cells containing enkephalin) to 98% (percent of neurotensin cells containing NPY) of all identified peptides with each other in hamster IGL neurons (Morin and Blanchard, 2001). 8.3.2. Connectivity and relationship to sleep, visuomotor and vestibular systems Harrington (1997) has reviewed the substantial literature concerning the ventral part of the lateral geniculate complex. This review was titled, with a good deal of foresight, “The ventral lateral geniculate nucleus and intergeniculate leaflet: interrelated structures in the visual and circadian systems.” Most of the literature cited in that review was unaware that an “intergeniculate leaflet” existed, let alone the possibility that it might have functions different from those of the ventral lateral geniculate. At the time of the review, it was established that the IGL contributes to the photic regulation of circadian period and entrainment, and that IGL lesions could block the ability of certain nonphotic stimuli to induce phase responses normally consistent with an NPY-type PRC (Janik and Mrosovsky, 1994; Wickland and Turek, 1994; see Morin, 1994; Harrington, 1997 for reviews). The prediction in Harrington's review, evident in the title, that the IGL has functions beyond those of circadian rhythm BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 regulation has not yet been directly established, but the groundwork is now in place for doing so. The most important anatomical feature of the IGL appears to be the enormity of its connections with the rest of the brain (cf., Fig. 4, which only includes a portion of the ascending projections). Not only are they widespread, but they are generally bilateral and often reciprocal. The magnitude of IGL connectivity was initially suggested by an investigation of hypothalamic-IGL connectivity in the gerbil (Mikkelsen, 1990). This study, and others in hamster (Morin and Blanchard, 1999) and rat (Horvath, 1998; Moore et al., 2000) revealed a major efferent target extending from the lateral optic chiasm medially through the lateroanterior hypothalamus to the midline, including the SCN, and dorsally to include the subparaventricular zone and adjacent perifornical anterior hypothalamus. In addition, IGL projections extend to several divisions of the bed nucleus of the stria terminalis and variety of basal forebrain areas extending laterally as far as the piriform cortex (Morin and Blanchard, 1999). Projections to nuclei of the midline thalamus are also fairly extensive (Morin and Blanchard, 1999; Moore et al., 2000). Many of the same regions also send projections back to the IGL (Morin and Blanchard, 1999; Vrang et al., 2003). Comparison of regions receiving retinal projections reveals convergence with IGL efferents. Four distinct routes providing retinal innervation of rostral diencephalon have been described (Johnson et al., 1988a; Morin and Blanchard, 1999): (a) to the SCN and anterior hypothalamus; (b) to the ventral lateral hypothalamic area; (c) rostrally to the ventral preoptic area and (d) to the bed nucleus of the stria terminalis (Cooper et al., 1994) (earlier identified as projecting to the anterodorsal thalamic nucleus; Johnson et al., 1988a). With very few exceptions, IGL projections innervate the retinorecipient regions, although the extent of IGL projections is substantially greater than those from the retina (Morin and Blanchard, 1999). In a similar vein, areas receiving IGL input are also very likely to receive projections from the SCN (Morin et al., 1994) and from the olfactory system (Cooper et al., 1994). IGL connections with the midbrain and brainstem are just as extensive as those for the forebrain. The concept of a “subcortical visual shell” has been invoked to facilitate discussion of connectivity of the IGL (Morin and Blanchard, 1998). The subcortical visual shell consists of 12 contiguous retinorecipient nuclei of the thalamus and midbrain. All but those projecting to cortex (lateral posterior and dorsal lateral geniculate nuclei) receive both ipsi- and contralateral projections from the IGL. In addition, most of the regions also supply reciprocal input to the IGL bilaterally (Morin and Blanchard, 1998), although ipsilateral connections are more robust. The functional importance of IGL connections with nuclei of the subcortical visual shell has not yet been tested. However, the presence of bilaterally symmetrical connectivity strongly suggests involvement of the IGL in the regulation of visuomotor function (for a review, see Morin and Blanchard, 1998; Horowitz et al., 2004). The further suggestion has been made that the IGL may contribute to two discrete classes of events, one concerning visuomotor, and the other circadian rhythm, regulation (Horowitz et al., 2004, 2005; Morin and Blanchard, 2005). The 35 IGL has at least two classes of neurons identified by their projections to the SCN, one being so connected and the other not. Those IGL cells that contribute GHT input to the SCN appear not to receive any input from the pretectum or superior colliculus despite abundant IGL-afferent projections from those areas (Morin and Blanchard, 1998; Horowitz et al., 2004). This further suggests that photic input to the pretectum and tectum does not influence circadian rhythmicity by passing through the IGL and GHT. This inference is supported by lesion studies indicating that loss of the IGL, but not visual midbrain areas afferent to the IGL, modifies circadian rhythm response to light (Morin and Pace, 2002). There have been recent reports describing brainstem origins of IGL-afferent connections (Horowitz et al., 2004; Vrang et al., 2003). Two of these, a serotonergic input from the dorsal raphe and a noradrenergic projection from the locus coeruleus, have been previously documented (MeyerBernstein and Morin, 1996; Kromer and Moore, 1980). Other regions sending projections to the IGL include several nuclei of the oculomotor complex, the caudal cuneiform, pedunculopontine and laterodorsal tegmental nuclei (Horowitz et al., 2004; Vrang et al., 2003). In addition, projections from the pararubral nucleus (which also is retinorecipient) and vestibular nuclei to the IGL have been described (Horowitz et al., 2004). Several of these regions, including the medial vestibular nucleus, locus coeruleus, divisions of the dorsal tegmental and pontine tegmental nuclei and the dorsal raphe, are also recipients of projections from the hypocretin/orexin neurons in the lateral hypothalamic area (Horowitz et al., 2005; Mintz et al., 2001). These regions are also thought to be involved in the modulation of sleep and arousal processes (Saper et al., 2001). Most also receive projections directly from the IGL (however, the locus coeruleus and vestibular nuclei do not) (Morin and Blanchard, 2005). Thus, it seems likely that the IGL contributes to the regulation of sleep and arousal in addition to its role in circadian rhythm regulation and its hypothesized role as a mediator of visuomotor function. 8.4. Function 8.4.1. Neurophysiology A novel observation has been made concerning the firing rate of IGL neurons. In a series of paper, Lewandowski and colleagues have described a slow oscillation in the rat IGL that is not evident in adjacent thalamic structures. Maximal firing rate is approximately 20 Hz occurring as a burst that lasts about 70 s with the overall oscillation of firing having a period of about 106 s (Lewandowski et al., 2000). Unlike the firing pattern observed in the dorsal lateral geniculate, there is no correlation between IGL cell firing and the pattern of firing in the visual cortex. A lesion of one IGL does not disrupt rhythmicity in the other (Lewandowski et al., 2002). The origin of the oscillatory activity is unknown, but is not exclusively intrinsic to the IGL as it fails to persist in a slice The function of this burst oscillation is not known, nor is it even known to be related to circadian rhythm regulation. However, it is present only in the light with bursting suppressed by dark (Bina et al., 1993). The bursting pattern can be obliterated and rendered a tonic discharge pattern by bicuculline or picrotoxin applied 36 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 intraventricularly (Blasiak and Lewandowski, 2004a). In addition, the IGL burst discharge frequency is increased about 100% by lesions of the dorsal raphe nucleus (Blasiak and Lewandowski, 2003), although serotonin agonists injected intraperitoneally unexpectedly induce an increase in discharge frequency as well. The implications of the slow oscillatory bursting activity are not clear and may be related more to suspected visuomotor functions of the IGL. Intriguingly, the ultradian rhythm in IGL neuron burst discharge is very similar to what has been described as a burst pattern in SCN neurons (Miller and Fuller, 1992). About 20% of the cells recorded in and near the SCN burst at a rate of about 10 Hz with an average period of about 143 s. It may be that the IGL burst rhythm is derived from the SCN region because the IGL rhythm is lost in a slice preparation (Blasiak and Lewandowski, 2004b). 8.4.2. Nonphotic regulation of rhythmicity One major question concerning the influence of nonphotic stimuli on circadian rhythm regulation is whether it is mediated by activity (i.e., muscle/joint movement) or a correlate of activity. Dark pulses, triazolam and novel wheel locomotion each elicit large phase advances when administered at CT6 (see Morin, 1994; Mrosovsky, 1996) and each of these is usually associated with increased wheel running. At least two experiments have now demonstrated that different parts of the brain mediate the phase shifts elicited by different stimuli. In the clearest case (Marchant and Morin, 1999), the benzodiazepine, triazolam, failed to elicit phase shifts at any circadian time in hamsters sustaining neurotoxic lesions of the deep superior colliculus. Lesions of the pretectum were at least partially effective in eliminating such responses and, because of the lesion method, it has not been possible to fully distinguish between collicular and pretectal effects. However, lesions confined to the superficial layers of the superior colliculus had no effect on phase response to triazolam. In contrast to the ability of midbrain lesions to block phase shift responses to triazolam, the same lesions had no effect on phase shifts induced by prolonged running in a novel wheel. Thus, the neural substrates for triazolam and novel wheelinduced phase shifts are different, although each appears to require an intact GHT as a final common path conveying information to the SCN (Janik and Mrosovsky, 1994; Wickland and Turek, 1994; Johnson et al., 1988b; Marchant et al., 1997). These results support previous observations that chlordiazepoxide (a benzodiazepine) and fentanyl (an opiate mu opioid receptor agonist), neither of which enhances locomotion in hamsters, nevertheless induce equivalent phase shifts (Biello and Mrosovsky, 1993; Maywood et al., 1997; Meijer et al., 2000) to those induced by triazolam. The second experiment differentiating response to triazolam from response to novel wheel involved serotoninspecific neurotoxic lesions of the median or dorsal raphe nuclei. Loss of serotonin neurons from the median (but not the dorsal) raphe eliminated phase shifts in response to triazolam treatment (Meyer-Bernstein and Morin, 1998). Novel wheel access elicited equivalent phase shifts regardless of dorsal raphe, median raphe or control lesions. Thus, it is clear that while a final common path from the IGL to SCN mediates the phase shift response to two distinct stimuli, there are different anatomical substrates subserving those stimuli. Whether the ability of DD to elicit phase shift responses corresponding to the NPY-type PRC depends upon locomotion has not been determined. NPY, the first neuromodulator identified in the IGL (Card et al., 1983), has also received the greatest amount of attention with respect to IGL and GHT function. Stimulation of the IGL has been thought to elicit phase shifts because of NPY release from GHT terminals in the SCN (see Morin, 1994). This concept has been reaffirmed by antagonism of NPY activity in vivo by infusion of NPY antiserum onto the SCN which blocked phase shifts in response to novel wheel running (Biello et al., 1994). As indicated above, cells contributing to the hamster GHT also contain GABA, neurotensin and enkephalin. Neurotensin appears not to have been evaluated with respect to its ability to modify circadian rhythmicity in vivo. However, a CT6 application of neurotensin to the rat SCN in a slice preparation generally reduced neuron firing (Coogan et al., 2001), while inducing large phase advances of the firing rhythm (MeyerSpasche et al., 2002). A small literature exists concerning the function of enkephalin in the hamster GHT. The delta opioid receptor visualized by immunohistochemistry is densely distributed on cells in the SCN, particularly in the ventral and medial parts of the nucleus (Byku et al., 2000). A few cells in the IGL also are identifiable from the delta opioid receptor labeling. The observed distribution of receptor label in the SCN is consistent with the distribution of enkephalin terminals (Morin et al., 1992). Delta opioid agonists administered at CT8 or CT10 (but not at other times) will induce moderate phase advances that are not related to the amount of activity also induced by the drugs (Byku and Gannon, 2000a,b). Agonists of kappa or mu opioid receptors apparently do not elicit phase shifts, although other reports indicate that fentanyl, a mu opioid agonist, can generate an NPY-type PRC (Meijer et al., 2000; Vansteensel et al., 2003b). The delta (but not mu or kappa) opioid agonists are also able to prevent light-induced phase shifts when administered in advance of a light pulse at CT19 (Tierno et al., 2002). This result is consistent with a presynaptic inhibitory action on RHT terminals, as suggested by the receptor localization in the SCN (Byku et al., 2000). However, electrophysiological evidence suggests that the primary site of action of the effective delta opioid agonists might be outside the SCN (Cutler et al., 1999). The ability of delta opioid agonists to block light-induced phase shifts is similar to the effect of NPY. In vivo infusion of NPY onto the SCN greatly attenuates light-induced phase advances and delays (Lall and Biello, 2002, 2003a,b; Weber and Rea, 1997). In contrast, infusion of NPY antiserum onto the SCN augments phase advances to light during the late subjective night (Biello, 1995). Wheel running, which is thought to release NPY into the SCN through the GHT (Biello et al., 1994), appears to attenuate light-induced phase advances, but not delays (Mistlberger and Antle, 1998; Ralph and Mrosovsky, 1992). Inhibition of phase response to light is observable in the slice preparation if the light pulse is administered in vivo followed by removal of the brain and acute treatment of the SCN with NPY (Yannielli and Harrington, 2000). Similarly, NMDA stimulation of the SCN slice yields BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 large phase shifts that are blocked by coapplication of NPY (Yannielli and Harrington, 2001; Yannielli et al., 2004) and the NPY-blocked phase response to NMDA can be restored by simultaneous treatment with an NPY Y5 (but not Y1) receptor antagonist. NPY induces phase advances when applied by itself to the SCN slice during the subjective day, but no observable phase shift during the subjective night (Harrington and Schak, 2000; Biello et al., 1997b). In vivo studies using intracranial injections showed that a Y5 receptor antagonist enhances light-induced phase shifts. If the locomotor rhythm phase response to light is blocked by intracranial NPY, the shifts can be restored to normal by coinjection of NPY and the Y5 receptor antagonist (Lall and Biello, 2003a; Yannielli et al., 2004). Additional in vivo studies also demonstrated probable involvement of the Y5 receptor in the NPY suppression of light-induced phase shifts (Lall and Biello, 2003a) and lend credibility to the view that light and NPY (consequent to locomotor activity) normally act together to regulate phase shift magnitude (Lall and Biello, 2002). The Mistlberger lab has added a new twist to the topic of nonphotic control of circadian rhythm phase. Initially, there were two significant observations. One was that 24 h forced treadmill activity blocked light-induced phase delays, whereas simple treadmill activity simultaneous with the light pulse did not alter phase. The second observation yielded the converse result, namely, a shorter interval on the treadmill (6 h) blocked phase response to light (Mistlberger et al., 1997). Studies were then performed, using the Aschoff type II procedure, to elaborate on the ability of “sleep deprivation” to induce rhythm phase shifts. In these experiments, sleep deprivation was accomplished using gentle handling (not confounded by simultaneous locomotion) beginning at CT6. A 3 h procedure elicited a significant phase advance shift and a 1 h procedure yielded an even greater (about 150 min) shift (Antle and Mistlberger, 2000). The shifts appear to occur in about two thirds of the animals and it was observed that the fewer the number of interventions needed to keep an animal awake, the larger the phase shift to the sleep deprivation procedure. Additionally, exposure to light (10 lx) during a 3 h gentle handling interval effectively eliminated phase advances. This observation suggests that the phase shifts consequent to “gentle handling” are not caused by the absence of sleep, but rather by a correlate. Such a correlate could be “stress” generated by the sleep deprivation procedure. In a comprehensive series of experiments (Mistlberger et al., 2003), several of the stress-related issues consequent to sleep deprivation were clarified. In particular, stress as reflected by elevated cortisol levels, is not associated with phase advance shifts, nor are shifts elicited by restraint which also elevates cortisol (Mistlberger et al., 2003). Nor is sleep deprivation caused by the “pedestal over water” method associated with phase shifts. In contrast, mere access to a 1 m2 open field did cause phase shifts similar in magnitude to those resulting from gentle handling. Phase shift magnitude was related to the amount of forward locomotion and those animals classified as “shifters” engaged in considerably more locomotion than the nonshifters. The conclusion of these studies is that some level of locomotion is indeed necessary to achieve phase advance shifts (Mistlberger et al., 2003). Observations that such shifts occur following the simple 37 intraperitoneal injection of saline (Mead et al., 1992; Hastings et al., 1992) may reflect an action of this stimulus complex through an alternate route to the circadian clock not requiring locomotion for phase shifts (cf., benzodiazepine; Marchant and Morin, 1999; Biello and Mrosovsky, 1993), or the response to saline may in fact include a locomotor component not measured in those studies. One variation on the theme of nonphotic stimuli capable of inducing phase advances has involved use of “dark pulses” administered at CT6. The dark pulse is associated with increased locomotion and consequent phase shifts in hamsters housed in LL (Boulos and Morin, 1985; Boulos and Rusak, 1982). Phase advance responses can be greatly increased (up to 7 h) if the dark pulses coincide with the “gentle handling” paradigm for sleep deprivation (Mistlberger et al., 2002). Although the reference to dark pulses as a nonphotic stimulus implies that it is of the same class as benzodiazepines, novel wheel, gentle handling and saline injection, the mechanism of action of dark pulses is not necessarily the same. In particular, IGL lesions block the ability of triazolam (Johnson et al., 1988b), novel wheel (Janik and Mrosovsky, 1994; Wickland and Turek, 1994) and saline injection (Maywood et al., 1997) (gentle handling has not been tested) to elicit phase advances, but they apparently do not block such responses to dark pulses (Harrington and Rusak, 1986). The effect of restraint has also become more ambiguous than expected when considered in the context of dark pulses. Restraint during the midsubjective day has no effect on rhythm phase (Van Reeth et al., 1991; Rosenwasser and Dwyer, 2002). However, during the late subjective day, brief or 6 h restraint will induce phase delays. At both circadian times, restraint blocks the ability of a dark pulse to induce a phase advance (Rosenwasser and Dwyer, 2002; Van Reeth and Turek, 1989; Reebs et al., 1989). Thus, on the one hand, phase advances during the midsubjective day are blocked by unknown mechanism invoked by restraint, but one that is apparently not directly related to the circadian clock; on the other hand, blockade of the dark-induced phase advance at dusk may be the simple consequence of a restraint-induced phase shift of equal magnitude but opposite direction (Rosenwasser and Dwyer, 2002; Dwyer and Rosenwasser, 2000). 8.4.3. Photic regulation of rhythmicity One major function of the IGL is modulation of the circadian period during LL conditions (Pickard et al., 1987; Harrington and Rusak, 1986). However, the visual system projects to a variety of midbrain regions that connect with the IGL leaving the possibility that photic information effecting the circadian clock acts indirectly through, rather than directly in, the IGL. This perspective has been enhanced by the demonstration that visual midbrain structures are involved in circadian rhythm response to a benzodiazepine and that phase shift magnitude to a novel wheel stimulus is modulated by light (Marchant and Morin, 1999). IGL lesions reduce the circadian period lengthening effect of LL by about 50%, but do not alter the normal period in DD (Morin and Pace, 2002). This result suggests the IGL mediates the tonic effects of light. In contrast, large lesions of the pretectum and tectum that eliminate rhythm response to benzodiazepine treatment (Marchant and Morin, 1999) have no effect on circadian period in LL (Morin and Pace, 2002). 38 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 In mouse, IGL lesions lengthen the circadian period of DDhoused mice, but do not modify the period lengthening effect of LL (Pickard, 1994; Lewandowski and Usarek, 2002). In the rat, IGL lesions do not alter the circadian period in DD nor do they prevent LL-induced circadian rhythm disruption (Edelstein and Amir, 1999a). Period in LL before rhythm disruption was not assessed. Under skeleton photoperiod conditions (1 h of light from ZT0-1 and ZT11-12, rats with IGL lesions generally fail to entrain indicating the necessity of the IGL under these conditions; Edelstein and Amir, 1999b). Equivalent studies have not been done in other species. Just as NPY is able to attenuate light-induced phase shifts, effects of NPY on phase are also inhibited by light. Novel wheel-induced phase advances are greatly attenuated by a subsequent light pulse. Similarly, NPY-induced phase advances are curtailed by a light pulse (Biello and Mrosovsky, 1995). A glutamate agonist (NMDA), injected onto the SCN to mimic the effect of photic input, also greatly reduces the phase advance effects of NPY administered in the midsubjective day (Gamble et al., 2004). At CT20, NMDA injection onto the SCN induces phase advances (Mintz et al., 1999), but when given simultaneously with NPY, small phase delays occur (Gamble et al., 2004). This result, in conjunction with the observation that the effect occurs in the presence of TTX, suggests that NPY is acting postsynaptically to induce phase shifts, rather as a presynaptic inhibitor of RHT function. Glutamate receptor antagonists, known to modify SCN and rhythm response to light (Abe et al., 1992; Rea et al., 1993b; Vindlacheruvu et al., 1992), fail to alter light-induced FOS expression in the rat IGL (Edelstein and Amir, 1998). This is consistent with the likelihood that there appears to be little or no glutamatergic innervation of the IGL (Fujiyama et al., 2003). 9. Serotonin and midbrain raphe contribution to circadian rhythm regulation The 1994 review (Morin, 1994) fell victim to the typical problem of being partially out of date by the time it was published. No topic was more affected by this than serotonin and its effect on circadian rhythmicity. In the year of publication, research on the topic began to blossom and there have been several major reviews published (Morin, 1999; Rea and Pickard, 2000a; Mistlberger et al., 2000; Yannielli and Harrington, 2004). One of the more important changes in perspective concerning the role of serotonin has been a change of focus away from the dorsal raphe nucleus, previously thought to be the source of SCN innervation by serotonin fibers (Smale et al., 1990; Azmitia and Segal, 1978). Anatomical studies, using three separate methods (anterograde and retrograde tract tracing of raphe projections, and lesion studies designed to destroy raphe projections), have unequivocally demonstrated that the median raphe sends mixed serotonergic and nonserotonergic projections to the SCN, and the dorsal raphe sends mixed serotonergic and nonserotonergic projections to the IGL (Moga and Moore, 1997; Meyer-Bernstein and Morin, 1996; HaySchmidt et al., 2003). This simple anatomy is complicated by the presence of reciprocal serotonergic and nonserotonergic connections between dorsal and median raphe nuclei (Tischler and Morin, 2003). 9.1. Effects of serotonin-specific neurotoxins The sense of the literature is that, in hamsters, serotonin has an inhibitory effect on rhythm response to light which, in animals lacking median raphe serotonin neurons, is evident as a larger amplitude PRC, greater prevalence of, and shorter latency to, light-induced rhythm splitting and longer circadian periods under LL (Meyer-Bernstein and Morin, 1996; Morin and Blanchard, 1991; Bradbury et al., 1997). No effects on rhythmicity are seen following destruction of only the dorsal raphe serotonergic cells. Most recently, evaluation of irradiance response curves in animals with neurotoxic lesions of median raphe serotonin neurons has shown similar sensitivities of lesioned and unlesioned controls to light, but a greater phase response by lesioned animals to high intensity (probably saturating) light (Muscat et al., 2005). The drug of abuse, MDMA (‘ecstasy’), is rapidly toxic to serotonin terminals (Battaglia et al., 1991). Treatment of rats with this drug for several days reduces phase advances of the rhythm in SCN firing rate by about 50% in response to 8-OHDPAT applied to a slice at ZT6 (Biello and Dafters, 2001). The effects of MDMA on rhythmicity have been extended to the evaluation of its effects on hamsters in vivo. After drug injections of MDMA, phase advances to 8-OH-DPAT administered at CT8 were blocked. Light-induced phase delays were convincingly reduced about 50% (Colbron et al., 2002), although the effect is not consistent with the literature showing that serotonin loss increases phase response to light. Damage to the serotonin terminals varies according to brain region, with some areas being completely unaffected by the drug (Battaglia et al., 1991). In the above MDMA studies, it is unclear as to which part of, or to what extent, the serotonin system has been damaged. 9.2. Serotonin receptor function A major development in the study of serotonergic effects on circadian rhythm regulation has been the increased focus on particular receptor types and their function. One critical development has been the recognition that 8-OH-DPAT, long thought to a 5HT1A-specific agonist and frequently used because of that specificity (see Morin, 1999), is actually specific to the 5HT1A, 5HT5A and 5HT7 receptors (Lovenberg et al., 1993; Sprouse et al., 2004a; and reviewed by Gannon, 2001). There are now reports indicating that the SCN contains the 5HT1A, 5HT1B, 5HT2A, 5HT2C, 5HT5A and 5HT7 receptor types (Sprouse et al., 2004a,b; Belenky and Pickard, 2001; Duncan et al., 1999, 2000; Prosser et al., 1993; Oliver et al., 2000; Moyer and Kennaway, 1999). A useful review of the evidence favoring the presence and function of 5HT7 receptors in the SCN was published in 2001 (Gannon, 2001), but needs to be interpreted in the context of subsequent information. The 5HT5A receptor has most recently been appreciated as a serotonin receptor type contributing to circadian rhythm regulation. It is distributed in the dorsal and median raphe, as well as in the SCN and IGL (Duncan et al., 2000). Thus, the 5HT1A, 5HT5A and 5HT7 receptors are found in similar places and respond to similar agonists/antagonists thereby increasing the difficulty of discerning receptor-specific function. One suggestion is that 8-OH-DPAT injection into the dorsal raphe BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 induces phase shifts via 5HT7 (but not 5HT5A) receptor activation (Duncan et al., 2004), whereas systemic injection of the drug induces phase shifts by activating the 5HT1A receptor (Tominaga et al., 1992). The phase response of hamsters to dorsal raphe application of serotonin 5HT1A/5A/ 7 agonists diminishes with age (Duncan et al., 2004; Penev et al., 1995), and this may be the result of specific loss of available 5HT7 receptors (Duncan et al., 1999). In the rat, the 5HT2C receptor may contribute to the regulation of circadian rhythm phase. The receptor type is present in the SCN (Moyer and Kennaway, 1999). Agonists of the 5HT2C receptor are able to induce phase delays in rats and induction of FOS in SCN neurons at CT18, but not at CT6 (Kennaway and Moyer, 1998), and generally mimic the effects of light. The suggestion has been made (Kennaway and Moyer, 1998) that previous observations of quipazine-induced phase shifts in rat (Prosser et al., 1993; Kennaway and Moyer, 1998) may have been the result of that drug's affinity for the 5HT2 class of receptors. Kennaway and Moyer (1998) suggest that the literature is ambiguous with respect to the certainty that the rat SCN contains 5HT1A and 5HT7 receptors. Further, they suggest the possibility that light may act through the known retina-dorsal raphe projection in the rat (not evident in the hamster; see Section 4.2) and it is the modulation of the 5HT2C receptors on this pathway that results in light-like phase response and FOS induction (Kennaway and Moyer, 1998). Consistent with this view is the observation that reduction of serotonin in rats by pCPA injection attenuates light-induced FOS (Moyer and Kennaway, 2000), suggesting that normal serotonin innervation is necessary for light-induced FOS. Agonist activation of 5HT2A/C receptors induces substantially (but not completely) light-like FOS expression in the rat SCN (Kennaway et al., 2001). The investigators suggest that, in the rat, but probably not in the mouse or hamster, there are two pathways by which light can modulate SCN rhythmicity. One of these is the RHT, while the other involves the retinal projection to the dorsal raphe which, it is hypothesized, sends a direct serotonergic projection to the SCN that effects glutamate release at that location (Kennaway et al., 2001). A dorsal raphe projection to the SCN is probably absent in the rat (Moga and Moore, 1997), as it is in hamster, but there is abundant dorsal raphe innervation of adjacent hypothalamus (Moga and Moore, 1997; Vertes, 1991) and peri-SCN neurons modulated by serotonin from the dorsal raphe may provide glutamate signaling to the SCN. Involvement of the 5HT2C receptor in rat rhythm regulation is further supported by the observation that agonists specific for this type of receptor induce expression of FOS, Per1 and Per2 in the SCN. This effect was more robust at ZT16 than ZT22 (Varcoe et al., 2003). The issue of species differences has been addressed by direct comparison of mouse and hamster responses to a variety of 5HT1A, 1B, 2 or 7 agonists. The results are complex with differing phase and spontaneous Fos-induction responses in each species, plus differing effects on lightinduced phase shifts and Fos-induction. In addition, response has also been shown to be related to whether the drug was injected centrally (SCN) or administered peripherally (Antle et al., 2003b). One aspect of the complexity of serotonergic activity relative to the effects of light is the demonstration that phase response to agonists can vary enormously depend- 39 ing upon prior light exposure (Knoch et al., 2004). Hamsters exposed to LL for as little as 1 day can have very large phase shifts in response to intracranial NPY given at CT6, a sleep deprivation procedure at ZT18 or 8-OH-DPAT at ZT18 or ZT21 (Aschoff type 2 test). LL exposure also eliminates the early night time peak in peri-SCN serotonin and reduces the overall level to approximately the typical late afternoon levels seen under LD conditions (Knoch et al., 2004). The 5HT1B receptor has received substantial attention with respect to its role in rhythm regulation. The receptor is identifiable in the SCN, specifically on terminals of the RHT (Belenky and Pickard, 2001; Pickard et al., 1999) and there is an approximately 35% reduction in 5HT1B binding sites in the SCN after bilateral enucleation (Pickard et al., 1996; Manrique et al., 1999). 5HT1B receptor agonists markedly attenuate lightinduced phase shifts and the induction of FOS in the SCN, although cells in a central region that appears to include the CALB neurons continue to express FOS (Pickard et al., 1996, 1999; Pickard and Rea, 1997; Shimazoe et al., 2004). Lightinduced suppression of nocturnal melatonin is also alleviated by treatment with 5HT1B agonists (Rea and Pickard, 2000b). Infusion of receptor agonist directly onto the SCN eliminated phase response to light (Pickard et al., 1996). Use of the 5HT1B receptor knockout mouse has yielded substantial additional data. Phase shifts elicited by light in this strain are not modified by 5HT1B receptor agonists. Electrical stimulation of the optic nerve or glutamate application to the bath elicit excitatory postsynaptic currents (EPSCs) in SCN neurons recorded in a slice preparation. Agonists for the 5HT1B receptor greatly reduce EPSCs in the wild-type mouse, but have no effect in the 5HT1B receptor KO mouse (Smith et al., 2001; Pickard et al., 1999). Although the circadian period of wild-type mice does not differ from that of the 5HT1B KO mice, the period is longer in LL consistent with the view that this strain lacks inhibitory control of retinal input to the circadian visual system (Sollars et al., 2002). Most recently, bicucullinesensitive miniature inhibitory currents were recorded on SCN (which are predominantly GABAergic; Moore and Speh, 1993; Morin and Blanchard, 2001). Frequency of the inhibitory currents is reduced by a 5HT1B receptor agonist, but the agonist had no effect on the currents in SCN neurons of 5HT1B KO mice (Bramley et al., 2005). The authors suggest that abundant 5HT1B receptors are present presynaptically on GABA terminals, and thereby modulate GABA release within the SCN. 9.3. Regulation and consequences of serotonin release Extracellular serotonin increases abruptly from daytime levels to higher night time levels in both the peri-SCN and peri-IGL region (Dudley et al., 1998; Grossman et al., 2004). Similarly, extracellular serotonin increases in both the SCN and IGL in response to certain nonphotic stimuli (Dudley et al., 1998; Grossman et al., 2000, 2004). Serotonin availability in these nuclei is also augmented by electrical stimulation of the dorsal raphe nucleus (Grossman et al., 2004; Dudley et al., 1999; Glass et al., 2000b, 2003b). Despite these effects on serotonin release, it is not yet known to what extent the stimulation-induced transmitter increase in either the SCN or IGL contributes to circadian rhythm phase control. Studies involving electrical stimulation 40 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 of the dorsal or median raphe in which serotonin neurons have been destroyed have not been performed. Arguing against a normal effect of serotonin on rhythm phase control is the observation that treatment of hamsters with a 5HT1A receptor antagonist elevates serotonin release in the peri-SCN region to levels comparable to those seen during novel wheel access or raphe electrical stimulation without augmenting phase shifts in response to novel wheel activity (Antle et al., 2000). Nor do systemic or SCN injections of 5HT1/2, 5HT1A and 5HT2/7 receptor antagonists alter novel wheel-induced phase shifts (Antle et al., 1998). Consistent with those results is the observation that the serotonergic median raphe projections appear to be essential for triazolam-induced phase shifts, but not for those induced by novel wheel activity (Meyer-Bernstein and Morin, 1998). It is possible that the electrical stimulation activates nonserotonergic raphe cells projecting to the SCN or IGL (Meyer-Bernstein and Morin, 1996). The most directly related data on the topic come from the mouse in which a serotoninspecific neurotoxin was injected intracranially into the region of the SCN (Marchant et al., 1997; Edgar et al., 1997) and, in combination with IGL lesions, block activity-related phase control in the mouse. The suggestion is that the serotonergic projections to the SCN destroyed by the neurotoxin are necessary for such phase control. However, in the experiments, serotonin depletion occurred widely within the diencephalon and localization to the SCN cannot be assured. In the hamster, small injections of neurotoxin that destroyed nearly all serotonergic innervation to the SCN without damaging most of the surrounding hypothalamus had very little effect on circadian rhythmicity compared to the consequences of loss of all median raphe projections (Meyer-Bernstein and Morin, 1996; Meyer-Bernstein et al., 1997; Bobrzynska et al., 1996). There are two implications of these studies. One is that median raphe projections to non-SCN targets (also destroyed by intraraphe neurotoxin injection) may contribute to the regulation of hamster circadian rhythmicity. The other is that the dense serotonergic projection to the SCN (destroyed by the intra-SCN neurotoxin) may be minimally involved in such regulation. One perplexing issue has been the fact that electrical stimulation of the dorsal or the median raphe nuclei yields similar release of serotonin in the SCN (Dudley et al., 1999) and stimulation of either nucleus during the subjective day elicits equivalent magnitude phase advances (Meyer-Bernstein and Morin, 1999). A model has been developed (Glass et al., 2003b) that provides a reasonable reconciliation of behavioral, pharmacological and anatomical data (Fig. 5; see Glass et al., 2003b for an extensive explanation and presentation of the data implicating GABA). The similar effectiveness of stimulation occurring in either dorsal or median raphe may be possible because of the serotonergic–nonserotonergic interconnection between the two nuclei (Tischler and Morin, 2003). 9.4. Serotonergic potentiation of light-induced phase shifts It is generally the case that a manipulation during the subjective night that modifies phase advances will also modify phase delays. Such is not true for the drug BMY 7378. Administered at CT19, this drug potentiates the phase shifting effect of light given shortly thereafter. However, no such effect Fig. 5 – Diagrammatic conceptualization of the functional serotonergic circuitry governing circadian rhythm regulation. Based on an unpublished update provided courtesy of Dr. J.D. Glass, modified from the previous version (Glass et al., 2003b). The presumptive intra-IGL GABA connection is based upon unpublished work from the Glass laboratory. There are data for (Glass et al., 2003b) and against (Meyer-Bernstein and Morin, 1998) the likelihood that behavioral input induces phase shifts by acting through the serotonin neurons of the dorsal raphe nucleus. occurs when the drug/light combination occurs at CT14. The reason for this is not clear, although it may result from cancellation of light-induced phase delays by drug-induced phase advances when each is administered at CT14. At CT19, BMY 7378 alone induces small (1 h) phase advances, but the CT19 combination of light and drug yields shifts more than 300% larger than those induced by light alone (light-induced shifts are about 1.5 h; potentiated shifts are about 5 h; Gannon, 2003). This phenomenon is similar to what has been obtained with other drugs with different effects on phase response in the absence of light (Gannon, 2003; Rea et al., 1995; Moriya et al., 1998). For example, NAN-190 administered alone at CT14 or CT19-20 has essentially no effect on rhythm phase (Rea et al., 1995; Moriya et al., 1996), but potentiates both phase advances and delays when administered at those times in combination with light (Rea et al., 1995). One interpretation (Gannon, 2003) is that the drugs, which have mixed agonist/antagonist properties, facilitate light-induced phase shifts by acting as agonists on presynaptic 5HT1A receptors of median raphe serotonin cell dendrites to inhibit release of serotonin in the SCN. Elsewhere in the brain, the drugs act on postsynaptic 5HT1A receptors which are, presumably, antagonized by the drugs. WAY 100635, a 5HT1A antagonist, has no effect on light-induced phase advances (Smart and Biello, 2001), and it is thought that the particular combination of raphe cell autoreceptor activation plus the antagonism of SCN postsynaptic receptors is necessary for the large light-induced phase advances potentiated by the mixed agonist/antagonists BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 41 (Gannon, 2003). If this hypothesis is correct, then it may be possible to mimic the effect of the mixed agonist/antagonist drugs by concomitant injection of a 5HT1A antagonist and a 5HT1A agonist known to act on raphe autoreceptors. The complex control of serotonergic involvement in rhythm regulation is further suggested by the observation that depletion of brain serotonin (88% lower in the peri-SCN region) by systemic pCPA treatment greatly augments phase shifts in response to SCN application of serotonin or the 5HT1A/7 receptor agonist, 8-OH-DPAT (shifts are about 100 min with pCPA treatment vs. 30 min without) (Ehlen et al., 2001). The results suggest that a necessary condition for normal phase response to agonists is lack of activity at the postsynaptic receptors. Short-term changes in postsynaptic receptor number or sensitivity to endogenous or exogenous agonists may be critical to their effect on rhythm phase (see Ehlen et al., 2001 for a discussion). In animals pretreated with pCPA, phase advances resulting from 8-OH-DPAT infusion onto the SCN at CT6 were reduced by about 68% in animals treated with 5HT7 receptor antagonists (Ehlen et al., 2001). On the one hand, the result supports the view that 5HT7 receptors mediate the effect of serotonin agonists applied to the SCN. On the other, there is a significant residual phase shift effect of 8-OH-DPAT that may be attributable to its affinity for the 5HT1A receptor. It is also interesting that light stimulation simultaneous with 8-OHDPAT (i.e., CT6) in pCPA treated animals completely blocks the phase shifting effect of the 5HT1A/7 agonist (Ehlen et al., 2001). The in vivo result is consistent with observations that glutamate application to the SCN slice preparation, which presumably mimics the excitatory effect of light exposure, blocks the phase shifting effect of NPY or serotonin (Biello et al., 1997a; Prosser, 1998). The presumption is that the action of serotonin takes place in the SCN, although there is evidence for IGL involvement in the response to 8-OH-DPAT as well (Challet et al., 1998b). 10. Failure to re-entrain It is axiomatic that an entrained animal will re-entrain to a shifted light–dark cycle. In this era of transgenic animals specifically designed to show abnormal behavioral traits that will allow novel probing of the way things usually work, it is ironic that one of the more provocative developments during the last decade has been the discovery that a standard laboratory rodent, the Siberian hamster, is a model for failure to re-entrain. The initial observation occurred in both young and old animals entrained initially to an LD 16:8 photoperiod, then subjected to a 5 h delay of the photoperiod. About 90% of animals had free-running locomotor and body temperature rhythms for many weeks after the shift or became arrhythmic (Ruby et al., 1996, 1997) (Fig. 6). Melatonin treatment, known to modify the phase angle of entrainment in Siberian hamsters (Margraf and Lynch, 1993), reduced the percentage of animals failing to re-entrain to a shifted photoperiod (Ruby et al., 1997). Analysis of melatonin levels has shown that animals subjected to a 5 h phase delay have very low circulating melatonin at a time that it is expected to be high (Ruby et al., 2000), suggesting that the Fig. 6 – Failure to re-entrain to a shifted photoperiod by Siberian hamsters. The initial photoperiod was LD16:8 (white and black bars at the top of each record; lights off = ZT12) which, on the day of the arrowhead, was changed. On that day at ZT17, the lights were turned on for 2 h. The next day, the LD16:8 photoperiod was delayed by 3 h. Shading areas indicate the times of lights off under the modified photoperiod. This combination of light manipulations prevents re-entrainment in most animals. The expected re-entrainment (A) sometimes follows the phase shift, but is less likely to occur than the lack of re-entrainment (B, C). One case (C) demonstrates neither re-entrainment nor a clear free-running circadian rhythm, and a significant percentage of animals become arrhythmic. Unpublished data courtesy of Dr. N.F. Ruby. 42 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 absence of melatonin may contribute to the inability to achieve a stable phase angle of entrainment after the shift. Siberian hamsters re-entrain properly to 1 or 3 h delays of photoperiod phase, and to 1 h advances, but much less well to 3 h advances. Generally, these animals do not re-entrain properly following 5 h advance or delay shifts in the photoperiod, although the effect is modifiable by the exact manner in which the photoperiod is shifted (Ruby et al., 1998, 2004). If Siberian hamsters are exposed to a 5 h phase delay in the photoperiod, then immediately placed in DD for 14 days, all animals will reentrain when the shifted photoperiod is resumed. If DD exposure is delayed for several days, re-entrainment probability is reduced to less than 50% (Ruby et al., 2002b). The Siberian hamster is noted for the existence of individuals in the general population that fail to undergo gonadal regression when exposed to a short day photoperiod and breeding has selected this trait in a strain of animals identified as ‘nonresponders.’ Photoperiodic time measurement for reproduction control relies on the use of a circadian clock (Elliott et al., 1972; Rusak and Morin, 1976; Stetson and WatsonWhitmyre, 1976). Comparison of the responder and nonresponder strains with the phase shift paradigm has shown that they also have the traits of re-entrainment and nonreentrainment, respectively (Ruby et al., 2004). Most recently, Siberian hamsters that fail to re-entrain to a 5 h photoperiod shift have been exposed to light to induce SCN gene activity. In animals that re-entrain, Fos and Per1 gene activity is induced, as expected, in SCN neurons. In contrast, gene induction by light apparently does not occur in animals that fail to re-entrain (Barakat et al., 2004). This is conceivably related to the absence of light-induced gene expression in golden hamsters showing split rhythms in LL (De la Iglesia et al., 2000). The collective results of these studies indicate that the lighting conditions dictate subsequent response of the Siberian hamster circadian system to light. The most extreme conclusion is that a consequence of the 5 h phase shift renders the circadian rhythm system unresponsive to the effects of light. 11. Masking Mrosovsky (1999) is synonymous with masking and has provided a substantial review of the topic. Masking is important to the study of circadian rhythms for several reasons. One is purely technical to the extent that, under a variety of circumstances in which there are questions concerning whether the evident rhythm is imposed by the environment or is from endogenous sources, tests must be conducted to obtain an explicit answer. For example, mice lacking the genes coding for cryptochrome1 (Cry1) and cryptochrome2 (Cry2) show nocturnal locomotor activity under LD conditions, but are arrhythmic under DD (Albus et al., 2002; Van der Horst et al., 1999). The transfer into DD is the test of persistent rhythmicity with the same initial phase as seen under LD conditions. In the case of the Cry1/2 KO mice, the result of the transfer demonstrated that the absence of wheel running during the light portion of the previous daily photoperiod occurred because the running was strongly suppressed by the negative masking effects of light. A second major reason for studying masking concerns the anatomy of the visual system. Light-induced masking is a visual function, but the visual system anatomy contributing to it has not been satisfactorily elucidated. Normal mice generate a characteristic irradiance response curve when tested for light-induced masking of running wheel revolutions. Through a middle range of intensities, the stronger the stimulus, the greater the extent of negative masking. With very dim stimuli, activity level actually increases slightly (positive masking; see Mrosovsky, 1999; Redlin and Mrosovsky, 1999a). At the level of the retina, both classical and melanopsin photoreceptors have been shown to contribute to masking (Hattar et al., 2003; Panda et al., 2003). Melanopsin cells influence masking responses to the higher light intensities (Mrosovsky and Hattar, 2003). Loss of rod function in mice eliminates the positive masking seen at dim illumination levels, but for some strains, there may also be a significant reduction in the masking response to higher intensities (Mrosovsky et al., 1999, 2000). The analysis of visual projections contributing to negative masking remains incomplete. Mrosovsky and colleagues have evaluated contributions of the visual cortex, superior colliculus, lateral geniculate and SCN without obtaining data demonstrating that one of these areas is necessary for negative masking. In fact, the most common effect of lesions is actually increased masking. This is true for lesions of the visual cortex, superior colliculus, dorsal lateral geniculate and IGL, although it has not yet been possible to distinguish the effects of dorsolateral geniculate lesions from lesions aimed at the IGL (Edelstein et al., 2001; Redlin et al., 1999, 2003). Lesions of the SCN render animals arrhythmic and masking experiments must, of necessity, be conducted differently from those in which animals have normal circadian locomotor rhythms. When SCN lesioned animals are given access to running wheels in a 3.5 h light:3.5 h dark schedule, about 90% of their wheel revolutions occur during the dark intervals and the same animals show a strong preference for darkened vs. a lighted chamber (Redlin and Mrosovsky, 1999b). These results indicate that negative masking is not abolished by SCN lesions. By inference, it would also appear that retinal projections passing through the SCN to the subparaventricular zone and dorsomedial hypothalamus are not necessary for masking because they would also be destroyed by the lesions. However, these conclusions must be tempered by a recent report indicating loss of negative masking in SCN-lesioned hamsters (Li et al., 2005). This study did not address possible masking functions of retinal projections destroyed while passing through and around the SCN to other hypothalamic sites. Despite the absence of a demonstration that a particular structure is necessary for masking, the Mrosovsky work collectively supports the view that two visual processes govern the behavior. One, involving thalamus, midbrain and cortical visual structures, provides a normal inhibitory effect on the magnitude of the masking response to light. The other, presumably in one of the two dozen or so other retinorecipient brain regions (Morin and Blanchard, 1999; Horowitz et al., 2004) not yet tested, or in the SCN (Li et al., 2005), actually controls the negative masking response to light. As indicated above, the rhythmicity of Cry1 and Cry2 double KO mice under LD conditions has been explained as a BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 phenomenon of negative masking because these mice are arrhythmic under DD conditions (Albus et al., 2002; Van der Horst et al., 1999). This issue has also been tested in the standard fashion (1 h light pulse at various levels of illumination). The double KO animals show masking which is no different from the wild-type controls, i.e., they are not more sensitive to the activity suppressing effects of light than the wild-type animals (Mrosovsky, 2001). These mice show bouts of “predark” locomotion the magnitude of which may be related to the prevailing light intensity. These animals may have an altered phase angle of entrainment that requires specific plotting methods to discern (see Fig. 3 in Mrosovsky, 2001). 12. The future Circadian rhythmicity is a pervasive feature of mammalian physiology and behavior. Thus, it should not be surprising that discoveries concerning regulation of the circadian visual system have importance with respect to understanding the workings of the brain and body. In this context, the discovery of melanopsin as a photoreceptive molecule in a subset of ganglion cells is one of the great strides forward in the study of the circadian visual system during the last decade. However, as is already being demonstrated (Dacey et al., 2005), it is unlikely that the importance of these ganglion cells lies exclusively with photic regulation of circadian rhythmicity. The melanopsin cells of the retina are likely to project widely in the visual system and influence most, if not all, retinorecipient regions. It is clear that the visual regulation of circadian rhythms is influenced by the classical visual photoreceptors as well as the intrinsically photoreceptive ganglion cells. There is an opportunity for the circadian rhythm field to contribute to understanding of retinal function by establishing the anatomical and functional relationships between rods, cones and melanopsin cells with respect to rhythm response to light. The most obvious influence of circadian rhythm research on the understanding of normal bodily function may be derived from the large amount of research (most of which has not been considered by this review) concerned with the molecular regulation of clock-like activity in the SCN. These studies have discovered a new window into the exploration of mammalian physiology through the demonstration of oscillating clock genes in peripheral tissues. While not necessarily circadian oscillations themselves (but see Yoo et al., 2004 to the contrary), under normal conditions, the genes do oscillate with many of the same characteristics as those in the SCN (Yagita et al., 2001). The widespread presence of oscillating presumptive clock genes undoubtedly has enormous ramifications for the normal functioning of organs as well as in disease states. Within the SCN, systematic exploration of the relationship between cell phenotype and function has been initiated. Part of this effort has focused on cell level analysis of gene expression. Another has sought to describe the rhythmic activity of particular cell types. Both have been directed, at least partially, toward the intrinsic organization of the whole SCN. The overall research effort has barely addressed a small fraction of the cell 43 types and already there is typical scientific confusion with one method showing an unexpected absence of rhythmicity in certain cells (Hamada et al., 2001), while another method demonstrates an unexpected form of rhythmicity in the same type of cell (Hamada et al., 2003). This topic is rich and likely to contribute substantially to such issues as the bimodal rhythmicity observed in horizontal slices through the hamster SCN, function-specific rhythm regulation and the general problem of specifying which cells are pacemakers. Research focused on the IGL component of the circadian visual system has refined the view that it contributes to both photic and nonphotic regulation of rhythmicity. However, the research on IGL connectivity has pointed toward a function for this nucleus that may extend well beyond its role in circadian rhythm regulation. In particular, the IGL appears to have widespread, bilateral and often reciprocal connections with brain regions involved in the regulation of sleep, visuomotor and vestibular system activities. The nature of the functional relationships among these systems remains to be seen, as is the circadian system role of the IGL with respect to their regulation. The serotonergic system influence on rhythm regulation has been substantially elucidated, but large parts of that influence remain a mystery. One characteristic, in particular, is compelling and not yet understood. Certain manipulations of the serotonergic system contribute to very large, rapid phase shifts to photic stimuli. How the circadian clock can overcome the inertia of a stable, entrained system is not understood, but the presence of such large shifts gives hope that there may be forthcoming manipulations that can reliably overcome the detrimental effects of jet lag or modify the severity of timing disorders such as advanced sleep phase syndrome. The last decade of research on the circadian visual system has been extremely exciting and productive. The current decade will see this trend continue unabated with a much enhanced recognition of the contributions made by investigators in the field of circadian visual system research to the understanding of general brain function and the normal physiology of bodily systems. Acknowledgments Supported by NIH grants NS22168 and MH64471 and National Space Biomedical Research Institute grant HPF0027 via Cooperative Agreement NCC9-58 with the National Aeronautics and Space Administration (to LPM) and NIH grants NS36607 and NS40782 (to CNA). REFERENCES Abe, H., Rusak, B., 1992. Stimulation of the hamster ventral lateral geniculate nucleus induces Fos-like immunoreactivity in suprachiasmatic nucleus cells. Neurosci. Lett. 148, 185–189. Abe, H., Rusak, B., Robertson, H.A., 1992. NMDA and non-NMDA receptor antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res. Bull. 28, 831–835. Abe, H., Honma, S., Shinohara, K., Honma, K., 1996. Substance P receptor regulates the photic induction of Fos-like protein in 44 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 the suprachiasmatic nucleus of Syrian hamsters. Brain Res. 708, 135–142. Abe, M., Herzog, E.D., Block, G.D., 2000. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. NeuroReport 11, 3261–3264. Abrahamson, E.E., Moore, R.Y., 2001. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 916, 172–191. Abrahamson, E.E., Leak, R.K., Moore, R.Y., 2001. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. NeuroReport 12, 435–440. Aggelopoulos, N.C., Meissl, H., 2000. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J. Physiol. 523, 211–222. Agostino, P.V., Ferreyra, G.A., Murad, A.D., Watanabe, Y., Golombek, D.A., 2004. Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem. Int. 44, 617–625. Aguilar-Roblero, R., Morin, L.P., Moore, R.Y., 1994. Morphological correlates of circadian rhythm restoration induced by transplantation of the suprachiasmatic nucleus in hamsters. Exp. Neurol. 130, 250–260. Aida, R., Moriya, T., Araki, M., Akiyama, M., Wada, K., Wada, E., Shibata, S., 2002. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol. Pharmacol. 61, 26–34. Aioun, J., Chambille, I., Peytevin, J., Martinet, L., 1998. Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res. 291, 239–253. Akasu, T., Shoji, S., Hasuo, H., 1993. Inward rectifier and low-threshold calcium currents contribute to the spontaneous firing mechanism in neurons of the rat suprachiasmatic nucleus. Pflugers Arch. 425, 109–116. Albers, H.E., Ferris, C.F., 1984. Neuropeptide Y: role in light–dark entrainment of hamster circadian rhythms. Neurosci. Lett. 50, 163–168. Albers, H.E., Ferris, C.F., Leeman, S.E., Goldman, B.D., 1984. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science 223, 833–835. Albers, H.E., Liou, S.-Y., Stopa, E.G., Zoeller, R.T., 1991. Interaction of colocalized neuropeptides: functional significance in the circadian timing system. J. Neurosci. 11, 846–851. Albus, H., Bonnefont, X., Chaves, I., Yasui, A., Doczy, J., Van der Horst, G.T.J., Meijer, J.H., 2002. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr. Biol. 12, 1130–1133. Alleva, J.J., Waleski, M.V., Alleva, F.R., 1971. A biological clock controlling the estrous cycles of the hamster. Endocrinology 88, 1368–1379. Altman, J., Bayer, S.A., 1979a. Development of the diencephalon in the rat: V. Thymidine-radiographic observations on internuclear and intranuclear gradients in the thalamus. J. Comp. Neurol. 188, 473–500. Altman, J., Bayer, S.A., 1979b. Development of the diencephalon in the rat: IV. Quantitative study of the time of origin of neurons and the internuclear chronological gradients in the thalamus. J. Comp. Neurol. 188, 455–472. Amir, S., 1992. Blocking NMDA receptors or nitric oxide production disrupts light transmission to the suprachiasmatic nucleus. Brain Res. 586, 336–339. Amir, S., Edelstein, K., 1997. A blocker of nitric oxide synthase, NG-nitro-L-arginine methyl ester, attenuates light-induced Fos protein expression in rat suprachiasmatic nucleus. Neurosci. Lett. 224, 29–32. Antle, M.C., Mistlberger, R.E., 2000. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neurosci. 20, 9326–9332. Antle, M.C., Marchant, E.G., Niel, L., Mistlberger, R.E., 1998. Serotonin antagonists do not attenuate activity-induced phase shifts of circadian rhythms in the Syrian hamster. Brain Res. 813, 139–149. Antle, M.C., Glass, J.D., Mistlberger, R.E., 2000. 5-HT1A autoreceptor antagonist-induced 5-HT release in the hamster suprachiasmatic nuclei: effects on circadian clock resetting. Neurosci. Lett. 282, 97–100. Antle, M.C., Foley, D.K., Foley, N.C., Silver, R., 2003a. Gates and oscillators: a network model of the brain clock. J. Biol. Rhythms 18, 339–350. Antle, M.C., Ogilvie, M.D., Pickard, G.E., Mistlberger, R.E., 2003b. Response of the mouse circadian system to serotonin 1A/2/7 agonists in vivo: surprisingly little. J. Biol. Rhythms 18, 145–158. Antle, M.C., Kriegsfeld, L.J., Silver, R., 2005. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J. Neurosci. 25, 2447–2454. Artinian, L.R., Ding, J.M., Gillette, M.U., 2001. Carbon monoxide and nitric oxide: interacting messengers in muscarinic signaling to the brain's circadian clock. Exp. Neurol. 171, 293–300. Arvanitogiannis, A., Amir, S., 1999. Resetting the rat circadian clock by ultra-short light flashes. Neurosci. Lett. 261, 159–162. Arvanitogiannis, A., Robinson, B., Beaulé, C., Amir, S., 2000. Calbindin-D28k immunoreactivity in the suprachiasmatic nucleus and the circadian response to constant light in the rat. Neuroscience 99, 397–401. Aston-Jones, G., Chen, S., Zhu, Y., Oshinsky, M.L., 2001. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 4, 732–738. Aton, S.J., Colwell, C.S., Harmar, A.J., Waschek, J., Herzog, E.D., 2005. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 8, 476–483. Aujard, F., Herzog, E.D., Block, G.D., 2001. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106, 255–261. Azmitia, E.C., Segal, M., 1978. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641–668. Bamshad, M., Aoki, V.T., Adkison, M.G., Warren, W.S., Bartness, T.J., 1998. Central nervous system origins of the sympathetic outflow to white adipose tissue. Am. J. Physiol. 275, R291–R299. Bamshad, M., Song, C.K., Bartness, T.J., 1999. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 276, R1569–R1578. Barakat, M.T., O'Hara, B.F., Cao, V.H., Larkin, J.E., Heller, H.C., Ruby, N.F., 2004. Light pulses do not induce c-fos or per1 in the SCN of hamsters that fail to reentrain to the photocycle. J. Biol. Rhythms 19, 287–297. Bartness, T.J., Song, C.K., Demas, G.E., 2001. SCN efferents to peripheral tissues: implications for biological rhythms. J. Biol. Rhythms 16, 196–204. Baslow, M.H., 2000. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J. Neurochem. 75, 453–459. Battaglia, G., Sharkey, J., Kuhar, M.J., de Souza, E.B., 1991. Neuroanatomic specificity and time course of alterations in rat brain serotonergic pathways induced by MDMA (3,4-methylenedioxymethamphetamine): assessment using quantitative autoradiography. Synapse 8, 249–260. Beaulé, C., Amir, S., 2002. Effect of 192 IgG-saporin on circadian activity rhythms, expression of p75 neurotrophin receptors, BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 calbindin-D28K, and light-induced fos in the suprachiasmatic nucleus in rats. Exp. Neurol. 176, 377–389. Beaulé, C., Arvanitogiannis, A., Amir, S., 2001a. Entrainment to a discrete light pulse at dawn is associated with acute suppression of FOS in the shell region of the suprachiasmatic nucleus. Soc. Neurosci. (abstract 182.6). Beaulé, C., Arvanitogiannis, A., Amir, S., 2001b. Light suppresses fos expression in the shell region of the suprachiasmatic nucleus at dusk and dawn: implications for photic entrainment of circadian rhythms. Neuroscience 106, 249–254. Beaule, C., Houle, L.M., Amir, S., 2003a. Expression profiles of PER2 immunoreactivity within the shell and core regions of the rat suprachiasmatic nucleus: lack of effect of photic entrainment and disruption by constant light. J. Mol. Neurosci. 21, 133–147. Beaulé, C., Robinson, B., Lamont, E.W., Amir, S., 2003b. Melanopsin in the circadian timing system. J. Mol. Neurosci. 21, 73–89. Belenky, M.A., Pickard, G.E., 2001. Subcellular distribution of 5-HT (1B) and 5-HT(7) receptors in the mouse suprachiasmatic nucleus. J. Comp. Neurol. 432, 371–388. Belenky, M.A., Smeraski, C.A., Provencio, I., Sollars, P.J., Pickard, G.E., 2003. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J. Comp. Neurol. 460, 380–393. Bergstrom, A.L., Hannibal, J., Hindersson, P., Fahrenkrug, J., 2003. Light-induced phase shift in the Syrian hamster (Mesocricetus auratus) is attenuated by the PACAP receptor antagonist PACAP6-38 or PACAP immunoneutralization. Eur. J. Neurosci. 18, 2552–2562. Berson, D.M., Dunn, F.A., Takao, M., 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. Best, J.D., Maywood, E.S., Smith, K.L., Hastings, M.H., 1999. Rapid resetting of the mammalian circadian clock. J. Neurosci. 19, 828–835. Biello, S.M., 1995. Enhanced photic phase shifting after treatment with antiserum to neuropeptide Y. Brain Res. 673, 25–29. Biello, S.M., Dafters, R.I., 2001. MDMA and fenfluramine alter the response of the circadian clock to a serotonin agonist in vitro. Brain Res. 920, 202–209. Biello, S.M., Mrosovsky, N., 1993. Circadian phase-shifts induced by chlordiazepoxide without increased locomotor activity. Brain Res. 622, 58–62. Biello, S.M., Mrosovsky, N., 1995. Blocking the phase-shifting effect of neuropeptide Y with light. Proc. R. Soc. Lond., B Biol. Sci. 259, 179–187. Biello, S.M., Janik, D., Mrosovsky, N., 1994. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience 62, 273–279. Biello, S.M., Golombek, D.A., Harrington, M.E., 1997a. Neuropeptide Y and glutamate block each other's phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience 77, 1049–1057. Biello, S.M., Golombek, D.A., Schak, K.M., Harrington, M.E., 1997b. Circadian phase shifts to neuropeptide Y in vitro: cellular communication and signal transduction. J. Neurosci. 17, 8468–8475. Bina, K.G., Rusak, B., 1996. Muscarinic receptors mediate carbachol-induced phase shifts of circadian activity rhythms in Syrian hamsters. Brain Res. 743, 202–211. Bina, K.G., Rusak, B., Semba, K., 1993. Localization of cholinergic neurons in the forebrain and brainstem that project to the suprachiasmatic nucleus of the hypothalamus in rat. J. Comp. Neurol. 335, 295–307. Bina, K.G., Rusak, B., Semba, K., 1997. Sources of p75-nerve growth factor receptor-like immunoreactivity in the rat suprachiasmatic nucleus. Neuroscience 77, 461–472. Bittman, E.L., Goldman, B.D., 1979. Serum levels of gonadotrophins in hamsters exposed to short photoperiods: effects of adrenalectomy and ovariectomy. J. Endocrinol. 83, 113–118. Blasiak, T., Lewandowski, M.H., 2003. Dorsal raphe nucleus 45 modulates neuronal activity in rat intergeniculate leaflet. Behav. Brain Res. 138, 179–185. Blasiak, T., Lewandowski, M.H., 2004a. Blockade of GABAA receptors disrupts isoperiodic neuronal oscillations in the intergeniculate leaflet of the rat. Brain Res. 1009, 82–87. Blasiak, A., Lewandowski, M.H., 2004b. In vitro extracellular recording of spontaneous activity of the intergeniculate leaflet neurons. Brain Res. 1015, 82–86. Bobrzynska, K.J., Vrang, N., Mrosovsky, N., 1996. Persistence of nonphotic phase shifts in hamsters after serotonin depletion in the suprachiasmatic nucleus. Brain Res. 741, 205–214. Boer, G.J., van Esseveldt, K.E., van der Geest, B.A., Duindam, H., Rietveld, W.J., 1999. Vasopressin-deficient suprachiasmatic nucleus grafts re-instate circadian rhythmicity in suprachiasmatic nucleus-lesioned arrhythmic rats. Neuroscience 89, 375–385. Bos, N.P.A., Mirmiran, M., 1993. Effects of excitatory and inhibitory amino acids on neuronal discharges in the cultured suprachiasmatic nucleus. Brain Res. Bull. 31, 67–72. Botchkina, G.I., Morin, L.P., 1993. Development of the hamster serotoninergic system: cell groups and diencephalic projections. J. Comp. Neurol. 338, 405–431. Botchkina, G.I., Morin, L.P., 1995. Specialized neuronal and glial contributions to development of the hamster lateral geniculate complex and circadian visual system. J. Neurosci. 15, 190–201. Boulos, Z., Morin, L.P., 1985. Entrainment of split circadian activity rhythms in hamsters. J. Biol. Rhythms 1, 1–15. Boulos, Z., Rusak, B., 1982. Circadian phase response curves for dark pulses in the hamster. J. Comp. Physiol. 146, 411–417. Bouskila, Y., Dudek, F.E., 1995. A rapidly activating type of outward rectifier K+ current and A-current in rat suprachiasmatic nucleus neurones. J. Physiol. 488 (Pt. 2), 339–350. Bradbury, M.J., Dement, W.C., Edgar, D.M., 1997. Serotonin-containing fibers in the suprachiasmatic hypothalamus attenuate light-induced phase delays in mice. Brain Res. 768, 125–134. Bramley, J.R., Sollars, P.J., Pickard, G.E., Dudek, F.E., 2005. 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. J. Neurophysiol. 93, 3157–3164. Bryant, D.N., LeSauter, J., Silver, R., Romero, M.T., 2000. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J. Biol. Rhythms 15, 103–111. Buijs, R.M., Chun, S.J., Niijima, A., Romijn, H.J., Nagai, K., 2001. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 431, 405–423. Burgoon, P.W., Lindberg, P.T., Gillette, M.U., 2004. Different patterns of circadian oscillation in the suprachiasmatic nucleus of hamster, mouse, and rat. J. Comp. Physiol., A 190, 167–171. Byku, M., Gannon, R.L., 2000a. SNC 80, a delta-opioid agonist, elicits phase advances in hamster circadian activity rhythms. NeuroReport 11, 1449–1452. Byku, M., Gannon, R.L., 2000b. Opioid induced non-photic phase shifts of hamster circadian activity rhythms. Brain Res. 873, 189–196. Byku, M., Legutko, R., Gannon, R.L., 2000. Distribution of δ opioid receptor immunoreactivity in the hamster suprachiasmatic nucleus and intergeniculate leaflet. Brain Res. 857, 1–7. Cagampang, F.R., Piggins, H.D., Sheward, W.J., Harmar, A.J., Coen, C.W., 1998a. Circadian changes in PACAP type 1 (PAC1) receptor mRNA in the rat suprachiasmatic and supraoptic nuclei. Brain Res. 813, 218–222. Cagampang, F.R., Sheward, W.J., Harmar, A.J., Piggins, H.D., Coen, C.W., 1998b. Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Brain Res. Mol. Brain Res. 54, 108–112. 46 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 Caillol, M., Devinoy, E., Lacroix, M.C., Schirar, A., 2000. Endothelial and neuronal nitric oxide synthases are present in the suprachiasmatic nuclei of Syrian hamsters and rats. Eur. J. Neurosci. 12, 649–661. Canbeyli, R.S., Lehman, M.N., Silver, R., 1991. Tracing SCN graft efferents with Dil. Brain Res. 554, 15–21. Card, J.P., Moore, R.Y., 1982. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J. Comp.Neurol. 206, 390–396. Card, J.P., Moore, R.Y., 1984. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience 13, 390–396. Card, J.P., Moore, R.Y., 1989. Organization of lateral geniculate–hypothalamic connections in the rat. J. Comp. Neurol. 284, 135–147. Card, J.P., Brecha, N., Moore, R.Y., 1983. Immunohistochemical localization of avian pancreatic polypeptide-like immunoreactivity in the rat hypothalamus. J. Comp. Neurol. 217, 123–136. Cassone, V.M., Speh, J.C., Card, J.P., 1988. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J. Biol. Rhythms 3, 71–91. Castel, M., Morris, J.F., 2000. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain's circadian pacemaker. J. Anat. 196, 1–13. Castel, M., Belenky, M., Cohen, S., Ottersen, O.P., Storm-Mathisen, J., 1993. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 5, 368–381. Challet, E., Naylor, E., Metzger, J.M., MacIntyre, D.E., Turek, F.W., 1998a. An NK1 receptor antagonist affects the circadian regulation of locomotor activity in golden hamsters. Brain Res. 800, 32–39. Challet, E., Scarbrough, K., Penev, P.D., Turek, F., 1998b. Roles of suprachiasmatic nuclei and intergeniculate leaflets in mediating the phase-shifting effects of a serotonergic agonist and their photic modulation during subjective day. J. Biol. Rhythms 12, 410–421. Challet, E., Dugovic, C., Turek, F.W., Olivier, V.R., 2001. The selective neurokinin 1 receptor antagonist R116301 modulates photic responses of the hamster circadian system. Neuropharmacology 40, 408–415. Chambille, I., Doyle, S., Servière, J., 1993. Photic induction and circadian expression of Fos-like protein. Immunohistochemical study in the retina and suprachiasmatic nuclei of hamster. Brain Res. 612, 138–150. Chen, D., Hurst, W.J., Ding, J.M., Faiman, L.E., Mayer, B., Gillette, M.U., 1997. Localization and characterization of nitric oxide synthase in the rat suprachiasmatic nucleus: evidence for a nitrergic plexus in the biological clock. J. Neurochem. 68, 855–861. Chen, D., Buchanan, G.F., Ding, J.M., Hannibal, J., Gillette, M.U., 1999. Pituitary adenylyl cyclase-activating peptide: a pivotal modulator of glutamatergic regulation of the suprachiasmatic circadian clock. Proc. Natl. Acad. Sci. U. S. A. 96, 13468–13473. Chevassus, A.L., Cooper, H.M., 1998. Is there a geniculohypothalamic tract in primates? A comparative immunohistochemical study in the circadian system of strepsirhine and haplorhine species. Brain Res. 805, 213–219. Christian, C.A., Harrington, M.E., 2002. Three days of novel wheel access diminishes light-induced phase delays in vivo with no effect on per1 induction by light. Chronobiol. Int. 19, 671–682. Cloues, R.K., Sather, W.A., 2003. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J. Neurosci. 23, 1593–1604. Colbron, S., Jones, M., Biello, S.M., 2002. MDMA alters the response of the circadian clock to a photic and non-photic stimulus. Brain Res. 956, 45–52. Colwell, C.S., 2000. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur. J. Neurosci. 12, 571–576. Colwell, C.S., Menaker, M., 1992. NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. J. Biol. Rhythms 7, 125–136. Colwell, C.S., Ralph, M.R., Menaker, M., 1990. Do NMDA receptors mediate the effects of light on circadian behavior. Brain Res. 523, 117–120. Colwell, C.S., Foster, R.G., Menaker, M., 1991. NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res. 554, 105–110. Colwell, C.S., Kaufman, C.M., Menaker, M., 1993a. Photic induction of Fos in the hamster suprachiasmatic nucleus is inhibited by baclofen but not by diazepam or bicucullin. Neurosci. Lett. 163, 177–181. Colwell, C.S., Kaufman, C.M., Menaker, M., Ralph, M.R., 1993b. Light-induced phase shifts and Fos expression in the hamster circadian system: the effects of anesthetics. J. Biol. Rhythms 8, 179–188. Colwell, C.S., Kaufman, C.M., Menaker, M., 1993c. Phase-shifting mechanisms in the mammalian circadian system: new light on the carbachol paradox. J. Neurosci. 13, 1454–1459. Colwell, C.S., Michel, S., Itri, J., Rodriguez, W., Tam, J., Lelievre, V., Hu, Z., Liu, X., Waschek, J.A., 2003. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 285, R939–R949. Colwell, C.S., Michel, S., Itri, J., Rodriguez, W., Tam, J., Lelievre, V., Hu, Z., Waschek, J.A., 2004. Selective deficits in the circadian light response in mice lacking PACAP. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 287, R1194–R1201. Coogan, A.N., Rawlings, N., Luckman, S.M., Piggins, H.D., 2001. Effects of neurotensin on discharge rates of rat suprachiasmatic nucleus neurons in vitro. Neuroscience 103, 663–672. Cooper, H.M., Parvopassu, F., Herbin, M., Magnin, M., 1994. Neuroanatomical pathways linking vision and olfaction in mammals. Psychoneuroendocrinology 19, 623–639. Coyle, J.T., 1997. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol. Dis. 4, 231–238. Crill, W.E., 1996. Persistent sodium current in mammalian central neurons. Annu. Rev. Physiol. 58, 349–362. Cutler, D.J., Munekawa, K., Mason, R., 1999. Electrophysiological effects of opioid receptor activation on Syrian hamster suprachiasmatic nucleus neurones in vitro. Brain Res. Bull. 50, 119–125. Cutler, D.J., Hardin, H., Rees, H., Maywood, E., Hastings, M.H., Shena, H.M., Martens, J.M., Harrer, C., Piggins, H.D., 2002. The mouse VPAC2 receptor confers SCN cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide (VIP) in vitro. Soc. Res. Biol. Rhythms 8, 37. Cutler, D.J., Haraura, M., Reed, H.E., Shen, S., Sheward, W.J., Morrison, C.F., Marston, H.M., Harmar, A.J., Piggins, H.D., 2003. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur. J. Neurosci. 17, 197–204. Dacey, D.M., Liao, H.W., Peterson, B.B., Robinson, F.R., Smith, V.C., Pokorny, J., Yau, K.W., Gamlin, P.D., 2005. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754. Daikoku, S., Hisano, S., Kagotani, Y., 1992. Neuronal associations in the rat suprachiasmatic nucleus demonstrated by immunoelectron microscopy. J. Comp. Neurol. 325, 559–571. Dardente, H., Poirel, V.J., Klosen, P., Pevet, P., Masson-Pevet, M., 2002. Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res. 958, 261–271. BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 Decker, K., Reuss, S., 1994. Nitric oxide-synthesizing neurons in the hamster suprachiasmatic nucleus: a combined NOS- and N. Brain Res. 666, 284–288. De Jeu, M., Pennartz, C.M.A., 1997. Functional characterization of the H-current in SCN neurons in subjective day and night: a whole-cell patch-clamp study in acutely prepared brain slices. Brain Res. 767, 72–80. De Jeu, M., Pennartz, C.M.A., 2002. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J. Neurophysiol. 87, 834–844. De Jeu, M., Hermes, N., Pennartz, C.M.A., 1998. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. NeuroReport 9, 3725–3729. De Jeu, M., Geurtsen, A., Pennartz, C.M.A., 2002. A Ba(2+)-sensitive K(+) current contributes to the resting membrane potential of neurons in rat suprachiasmatic nucleus. J. Neurophysiol. 88, 349–878. De la Iglesia, H.O., Schwartz, W.J., 2002. A subpopulation of efferent neurons in the mouse suprachiasmatic nucleus is also light responsive. NeuroReport 13, 857–860. De la Iglesia, H.O., Blaustein, J.D., Bittman, E.L., 1995. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. NeuroReport 6, 1715–1722. De la Iglesia, H.O., Meyer, J., Carpino Jr., A., Schwartz, W.J., 2000. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290, 799–801. De la Iglesia, H.O., Meyer, J., Schwartz, W.J., 2003. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J. Neurosci. 23, 7412–7414. De Vries, M.J., Meijer, J.H., 1991. Aspartate injections into the suprachiasmatic region of the Syrian hamster do not mimic the effects of light on the circadian activity rhythm. Neurosci. Lett. 127, 215–218. De Vries, M.J., Nunes Cardozo, B., Van der Want, J., De Wolf, A., Meijer, J.H., 1993. Glutamate immunoreactivity in terminals of the retinohypothalamic tract of the brown Norwegian rat. Brain Res. 612, 231–237. Devries, M.J., Lakke, E.J.F., 1995. Retrograde labeling of retinal ganglion-cells and brain neuronal subsets by [H-3]D-aspartate injection in the Syrian-hamster hypothalamus. Brain Res. Bull. 38, 349–354. Ding, J.M., Chen, D., Weber, E.T., Faiman, L.E., Rea, M.A., Gillette, M.U., 1994. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266, 1713–1717. Ding, J.M., Faiman, L.E., Hurst, W.J., Kuriashkina, L.R., Gillette, M.U., 1997. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J. Neurosci. 17, 667–675. Dkhissi-Benyahya, O., Sicard, B., Cooper, H.M., 2000. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity. J. Neurosci. 20, 7790–7797. Doan, A., Urbanski, H.F., 1994. Diurnal expression of Fos in luteinizing hormone-releasing hormone neurons of Syrian hamsters. Biol. Reprod. 50, 301–308. Donaldson, J.A., Stephan, F.K., 1982. Entrainment of circadian rhythms: retinofugal pathways and unilateral suprachiasmatic nucleus lesions. Physiol. Behav. 29, 1161–1169. Dudley, T.E., DiNardo, L.A., Glass, J.D., 1998. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J. Neurosci. 18, 5045–5052. Dudley, T.E., DiNardo, L.A., Glass, J.D., 1999. In vivo assessment of the midbrain raphe nuclear regulation of serotonin release in the hamster suprachiasmatic nucleus. J. Neurophysiol. 81, 1468–1477. Duncan, M.J., Short, J., Wheeler, D.L., 1999. Comparison of the 47 effects of aging on 5-HT7 and 5-HT1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res. 829, 39–45. Duncan, M.J., Jennes, L., Jefferson, J.B., Brownfield, M.S., 2000. Localization of serotonin5A receptors in discrete regions of the circadian timing system in the Syrian hamster. Brain Res. 869, 178–185. Duncan, M.J., Grear, K.E., Hoskins, M.A., 2004. Aging and SB-269970-A, a selective 5-HT7 receptor antagonist, attenuate circadian phase advances induced by microinjections of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 1008, 40–48. Dwyer, S.M., Rosenwasser, A.M., 2000. Effects of light intensity and restraint on dark-pulse-induced circadian phase shifting during subjective night in Syrian hamsters. J. Biol. Rhythms 15, 491–500. Ebling, F.J.P., 1996. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog. Neurobiol. 50, 109–123. Edelstein, K., Amir, S., 1998. Glutamatergic antagonists do not attenuate light-induced Fos protein in rat intergeniculate leaflet. Brain Res. 810, 264–268. Edelstein, K., Amir, S., 1999a. The intergeniculate leaflet does not mediate the disruptive effects of constant light on circadian rhythms in the rat. Neuroscience 90, 1093–1101. Edelstein, K., Amir, S., 1999b. The role of the intergeniculate leaflet in entrainment of circadian rhythms to a skeleton photoperiod. J. Neurosci. 19, 372–380. Edelstein, K., Mrosovsky, N., 2001. Behavioral responses to light in mice with dorsal lateral geniculate lesions. Brain Res. 918, 107–112. Edelstein, K., De la Iglesia, H.O., Schwartz, W.J., Mrosovsky, N., 2003a. Behavioral arousal blocks light-induced phase advances in locomotor rhythmicity but not light-induced Per1 and fos expression in the hamster suprachiasmatic nucleus. Neuroscience 118, 253–261. Edelstein, K., De la Iglesia, H.O., Mrosovsky, N., 2003b. Period gene expression in the suprachiasmatic nucleus of behaviorally decoupled hamsters. Brain Res. Mol. Brain Res. 114, 40–45. Edgar, D.M., Reid, M.S., Dement, W.C., 1997. Serotonergic afferents mediate activity-dependent entrainment of the mouse circadian clock. Am. J. Physiol. 273, R265–R269. Ehlen, J.C., Grossman, G.H., Glass, J.D., 2001. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J. Neurosci. 21, 5351–5357. Elliott, J.A., Stetson, M.H., Menaker, M., 1972. Regulation of testis function in golden hamsters: a circadian clock measures photoperiodic time. Science 178, 771–773. Enaker, M., Roberts, R., Elliott, J., Underwood, H., 1970. Extraretinal light perception in the sparrow: 3. The eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. U. S. A. 67, 320–325. Erhardt, C., Galani, R., Jeltsch, H., Cassel, J.C., Klosen, P., Menet, J.S., Pevet, P., Challet, E., 2004. Modulation of photic resetting in rats by lesions of projections to the suprachiasmatic nuclei expressing p75 neurotrophin receptor. Eur. J. Neurosci. 19, 1773–1788. Erlander, M.G., Tillakaratne, N.J., Feldblum, S., Patel, N., Tobin, A.J., 1991. Two genes encode distinct glutamate decarboxylases. Neuron 7, 91–100. Fedorkova, L., Rutishauser, U., Prosser, R., Shen, H., Glass, J.D., 2002. Removal of polysialic acid from the SCN potentiates nonphotic circadian phase resetting. Physiol. Behav. 77, 361–369. Ferguson, S.A., Kennaway, D.J., 1999. Emergence of altered circadian timing in a cholinergically supersensitive rat line. Am. J. Physiol. 277, R1171–R1178. Ferguson, S.A., Kennaway, D.J., Moyer, R.W., 1999. Nicotine phase shifts the 6-sulphatoxymelatonin rhythm and induces c-Fos in the SCN of rats. Brain Res. Bull. 48, 527–538. 48 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 Ferreyra, G.A., Cammarota, M.P., Golombek, D.A., 1998. Photic control of nitric oxide synthase activity in the hamster suprachiasmatic nuclei. Brain Res. 797, 190–196. Field, M.D., Maywood, E.S., O'Brien, J.A., Weaver, D.R., Reppert, S.M., Hastings, M.H., 2000. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 25, 437–447. Fite, K.V., Janusonis, S., 2001. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus). Brain Res. 895, 139–145. Fite, K.V., Janusonis, S., Foote, W., Bengston, L., 1999. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J. Comp. Neurol. 414, 469–484. Fite, K.V., Birkett, M.A., Smith, A., Janusonis, S.F., McLaughlin, S., 2003. Retinal ganglion cells projecting to the dorsal raphe and lateral geniculate complex in Mongolian gerbils. Brain Res. 973, 146–150. Foote, W.E., Taber-Pierce, E., Edwards, L., 1978. Evidence for a retinal projection to the midbrain raphe of the cat. Brain Res. 156, 135–140. Foster, R.G., Provencio, I., Hudson, D., Fiske, S., De Grip, W., Menaker, M., 1991. Circadian photoreception in the retinally degenerate mouse (rd/rd). J. Comp. Physiol., A 169, 39–50. Foster, R.G., Provencio, I., Bovee-Geurts, P.H., DeGrip, W.J., 2003. The photoreceptive capacity of the developing pineal gland and eye of the golden hamster (Mesocricetus auratus). J. Neuroendocrinol. 15, 355–363. Freedman, M.S., Lucas, R.J., Soni, B., Von Schantz, M., Munoz, M., David-Gray, Z., Foster, R.G., 1999. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504. Freeman, M.E., Kanyicska, B., Lerant, A., Nagy, G., 2000. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80, 1523–1631. Fujiyama, F., Hioki, H., Tomioka, R., Taki, K., Tamamaki, N., Nomura, S., Okamoto, K., Kaneko, T., 2003. Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. J. Comp. Neurol. 465, 234–249. Fukuhara, C., Brewer, J.M., Dirden, J.C., Bittman, E.L., Tosini, G., Harrington, M.E., 2001. Neuropeptide Y rapidly reduces Period 1 and Period 2 mRNA levels in the hamster suprachiasmatic nucleus. Neurosci. Lett. 314, 119–122. Gamble, K.L., Novak, C.M., Paul, K.N., Albers, H.E., 2003. Tetrodotoxin blocks the circadian effects of NMDA during the day but not at night. NeuroReport 14, 641–644. Gamble, K.L., Novak, C.M., Albers, H.E., 2004. Neuropeptide Y and N-methyl-D-aspartic acid interact within the suprachiasmatic nuclei to alter circadian phase. Neuroscience 126, 559–565. Gannon, R.L., 2001. 5HT7 receptors in the rodent suprachiasmatic nucleus. J. Biol. Rhythms 16, 19–24. Gannon, R.L., 2003. Serotonergic serotonin1a mixed agonists/ antagonists elicit large-magnitude phase shifts in hamster circadian wheel-running rhythms. Neuroscience 119, 567–576 (Ref Type: Generic). Gaston, S., Menaker, M., 1968. Pineal function: the biological clock in the sparrow? Science 160, 1125–1127. Gerfen, C.R., Sawchenko, P.E., 1984. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 290, 219–238. Geusz, M.E., Fletcher, C., Block, G.D., Straume, M., Copeland, N.G., Jenkins, N.A., Kay, S.A., Day, R.N., 1997. Long-term monitoring of circadian rhythms in c-fos gene expression from suprachiasmatic nucleus cultures. Curr. Biol. 7, 758–766. Gillespie, C.F., Huhman, K.L., Babagbemi, T.O., Albers, H.E., 1996. Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J. Biol. Rhythms 11, 137–144. Gillespie, C.F., Mintz, E.M., Marvel, C.L., Huhman, K.L., Albers, H.E., 1997. GABA(A) and GABA(B) agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res. 759, 181–189. Gillespie, C.F., Van der Beek, E.M., Mintz, E.M., Mickley, N.C., Jasnow, A.M., Huhman, K.L., Albers, H.E., 1999. GABAergic regulation of light-induced c-Fos immunoreactivity within the suprachiasmatic nucleus. J. Comp. Neurol. 411, 683–692. Gillette, M.U., 1991. SCN electrophysiology in vitro: rhythmic activity and endogenous clock properties. In: Klein, D.C., Moore, R.Y., Reppert, S.M. (Eds.), Suprachiasmatic Nucleus: The Mind's Clock. Oxford Univ. Press, New York, pp. 125–143. Gillette, M.U., DeMarco, S.J., Ding, J.M., Gallman, E.A., Faiman, L.E., Liu, C., McArthur, A.J., Medanic, M., Richard, D., Tcheng, T.K., Weber, E.T., 1993. The organization of the suprachiasmatic circadian pacemaker of the rat and its regulation by neurotransmitters and modulators. J. Biol. Rhythms 8, S53–S58. Gillette, M.U., Buchanan, G.F., Artinian, L., Hamilton, S.E., Nathanson, N.M., Liu, C., 2001. Role of the M1 receptor in regulating circadian rhythms. Life Sci. 68, 2467–2472. Ginty, D.D., Kornhauser, J.M., Thompson, M.A., Bading, H., Mayo, K.E., Takahashi, J.S., Greenberg, M.E., 1993. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260, 238–241. Glass, J.D., Chen, L., 1999. Serotonergic modulation of astrocytic activity in the hamster suprachiasmatic nucleus. Neuroscience 94, 1253–1259. Glass, J.D., Hauser, U.E., Blank, J.L., Selim, M., Rea, M.A., 1993. Differential timing of amino acid and 5-HIAA rhythms in suprachiasmatic hypothalamus. Am. J. Physiol. 265, R504–R511. Glass, J.D., Lee, W., Shen, H., Watanabe, M., 1994. Expression of immunoreactive polysialylated neural cell adhesion molecule in the suprachiasmatic nucleus. Neuroendocrinology 60, 87–95. Glass, J.D., Shen, H., Fedorkova, L., Chen, L., Tomasiewicz, H., Watanabe, M., 2000a. Polysialylated neural cell adhesion molecule modulates photic signaling in the mouse suprachiasmatic nucleus. Neurosci. Lett. 280, 207–210. Glass, J.D., DiNardo, L.A., Ehlen, J.C., 2000b. Dorsal raphe nuclear stimulation of SCN serotonin release and circadian phase-resetting. Brain Res. 859, 224–232. Glass, J.D., Watanabe, M., Fedorkova, L., Shen, H., Ungers, G., Rutishauser, U., 2003a. Dynamic regulation of polysialylated neural cell adhesion molecule in the suprachiasmatic nucleus. Neuroscience 117, 203–211. Glass, J.D., Grossman, G.H., Farnbauch, L., DiNardo, L., 2003b. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J. Neurosci. 23, 7451–7460. Goel, N., Lee, T.M., Smale, L., 1999. Suprachiasmatic nucleus and intergeniculate leaflet in the diurnal rodent Octodon degus: retinal projections and immunocytochemical characterization. Neuroscience 92, 1491–1509. Gooley, J.J., Saper, C.B., 2003. A broad role for melanopsin in non-visual photoreception based on neuroanatomical evidence in rats. J. Neurosci. 23, 7093–7106. Gooley, J.J., Lu, J., Chou, T.C., Scammell, T.E., Saper, C.B., 2001. Melanopsin in cells of origin of the retinohypothalamic tract. Nat. Neurosci. 4, 1165. Gorman, M.R., Kendall, M., Elliott, J.A., 2005. Scotopic illumination enhances entrainment of circadian rhythms to lengthening light:dark cycles. J. Biol. Rhythms 20, 38–48. Graham, E.S., Turnbull, Y., Fotheringham, P., Nilaweera, K., Mercer, J.G., Morgan, P.J., Barrett, P., 2003. Neuromedin U and Neuromedin U receptor-2 expression in the mouse and rat BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 hypothalamus: effects of nutritional status. J. Neurochem. 87, 1165–1173. Graham, E.S., Littlewood, P., Turnbull, Y., Mercer, J.G., Morgan, P.J., Barrett, P., 2005. Neuromedin-U is regulated by the circadian clock in the SCN of the mouse. Eur. J. Neurosci. 21, 814–819. Gribkoff, V.K., Pieschl, R.L., Wisialowski, T.A., Park, W.K., Strecker, G.J., De Jeu, M.T.G., Pennartz, C.M.A., Dudek, F.E., 1999. A reexamination of the role of GABA in the mammalian suprachiasmatic nucleus. J. Biol. Rhythms 14, 126–130. Grossman, G.H., Mistlberger, R.E., Antle, M.C., Ehlen, J.C., Glass, J.D., 2000. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. NeuroReport 11, 1929–1932. Grossman, G.H., Farnbauch, L., Glass, J.D., 2004. Regulation of serotonin release in the Syrian hamster intergeniculate leaflet region. NeuroReport 15, 103–106. Guido, M.E., Goguen, D., De Guido, L., Robertson, H.A., Rusak, B., 1999. Circadian and photic regulation of immediate-early gene expression in the hamster suprachiasmatic nucleus. Neuroscience 90, 555–571. Haak, L.L., Heller, H.C., van den Pol, A.N., 1997. Metabrotropic glutamate receptor activation modulates kainate and serotonin calcium response in astrocytes. J. Neurosci. 17, 1825–1837. Hamada, T., Yamanouchi, S., Watanabe, A., Shibata, S., Watanabe, S., 1999. Involvement of glutamate release in substance P-induced phase delays of suprachiasmatic neuron activity rhythm in vitro. Brain Res. 836, 190–193. Hamada, T., LeSauter, J., Venuti, J.M., Silver, R., 2001. Expression of Period genes: rhythmic and non-rhythmic compartments of the suprachiasmatic nucleus pacemaker. J. Neurosci. 21, 7742–7750. Hamada, T., LeSauter, J., Lokshin, M., Romero, M.T., Yan, L.L., Venuti, J.M., Silver, R., 2003. Calbindin influences response to photic input in suprachiasmatic nucleus. J. Neurosci. 23, 8820–8826. Hamada, T., Antle, M.C., Silver, R., 2004a. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur. J. Neurosci. 19, 1741–1748. Hamada, T., Antle, M.C., Silver, R., 2004b. The role of Period1 in non-photic resetting of the hamster circadian pacemaker in the suprachiasmatic nucleus. Neurosci. Lett. 362, 87–90. Hanada, R., Teranishi, H., Pearson, J.T., Kurokawa, M., Hosoda, H., Fukushima, N., Fukue, Y., Serino, R., Fujihara, H., Ueta, Y., Ikawa, M., Okabe, M., Murakami, N., Shirai, M., Yoshimatsu, H., Kangawa, K., Kojima, M., 2004. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat. Med. 10, 1067–1073. Hannibal, J., 2002a. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 309, 73–88. Hannibal, J., 2002b. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J. Comp. Neurol. 453, 389–417. Hannibal, J., Fahrenkrug, J., 2002. Immunoreactive substance P is not part of the retinohypothalamic tract in the rat. Cell Tissue Res. 309, 293–299. Hannibal, J., Fahrenkrug, J., 2004. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 316, 99–113. Hannibal, J., Mikkelsen, J.D., Clausen, H., Holst, J.J., Wulff, B.S., Fahrenkrug, J., 1995. Gene expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat hypothalamus. Regul. Pept. 55, 133–148. Hannibal, J., Ding, J.M., Chen, D., Fahrenkrug, J., Larsen, P.J., Gillette, M.U., Mikkelsen, J.D., 1997. Pituitary adenylate cyclaseactivating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J. Neurosci. 17, 2637–2644. Hannibal, J., Moller, M., Ottersen, O.P., Fahrenkrug, J., 2000. PACAP 49 and glutamate are co-stored in the retinohypothalamic tract. J. Comp. Neurol. 418, 147–155. Hannibal, J., Vrang, N., Card, J.P., Fahrenkrug, J., 2001a. Lightdependent induction of cFos during subjective day and night in PACAP-containing ganglion cells of the retinohypothalamic tract. J. Biol. Rhythms 16, 457–470. Hannibal, J., Jamen, F., Nielsen, H.S., Journot, L., Brabet, P., Fahrenkrug, J., 2001b. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J. Neurosci. 21, 4883–4890. Hannibal, J., Hindersson, P., Knudsen, S.M., Georg, B., Fahrenkrug, J., 2002. The photopigment melanopsin is exclusively present in pituitary adenylated cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J. Neurosci. 22, RC191 (1-7). Harmar, A.J., Marston, H.M., Shen, S., Spratt, C., West, K.M., Sheward, W.J., Morrison, C.F., Dorin, J.R., Piggins, H.D., Reubi, J.-C., Kelly, J.S., Maywood, E., Hastings, M.H., 2002. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109, 497–508. Harrington, M.E., 1997. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci. Biobehav. Rev. 21, 705–727. Harrington, M.E., Rusak, B., 1986. Lesions of the thalamic intergeniculate leaflet alter hamster circadian rhythms. J. Biol. Rhythms 1, 309–325. Harrington, M.E., Schak, K.M., 2000. Neuropeptide Y phase advances the in vitro hamster circadian clock during the subjective day with no effect on phase during the subjective night. Can. J. Physiol. Pharm. 78, 87–92. Harrington, M.E., Hoque, S., Hall, A., Golombek, D.A., Biello, S., 1999. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J. Neurosci. 19, 6637–6642. Hartwich, M., Kalsbeek, A., Pevet, P., Nurnberger, F., 1994. Effects of illumination and enucleation on substance-P-immunoreactive structures in subcortical visual centers of golden-hamster and Wistar rat. Cell Tissue Res. 277, 351–361. Hastings, M.H., Mead, S.M., Vindlacheruvu, R.R., Ebling, F.J.P., Maywood, E.S., Grosse, J., 1992. Non-photic phase shifting of the circadian activity rhythm of Syrian hamsters: the relative potency of arousal and melatonin. Brain Res. 591, 20–26. Hastings, M.H., Field, M.D., Maywood, E.S., Weaver, D.R., Reppert, S.M., 1999. Differential regulation of mPER1 and mTIM proteins in the mouse suprachiasmatic nuclei: new insights into a core clock mechanism. J. Neurosci. 19, RC11. Hattar, S., Liao, H.W., Takao, M., Berson, D.M., Yau, K.W., 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070. Hattar, S., Lucas, R.J., Mrosovsky, N., Thompson, S., Douglas, R.H., Hankins, M.W., Lem, J., Biel, M., Hofmann, F., Foster, R.G., Yau, K.W., 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81. Hay-Schmidt, A., Vrang, N., Larsen, P.J., Mikkelsen, J.D., 2003. Projections from the raphe nuclei to the suprachiasmatic nucleus of the rat. J. Chem. Neuroanat. 25, 293–310. Hendrickson, A.E., Wagoner, N., Cowan, W.M., 1972. An autoradiographic and electron microscopic study of retinohypothalamic connections. Z. Zellforsch. 135, 1–26. Hertz, L., 2004. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem. Int. 45, 285–296. Herzog, E.D., Geusz, M.E., Khalsa, S.B., Straume, M., Block, G.D., 1997. Circadian rhythms in mouse suprachiasmatic nucleus explants on multimicroelectrode plates. Brain Res. 757, 285–290. Herzog, E.D., Takahashi, J.S., Block, G.D., 1998. Clock controls 50 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1, 708–713. Honma, S., Shirakawa, T., Katsuno, Y., Namihira, M., Honma, K., 1998a. Circadian periods of single suprachiasmatic neurons in rats. Neurosci. Lett. 250, 157–160. Honma, S., Ikeda, M., Abe, H., Tanahashi, Y., Namihira, M., Honma, K., Nomura, M., 1998b. Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem. Biophys. Res. Commun. 250, 83–87. Honrado, G.I., Johnson, R.S., Golombek, D.A., Spiegelman, B.M., Papaioannou, V.E., Ralph, M.R., 1996. The circadian system of c-fos deficient mice. J. Comp. Physiol., A 178, 563–570. Horikawa, K., Yokota, S., Fuji, K., Akiyama, M., Moriya, T., Okamura, H., Shibata, S., 2000. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J. Neurosci. 20, 5867–5873. Horowitz, S.S., Blanchard, J.H., Morin, L.P., 2004. Intergeniculate leaflet and ventral lateral geniculate nucleus afferent connections: an anatomical substrate for functional input from the vestibulo-visuomotor system. J. Comp. Neurol. 474, 227–245. Horowitz, S.S., Blanchard, J.H., Morin, L.P., 2005. Medial vestibular connections with the hypocretin (orexin) system. J. Comp. Neurol. 487, 127–146. Horvath, T.L., 1998. An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. J. Neurosci. 18, 1546–1558. Hsiao, K., Sachs, G.M., Schneider, G.E., 1984. A minute fraction of Syrian golden hamster retinal ganglion cells project bilaterally. J. Neurosci. 4, 359–367. Huang, R.C., 1993. Sodium and calcium currents in acutely dissociated neurons from rat suprachiasmatic nucleus. J. Neurophysiol. 70, 1692–1703. Hut, R.A., van der Zee, E.A., Jansen, K., Gerkema, M.P., Daan, S., 2002. Gradual reappearance of post-hibernation circadian rhythmicity correlates with numbers of vasopressin-containing neurons in the suprachiasmatic nuclei of European ground squirrels. J. Comp. Physiol., B 172, 59–70. Ichikawa, T., Hirata, Y., 1986. Organization of choline acetyltransferase-containing structure in the forebrain of the rat. J. Neurosci. 6, 281–292 (Ref Type: Abstract). Ikeda, M., Sugiyama, T., Wallace, C.S., Gompf, H.S., Yoshioka, T., Miyawaki, A., Allen, C.N., 2003. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron 38, 253–263. Ingram, C.D., Ciobanu, R., Coculescu, I.L., Tanasescu, R., Coculescu, M., Mihai, R., 1998. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog. Brain Res. 119, 351–364. Inouye, S.T., Kawamura, H., 1979. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. U. S. A. 76, 5962–5966. Isoldi, M.C., Rollag, M.D., de Lauro Castrucci, A.M., Provencio, I., 2005. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc. Natl. Acad. Sci. U. S. A. 102, 1217–1221. Itri, J., Colwell, C.S., 2003. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J. Neurophysiol. 90, 1589–1597. Jackson, A.C., Yao, G.L., Bean, B.P., 2004. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J. Neurosci. 24, 7985–7998. Jagota, A., De la Iglesia, H.O., Schwartz, W.J., 2000. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat. Neurosci. 3, 372–376. Janik, D., Mrosovsky, N., 1994. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 651, 174–182. Jhaveri, S., Edwards, M.A., Schneider, G.E., 1991. Initial stages of retinofugal axon development in the hamster: evidence for two distinct modes of growth. Exp. Brain Res. 87, 371–382. Jiang, Z.-G., Allen, C.N., North, R.A., 1995. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience 64, 813–819. Jiang, Z.-G., Yang, Y.-Q., Liu, Z.P., Allen, C.N., 1997. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J. Physiol. 499, 141–159. Jobst, E.E., Allen, C.N., 2002. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur. J. Neurosci. 16, 2469–2474. Jobst, E.E., Robinson, D.W., Allen, C.N., 2004. Potential pathways for intercellular communication within the calbindin subnucleus of the hamster suprachiasmatic nucleus. Neuroscience 123, 87–99. Johnson, R.F., Morin, L.P., Moore, R.Y., 1988a. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 462, 301–312. Johnson, R.F., Smale, L., Moore, R.Y., Morin, L.P., 1988b. Lateral geniculate lesions block circadian phase shift responses to a benzodiazepine. Proc. Natl. Acad. Sci. U. S. A. 85, 5301–5304. Kallo, I., Kalamatianos, T., Wiltshire, N., Shen, S., Sheward, W.J., Harmar, A.J., Coen, C.W., 2004. Transgenic approach reveals expression of the VPAC2 receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur. J. Neurosci. 19, 2201–2211. Kalsbeek, A., Teclemariam-Mesbah, R., Pevet, P., 1993. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus). J. Comp. Neurol. 332, 293–314. Kalsbeek, A., Fliers, E., Franke, A.N., Wortel, J., Buijs, R.M., 2000. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 141, 3832–3841. Karatsoreos, I.N., Yan, L., LeSauter, J., Silver, R., 2004. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J. Neurosci. 24, 68–75. Kawaguchi, C., Tanaka, K., Isojima, Y., Shintani, N., Hashimoto, H., Baba, A., Nagai, K., 2003. Changes in light-induced phase shift of circadian rhythm in mice lacking PACAP. Biochem. Biophys. Res. Commun. 310, 169–175. Kawamoto, K., Nagano, M., Kanda, F., Chihara, K., Shigeyoshi, Y., Okamura, H., 2003. Two types of VIP neuronal components in rat suprachiasmatic nucleus. J. Neurosci. Res. 74, 852–857. Kennaway, D.J., Moyer, R.W., 1998. Serotonin 5-HT2c agonists mimic the effect of light pulses on circadian rhythms. Brain Res. 806, 257–270. Kennaway, D.J., Moyer, R.W., Voultsios, A., Varcoe, T.J., 2001. Serotonin, excitatory amino acids and the photic control of melatonin rhythms and SCN c-FOS in the rat. Brain Res. 897, 36–43. Kennaway, D.J., Voultsios, A., Varcoe, T.J., Moyer, R.W., 2003. Melatonin and activity rhythm responses to light pulses in mice with the Clock mutation. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 284, R1231–R1240. Kim, Y.I., Dudek, F.E., 1992. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: inhibitory synaptic mechanisms. J. Physiol. (London) 458, 247–260. Kim, Y.I., Dudek, F.E., 1993. Membrane properties of rat suprachiasmatic nucleus neurons receiving optic nerve input. J. Physiol. (London) 464, 229–243. Kim, Y.I., Kim, S.H., Kim, D.Y., Lee, H.W., Shin, H.C., Chung, J.M., BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 Han, H.C., Na, H.S., Hong, S.K., 1999. Electrophysiological evidence for the role of substance P in retinohypothalamic transmission in the rat. Neurosci. Lett. 274, 99–102. Kim, D.Y., Kang, H.C., Shin, H.C., Lee, K.J., Yoon, Y.W., Han, H.C., Na, H.S., Hong, S.K., Kim, Y.I., 2001. Substance P plays a critical role in photic resetting of the circadian pacemaker in the rat hypothalamus. J. Neurosci. 21, 4026–4031. King, V.M., Chahad-Ehlers, S., Shen, S., Harmar, A.J., Maywood, E.S., Hastings, M.H., 2003. A hVIPR transgene as a novel tool for the analysis of circadian function in the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 17, 822–832. Kiss, J., Halasz, B., 1996. Synaptic contacts between cholinergic afferents and suprachiasmatic neurones of the rat. NeuroReport 7, 1961–1964. Knoch, M.E., Gobes, S.M., Pavlovska, I., Su, C., Mistlberger, R.E., Glass, J.D., 2004. Short-term exposure to constant light promotes strong circadian phase-resetting responses to nonphotic stimuli in Syrian hamsters. Eur. J. Neurosci. 19, 2779–2790. Koibuchi, N., Sakai, M., Watanabe, K., Yamaoka, S., 1992. Change in Fos-like immunoreactivity in the suprachiasmatic nucleus in the adult male rat. NeuroReport 3, 501–504. Kononenko, N.I., Dudek, F.E., 2004. Mechanism of irregular firing of suprachiasmatic nucleus neurons in rat hypothalamic slices. J. Neurophysiol. 91, 267–273. Kononenko, N.I., Shao, L.R., Dudek, F.E., 2004. Riluzole-sensitive slowly inactivating sodium current in rat suprachiasmatic nucleus neurons. J. Neurophysiol. 91, 710–718. Kopp, M.D., Schomerus, C., Dehghani, F., Korf, H.W., Meissl, H., 1999. Pituitary adenylate cyclase-activating polypeptide and melatonin in the suprachiasmatic nucleus: effects on the calcium signal transduction cascade. J. Neurosci. 19, 206–219. Kornhauser, J.M., Nelson, D.E., Mayo, K.E., Takahashi, J.S., 1990. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron 5, 127–134. Kriegsfeld, L.J., Demas, G.E., Lee Jr., S.E., Dawson, T.M., Dawson, V. L., Nelson, R.J., 1999. Circadian locomotor analysis of male mice lacking the gene for neuronal nitric oxide synthase (nNOS−/−). J. Biol. Rhythms 14, 20–27. Kriegsfeld, L.J., Drazen, D.L., Nelson, R.J., 2001. Circadian organization in male mice lacking the gene for endothelial nitric oxide synthase (eNOS−/−). J. Biol. Rhythms 16, 142–148. Kriegsfeld, L.J., LeSauter, J., Silver, R., 2004. Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus. J. Neurosci. 24, 2449–2457. Kromer, L.F., Moore, R.Y., 1980. A study of the organization of the locus coeruleus projections to the lateral geniculate nucleus in the albino rat. Neuroscience 5, 255–271. Krout, K.E., Kawano, J., Mettenleiter, T.C., Loewy, A.D., 2002. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience 110, 73–92. Kuhlman, S.J., McMahon, D.G., 2004. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur. J. Neurosci. 20, 1113–1117. Kuhlman, S.J., Quintero, J.E., McMahon, D.G., 2000. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. NeuroReport 11, 1479–1482. Kuhlman, S.J., Silver, R., LeSauter, J., Bult-Ito, A., McMahon, D.G., 2003. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J. Neurosci. 23, 1441–1450. Ladenheim, E.E., Jensen, R.T., Mantey, S.A., Moran, T.H., 1992. Distinct distributions of two bombesin receptor subtypes in the rat central nervous system. Brain Res. 593, 168–178. Ladenheim, E.E., Jensen, R.T., Mantey, S.A., Taylor, J.E., Coy, D.H., Moran, T.H., 1993. Bombesin receptor antagonists differentiate receptor subtypes in rat brain. Eur. J. Pharmacol. 235, 121–125. Laemle, L.K., Ottenweller, J.E., 2001. The relationship between 51 circadian rhythmicity and vasoactive intestinal polypeptide in the suprachiasmatic nucleus of congenitally anophthalmic mice. Brain Res. 917, 105–111. Lall, G.S., Biello, S.M., 2002. Attenuation of phase shifts to light by activity or neuropeptide Y: a time course study. Brain Res. 957, 109–116. Lall, G.S., Biello, S.M., 2003a. Attenuation of circadian light induced phase advances and delays by neuropeptide Y and a neuropeptide Y Y1/Y5 receptor agonist. Neuroscience 119, 611–618. Lall, G.S., Biello, S.M., 2003b. Neuropeptide Y, GABA and circadian phase shifts to photic stimuli. Neuroscience 120, 915–921. Land, P.W., Kyonka, E., Shamalla-Hannah, L., 2004. Vesicular glutamate transporters in the lateral geniculate nucleus: expression of VGLUT2 by retinal terminals. Brain Res. 996, 251–254. Larsen, P.J., 1999. Tracing autonomic innervation of the rat pineal gland using viral transneuronal tracing. Microsc. Res. Tech. 46, 296–304. Larsen, P.J., Enquist, L.W., Card, J.P., 1998. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur. J. Neurosci. 10, 128–145. Leak, R.K., Moore, R.Y., 2001. Topographic organization of suprachiasmatic nucleus projection neurons. J. Comp. Neurol. 433, 312–334. Leak, R.K., Card, J.P., Moore, R.Y., 1999. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res. 819, 23–32. Leanza, G., Nilsson, O.G., Wiley, R.G., Bjorklund, A., 1995. Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: behavioural, biochemical and stereological studies in the rat. Eur. J. Neurosci. 7, 329–343. Leathers, V.L., Linse, S., Forsen, S., Norman, A.W., 1990. Calbindin-D28K, a 1 alpha,25-dihydroxyvitamin D3-induced calcium-binding protein, binds five or six Ca2+ ions with high affinity. J. Biol. Chem. 265, 9838–9841. Lee, H.S., Nelms, J.L., Nguyen, M., Silver, R., Lehman, M.N., 2003. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat. Neurosci. 6, 111–112. Lehman, M.N., Silver, R., Gladstone, W.R., Kahn, R.M., Gibson, M., Bittman, E.L., 1987. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 7, 1626–1638. Lehman, M.N., LeSauter, J., Silver, R., 1998. Fiber outgrowth from anterior hypothalamic and cortical xenografts in the third ventricle. J. Comp. Neurol. 391, 133–145. LeSauter, J., Silver, R., 1998. Output signals of the SCN. Chronobiol. Int. 15, 535–550. LeSauter, J., Silver, R., 1999. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J. Neurosci. 19, 5574–5585. LeSauter, J., Lehman, M.N., Silver, R., 1996. Restoration of circadian rhythmicity by transplants of SCN “micropunches”. J. Biol. Rhythms 11, 163–171. LeSauter, J., Romero, P., Cascio, M., Silver, R., 1997. Attachment site of grafted SCN influences precision of restored circadian rhythm. J. Biol. Rhythms 12, 327–338. LeSauter, J., Stevens, P., Jansen, H., Lehman, M.N., Silver, R., 1999. Calbindin expression in the hamster SCN is influenced by circadian genotype and by photic conditions. NeuroReport 10, 3159–3163. LeSauter, J., Kriegsfeld, L.J., Hon, J., Silver, R., 2002. Calbindin-D28K cells selectively contact intra-SCN neurons. Neuroscience 111, 575–585. LeSauter, J., Yan, L., Vishnubhotla, B., Quintero, J.E., Kuhlman, S.J., McMahon, D.G., Silver, R., 2003. A short half-life GFP mouse 52 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 model for analysis of suprachiasmatic nucleus organization. Brain Res. 964, 279–287. Lewandowski, M.H., Usarek, A., 2002. Effects of intergeniculate leaflet lesions on circadian rhythms in the mouse. Behav. Brain Res. 128, 13–17. Lewandowski, M.H., Blasiak, T., Domoslawski, J., Wolkowska, A., 2000. Ultradian rhythmic neuronal oscillation in the intergeniculate leaflet. NeuroReport 11, 317–321. Lewandowski, M.H., Blasiak, T., Blasiak, A., 2002. Are ultra-slow isoperiodic oscillations in rat intergeniculate leaflet neurons dependent on reciprocal connection with its contralaterally located counterpart? Neurosci. Lett. 330, 243–246. Li, X., Gilbert, J., Davis, F.C., 2005. Disruption of masking by hypothalamic lesions in Syrian hamsters. J. Comp. Physiol., A Neuroethol. Sens. Neural Behav. Physiol. 191, 23–30. Ling, C., Schneider, G.E., Jhaveri, S., 1998. Target-specific morphology of retinal axon arbors in the adult hamster. Vis. Neurosci. 15, 559–579. Liou, S.Y., Albers, H.E., 1991. Single unit response of neurons within the hamster suprachiasmatic nucleus to neuropeptide Y. Brain Res. Bull. 27, 825–828. Liou, S.-Y., Shibata, S., Iwasaki, K., Ueki, S., 1986. Optic-nerve stimulation-induced increase of release of H-3 glutamate and H-3 aspartate but not H-3 GABA from the suprachiasmatic nucleus in slices of rat hypothalamus. Brain Res. Bull. 16, 527–531. Liou, S.-Y., Shibata, S., Albers, H.E., Ueki, S., 1990. Effects of GABA and anxiolytics on the single unit discharge of suprachiasmatic neurons in rat hypothalamic slices. Brain Res. Bull. 25, 103–107. Liu, C., Gillette, M.U., 1996. Cholinergic regulation of the suprachiasmatic nucleus circadian rhythm via a muscarinic mechanism at night. J. Neurosci. 16, 744–751. Liu, C., Reppert, S.M., 2000. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25, 123–128. Liu, C., Weaver, D.R., Strogatz, S.H., Reppert, S.M., 1997a. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell 91, 855–860. Liu, C., Ding, J.M., Faiman, L.E., Gillette, M.U., 1997b. Coupling of muscarinic cholinergic receptors and cGMP in nocturnal regulation of the suprachiasmatic circadian clock. J. Neurosci. 17, 659–666. Liu, Q.S., Xu, Q., Arcuino, G., Kang, J., Nedergaard, M., 2004. Astrocyte-mediated activation of neuronal kainate receptors. Proc. Natl. Acad. Sci. U. S. A. 101, 3172–3177. Lovenberg, T.W., Baron, B.M., De Lecea, L., Miller, J.D., Prosser, R.A., Rea, M.A., Foye, P.E., Racke, M., Slone, A.L., Siegel, B.W., Danielson, P.E., Sutcliffe, J.G., Erlander, M.G., 1993. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11, 449–458. Lucas, R.J., Foster, R.G., 1999. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology 140, 1520–1524. Lucas, R.J., Freedman, M.S., Munoz, M., Garcia-Fernández, J.-M., Foster, R.G., 1999. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284, 505–507. Lucas, R.J., Douglas, R.H., Foster, R.G., 2001. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 4, 621–626. Lucas, R.J., Hattar, S., Takao, M., Berson, D.M., Foster, R.G., Yau, K. W., 2003. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247. Madeira, M.D., Pereira, P.A., Silva, S.M., Cadete-Leite, A., Paula-Barbosa, M.M., 2004. Basal forebrain neurons modulate the synthesis and expression of neuropeptides in the rat suprachiasmatic nucleus. Neuroscience 125, 889–901. Mahoney, M.M., Nunez, A.A., Smale, L., 2000. Calbindin and Fos within the suprachiasmatic nucleus and the adjacent hypothalamus of Arvicanthis niloticus and Rattus norvegicus. Neuroscience 99, 565–575. Mai, L.M., Pan, J.T., 1993. Central administration of bombesin blocks the estrogen-induced afternoon prolactin surge. Neuroendocrinology 57, 40–44. Mai, L.M., Pan, J.T., 1995. Bombesin acts in the suprachiasmatic nucleus to affect circadian changes in tuberoinfundibular dopaminergic neuron activity and prolactin secretion. Endocrinology 136, 4163–4167. Major, D.E., Rodman, H.R., Libedinsky, C., Karten, H.J., 2003. Pattern of retinal projections in the California ground squirrel (Spermophilus beecheyi): anterograde tracing study using cholera toxin. J. Comp. Neurol. 463, 317–340. Manrique, C., Héry, F., Faudon, M., François-Bellan, A.M., 1999. Indirect evidence for an association of 5-HT1B binding sites with retinal and geniculate axon terminals in the rat suprachiasmatic nucleus. Synapse 33, 314–323. Marchant, E.G., Morin, L.P., 1999. The hamster circadian rhythm system includes nuclei of the subcortical visual shell. J. Neurosci. 19, 10482–10493. Marchant, E.G., Watson, N.V., Mistlberger, R.E., 1997. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. J. Neurosci. 17, 7974–7987. Margraf, R.R., Lynch, G.R., 1993. Melatonin injections affect circadian behavior and SCN neurophysiology in Djungarian hamsters. Am. J. Physiol. 264, R615–R621. Martinet, L., Bonnefond, C., Peytevin, J., Monnerie, R., Marcilloux, J.C., 1995. Vasoactive intestinal polypeptide in the suprachiasmatic nucleus of the mink (Mustela vison) could play a key role in photic induction. J. Neuroendocrinol. 7, 69–79. Maywood, E.S., Mrosovsky, N., 2001. A molecular explanation of interactions between photic and non-photic circadian clock-resetting stimuli. Gene Expr. Patterns 1, 27–31. Maywood, E.S., Smith, E., Hall, S.J., Hastings, M.H., 1997. A thalamic contribution to arousal-induced, non-photic entrainment of the circadian clock of the Syrian hamster. Eur. J. Neurosci. 9, 1739–1747. Maywood, E.S., Mrosovsky, N., Field, M.D., Hastings, M.H., 1999. Rapid down-regulation of mammalian Period genes during behavioral resetting of the circadian clock. Proc. Natl. Acad. Sci. U. S. A. 96, 15211–15216. Maywood, E.S., Okamura, H., Hastings, M.H., 2002. Opposing actions of neuropeptide Y and light on the expression of circadian clock genes in the mouse suprachiasmatic nuclei. Eur. J. Neurosci. 15, 216–220. McArthur, A.J., Coogan, A.N., Ajpru, S., Sugden, D., Biello, S.M., Piggins, H.D., 2000. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J. Neurosci. 20, 5496–5502. Mead, S., Ebling, F.J.P., Maywood, E.S., Humby, T., Herbert, J., Hastings, M.H., 1992. A nonphotic stimulus causes instantaneous phase advances of the light-entrainable circadian oscillator of the Syrian hamster but does not induce the expression of c-fos in the suprachiasmatic nuclei. J. Neurosci. 12, 2516–2522. Meijer, J.H., van der Zee, E.A., Dietz, M., 1988. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci. Lett. 86, 177–183. Meijer, J.H., Schaap, J., Watanabe, K., Albus, H., 1997. Multiunit activity recordings in the suprachiasmatic nuclei: in vivo vs. in vitro models. Brain Res. 753, 322–327. Meijer, J.H., Ruijs, A.C.J., Albus, H., Van de Geest, B., Duindam, H., Zwinderman, A.H., Dahan, A., 2000. Fentanyl, a μ-opioid receptor agonist, phase shifts the hamster circadian pacemaker. Brain Res. 868, 135–140. Melyan, Z., Tarttelin, E.E., Bellingham, J., Lucas, R.J., Hankins, M.W., BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 2005. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745. Menaker, M., 1968. Extraretinal light perception in the sparrow: I. Entrainment of the biological clock. Proc. Natl. Acad. Sci. U. S. A. 59, 414–421. Menaker, M., Keatts, H., 1968. Extraretinal light perception in the sparrow: II. Photoperiodic stimulation of testis growth. Proc. Natl. Acad. Sci. U. S. A. 60, 146–151. Menaker, M., Underwood, H., 1976. Extraretinal photoreception in birds. Photophysiology 23, 299–306. Menet, J.S., Vuillez, P., Pevet, P., 2003. Calbindin expression in the hamster suprachiasmatic nucleus depends on day-length. Neuroscience 122, 591–598. Meyer-Bernstein, E.L., Morin, L.P., 1996. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J. Neurosci. 16, 2097–2111. Meyer-Bernstein, E.L., Morin, L.P., 1998. Destruction of serotonergic neurons in the median raphe nucleus blocks circadian rhythm phase shifts to triazolam, but not to novel wheel access. J. Biol. Rhythms 13, 494–505. Meyer-Bernstein, E.L., Morin, L.P., 1999. Electrical stimulation of the median or dorsal raphe nuclei reduces light-induced FOS protein in the suprachiasmatic nucleus and causes circadian activity rhythm phase shifts. Neuroscience 92, 267–279. Meyer-Bernstein, E.L., Blanchard, J.H., Morin, L.P., 1997. The serotonergic projection from the median raphe nucleus to the suprachiasmatic nucleus modulates activity phase onset, but not other circadian rhythm parameters. Brain Res. 755, 112–120. Meyer-Bernstein, E.L., Jetton, A.E., Matsumoto, S.I., Markuns, J.F., Lehman, M.N., Bittman, E.L., 1999. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140, 207–218. Meyer-Spasche, A., Reed, H.E., Piggins, H.D., 2002. Neurotensin phase-shifts the firing rate rhythm of neurons in the rat suprachiasmatic nuclei in vitro. Eur. J. Neurosci. 16, 339–344. Mick, G., Maeno, H., Kiyama, H., Tohyama, M., 1994. Marginal topography of neurons expressing the substance P receptor in the rat suprachiasmatic nucleus. Mol. Brain Res. 21, 157–161. Mick, G., Shigemoto, R., Kitahama, K., 1995. Localization of substance P receptors in central neural structures controlling daily rhythms in nocturnal rodents. C. R. Acad. Sci. III 318, 209–217. Mikkelsen, J.D., 1990. Projections from the lateral geniculate nucleus to the hypothalamus of the Mongolian gerbil (Meriones unguiculatus): an anterograde and retrograde tracing study. J. Comp. Neurol. 299, 493–508. Mikkelsen, J.D., Larsen, P.J., 1993. Substance P in the suprachiasmatic nucleus of the rat: an immunohistochemical and in situ hybridization study. Histochemistry 100, 3–16. Mikkelsen, J.D., Vrang, N., 1994. A direct pretectosuprachiasmatic projection in the rat. Neuroscience 62, 497–505. Miller, J.D., Fuller, C.A., 1992. Isoperiodic neuronal activity in suprachiasmatic nucleus of the rat. Am. J. Physiol. 263, R51–R58. Miller, J.D., Morin, L.P., Schwartz, W.J., Moore, R.Y., 1996. New insights into the mammalian circadian clock [Review] [230 refs] Sleep 19, 641–667. Mintz, E.M., Albers, H.E., 1997. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res. 758, 245–249. Mintz, E.M., Marvel, C.L., Gillespie, C.F., Price, K.M., Albers, H.E., 1999. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J. Neurosci. 19, 5124–5130. Mintz, E.M., van den Pol, A.N., Casano, A.A., Albers, H.E., 2001. Distribution of hypocretin-(orexin) immunoreactivity in the 53 central nervous system of Syrian hamsters (Mesocricetus auratus). J. Chem. Neuroanat. 21, 225–238. Mintz, E.M., Jasnow, A.M., Gillespie, C.F., Huhman, K.L., Albers, H. E., 2002. GABA interacts with photic signaling in the suprachiasmatic nucleus to regulate circadian phase shifts. Neuroscience 109, 773–778. Mistlberger, R.E., Antle, M.C., 1998. Behavioral inhibition of light-induced circadian phase resettting is phase and serotonin dependent. Brain Res. 786, 31–38. Mistlberger, R.E., Landry, G.J., Marchant, E.G., 1997. Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci. Lett. 238, 5–8. Mistlberger, R.E., Antle, M.C., Glass, J.D., Miller, J.D., 2000. Behavioral and serotonergic regulation of circadian rhythms. Biol. Rhythm Res. 31, 240–283. Mistlberger, R.E., Belcourt, J., Antle, M.C., 2002. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: dark pulses revisited. J. Biol. Rhythms 17, 227–237. Mistlberger, R.E., Antle, M.C., Webb, I.C., Jones, M., Weinberg, J., Pollock, M.S., 2003. Circadian clock resetting by arousal in Syrian hamsters: the role of stress and activity. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 285, R917–R925. Moffett, J.R., 2003. Reductions in N-acetylaspartylglutamate and the 67 kDa form of gluamic acid decarboxylase immunoreactivities in the visual system of albino and pigmented rats after optic nerve transections. J. Comp. Neurol. 458, 221–239. Moffett, J.R., Williamson, L., Palkovits, M., Namboodiri, M.A.A., 1990. N-acetylaspartylglutamate: a transmitter candidate for the retinohypothalamic tract. Proc. Natl. Acad. Sci. U. S. A. 87, 8065–8069. Moffett, J.R., Williamson, L.C., Neale, J.H., Palkovits, M., Namboodin, M.A.A., 1991. Effect of optic nerve transection on N-acetylaspartylglutamate immunoreactivity in the primary and accessory optic projection systems in the rat. Brain Res. 538, 86–94. Moga, M.M., 1998. 192 IgG-saporin abolishes p75 neurotrophin receptor immunoreactivity in rat SCN. NeuroReport 9, 3197–3200. Moga, M.M., Moore, R.Y., 1996. Putative excitatory amino acid projections to the suprachiasmatic nucleus in the rat. Brain Res. 743, 171–177. Moga, M.M., Moore, R.Y., 1997. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J. Comp. Neurol. 389, 508–534. Moore, R.Y., 1983. Organization and function of a central nervous system oscillator: the suprachiasmatic hypothalamic nucleus. Fed. Proc. 42, 2783–2789. Moore, R.Y., 1989. The geniculohypothalamic tract in monkey and man. Brain Res. 486, 190–194. Moore, R.Y., 1993. Organization of the primate circadian system. J. Biol. Rhythms 8, S3–S9. Moore, R.Y., 1996. Entrainment pathways and the functional organization of the circadian timing system. In: Buijs, R., Kalsbeek, A., Romijn, H.J., Pennartz, C.M.A., Mirmiran, M. (Eds.), Hypothalamic Integration of Circadian Rhythms. Elsevier, Amsterdam, pp. 101–117. Moore, R.Y., Card, J.P., 1994. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J. Comp. Neurol. 344, 403–430. Moore, R.Y., Lenn, N.J., 1972. A retinohypothalamic projection in the rat. J. Comp. Neurol. 146, 1–14. Moore, R.Y., Speh, J.C., 1993. GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett. 150, 112–116. Moore, R.Y., Gustafson, E.L., Card, J.P., 1984. Identical immunoreactivity of afferents to the rat suprachiasmatic nucleus with antisera against avian pancreatic polypeptide, 54 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 molluscan cardiaoexcitatory peptide and neuropeptide Y. Cell Tissue Res. 236, 41–46. Moore, R.Y., Speh, J.C., Card, J.P., 1995. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J. Comp. Neurol. 352, 351–366. Moore, R.Y., Weis, R., Moga, M.M., 2000. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J. Comp. Neurol. 420, 398–418. Moore, R.Y., Speh, J.C., Leak, R.K., 2002. Suprachiasmatic nucleus organization. Cell Tissue Res. 309, 89–98. Mori, K., Miyazato, M., Ida, T., Murakami, N., Serino, R., Ueta, Y., Kojima, M., Kangawa, K., 2005. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. EMBO J. 24, 325–335. Morin, L.P., 1994. The circadian visual system. Brain Res. Rev. 67, 102–127. Morin, L.P., 1999. Serotonin and the regulation of mammalian circadian rhythmicity. Ann. Med. 31, 12–33. Morin, L.P., Blanchard, J.H., 1991. Depletion of brain serotonin by 5,7-DHT modifies hamster circadian rhythm response to light. Brain Res. 566, 173–185. Morin, L.P., Blanchard, J.H., 1995. Organization of the hamster intergeniculate leaflet: NPY and ENK projections to the suprachiasmatic nucleus, intergeniculate leaflet and posterior limitans nucleus. Vis. Neurosci. 12, 57–67. Morin, L.P., Blanchard, J.H., 1998. Interconnections among nuclei of the subcortical visual shell: the intergeniculate leaflet is a major constituent of the hamster subcortical visual system. J. Comp. Neurol. 396, 288–309. Morin, L.P., Blanchard, J.H., 1999. Forebrain connections of the hamster intergeniculate leaflet: comparison with those of ventral lateral geniculate nucleus and retina. Vis. Neurosci. 16, 1037–1054. Morin, L.P., Blanchard, J.H., 2001. Neuromodulator content of hamster intergeniculate leaflet neurons and their projection to the suprachiasmatic nucleus or visual midbrain. J. Comp. Neurol. 437, 79–90. Morin, L.P., Blanchard, J.H., 2005. Descending projections of the hamster intergeniculate leaflet: relationship to the sleep/ arousal and visuomotor systems. J. Comp. Neurol. 487, 204–216. Morin, L.P., Pace, L., 2002. The intergeniculate leaflet, but not the visual midbrain, mediates hamster circadian rhythm response to constant light. J. Biol. Rhythms 17, 217–226. Morin, L.P., Blanchard, J.H., Moore, R.Y., 1992. Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the hamster. Vis. Neurosci. 8, 219–230. Morin, L.P., Goodless-Sanchez, N., Smale, L., Moore, R.Y., 1994. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience 61, 391–410. Morin, L.P., Blanchard, J.H., Provencio, I., 2003. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet and visual midbrain: bifurcation and melanopsin immunoreactivity. J. Comp. Neurol. 465, 401–416. Moriya, T., Yamanouchi, S., Fukushima, T., Shimazoe, T., Shibata, S., Watanabe, S., 1996. Involvement of 5-HT1A receptor mechanisms in the inhibitory effects of methamphetamine on photic responses in the rodent suprachiasmatic nucleus. Brain Res. 740, 261–267. Moriya, T., Yoshinobu, Y., Ikeda, M., Yokota, S., Akiyama, M., Shibata, S., 1998. Potentiating action of MKC-242, a selective 5-HT1A receptor agonist, on the photic entrainment of the circadian activity rhythm in hamsters. Br. J. Pharmacol. 125, 1281–1287. Moyer, R.W., Kennaway, D.J., 1999. Immunohistochemical localization of serotonin receptors in the rat suprachiasmatic nucleus. Neurosci. Lett. 271, 147–150. Moyer, R.W., Kennaway, D.J., 2000. Serotonin depletion decreases light induced c-fos in the rat suprachiasmatic nucleus. NeuroReport 11, 1021–1024. Mrosovsky, N., 1996. Locomotor activity and non-photic influences on circadian clocks. Biol. Rev. Camb. Philos. Soc. 71, 343–372. Mrosovsky, N., 1999. Masking: history, definitions, and measurement. Chronobiol. Int. 16, 415–429. Mrosovsky, N., 2001. Further characterization of the phenotype of mCry1/mCry2-deficient mice. Chronobiol. Int. 18, 613–625. Mrosovsky, N., 2003. Contribution of classic photoreceptors to entrainment. J. Comp. Physiol., A 189, 69–73. Mrosovsky, N., Hattar, S., 2003. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol. Int. 20, 989–999. Mrosovsky, N., Foster, R.G., Salmon, P.A., 1999. Thresholds for masking responses to light in three strains of retinally degenerate mice. J. Comp. Physiol., A 184, 423–428. Mrosovsky, N., Salmon, P.A., Foster, R.G., McCall, M.A., 2000. Responses to light after retinal degeneration. Vision Res. 40, 575–578. Mrugala, M., Zlomanczuk, P., Jagota, A., Schwartz, W.J., 2000. Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 278, R987–R994. Munch, I.C., Moller, M., Larsen, P.J., Vrang, N., 2002. Light-induced c-fos expression in suprachiasmatic nuclei neurons targeting the paraventricular nucleus of the hamster hypothalamus: phase dependence and immunochemical identification. J. Comp. Neurol. 442, 48–62. Muscat, L., Morin, L.P., in press. Bionocular contributions to the responsiveness and integrative capacity of the circadian rhythm system to light. J. Biol. Rhythms. Muscat, L., Huberman, A.D., Jordan, C.L., Morin, L.P., 2003. Crossed and uncrossed retinal projections to the hamster circadian system. J. Comp. Neurol. 466, 513–524. Muscat, L., Tischler, R.C., Morin, L.P., 2005. Functional analysis of the role of the median raphe as a regulator of hamster circadian system sensitivity to light. Brain Res. 1044 (1), 59–66. Nagano, M., Adachi, A., Nakahama, K., Nakamura, T., Tamada, M., Meyer-Bernstein, E., Sehgal, A., Shigeyoshi, Y., 2003. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J. Neurosci. 23, 6141–6151. Nakahara, K., Hanada, R., Murakami, N., Teranishi, H., Ohgusu, H., Fukushima, N., Moriyama, M., Ida, T., Kangawa, K., Kojima, M., 2004. The gut-brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem. Biophys. Res. Commun. 318, 156–161. Nakamura, H., Itoh, K., 2004. Cytoarchitectonic and connectional organization of the ventral lateral geniculate nucleus in the cat. J. Comp. Neurol. 473, 439–462. Nakamura, W., Honma, S., Shirakawa, T., Honma, K., 2002. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat. Neurosci. 5, 399–400. Neale, J.H., Bzdega, T., Wroblewska, B., 2000. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J. Neurochem. 75, 443–452. Negroni, J., Nevo, E., Cooper, H.M., 1997. Neuropeptidergic organization of the suprachiasmatic nucleus in the blind mole rat (Spalax ehrenbergi). Brain Res. Bull. 44, 633–639. Nelson, D.E., Takahashi, J.S., 1991. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J. Physiol. (London) 439, 115–145. Nelson, D.E., Takahashi, J.S., 1999. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 277, R1351–R1361. Newman, L.A., Walker, M.T., Brown, R.L., Cronin, T.W., Robinson, P.R., 2003. Melanopsin forms a functional short-wavelength photopigment. Biochemistry 42, 12734–12738. BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 Novak, C.M., Albers, H.E., 2004a. Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 286, R820–R825. Novak, C.M., Albers, H.E., 2004b. Circadian phase alteration by GABA and light differs in diurnal and nocturnal rodents during the day. Behav. Neurosci. 118, 498–504. Novak, C.M., Ehlen, J.C., Huhman, K.L., Albers, H.E., 2004. GABA(B) receptor activation in the suprachiasmatic nucleus of diurnal and nocturnal rodents. Brain Res. Bull. 63, 531–535. Nunez, A.A., Bult, A., McElhinny, T.L., Smale, L., 1999. Daily rhythms of Fos expression in hypothalamic targets of the suprachiasmatic nucleus in diurnal and nocturnal rodents. J. Biol. Rhythms 14, 300–306. O'Hara, B.F., Edgar, D.M., Cao, V.H., Wiler, S.W., Heller, H.C., Kilduff, T.S., Miller, J.D., 1998. Nicotine and nicotinic receptors in the circadian system. Psychoneuroendocrinology 23, 161–173. Oliver, K.R., Kinsey, A.M., Wainwright, A., Sirinathsinghji, D.J., 2000. Localization of 5-ht(5A) receptor-like immunoreactivity in the rat brain. Brain Res. 867, 131–142. Otori, Y., Tominaga, K., Fukuhara, C., Yang, J., Yamazaki, S., Cagampang, F.R.A., Okamura, H., Inouye, S.-I.T., 1993. Substance P-like immunoreactivity in the suprachiasmatic nucleus of the rat. Brain Res. 619, 271–277. Panda, S., Sato, T.K., Castrucci, A.M., Rollag, M.D., DeGrip, W.J., Hogenesch, J.B., Provencio, I., Kay, S.A., 2002. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216. Panda, S., Provencio, I., Tu, D.C., Pires, S.S., Rollag, M.D., Castrucci, A.M., Pletcher, M.T., Sato, T.K., Wiltshire, T., Andahazy, M., Kay, S.A., Van Gelder, R.N., Hogenesch, J.B., 2003. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527. Panda, S., Nayak, S.K., Campo, B., Walker, J.R., Hogenesch, J.B., Jegla, T., 2005. Illumination of the melanopsin signaling pathway. Science 307, 600–604. Panula, P., Yang, H.Y., Costa, E., 1984. Comparative distribution of bombesin/GRP- and substance-P-like immunoreactivities in rat hypothalamus. J. Comp. Neurol. 224, 606–617. Paul, K.N., Fukuhara, C., Tosini, G., Albers, H.E., 2003. Transduction of light in the suprachiasmatic nucleus: evidence for two different neurochemical cascades regulating the levels of Per1 mRNA and pineal melatonin. Neuroscience 119, 137–144. Paul, K.N., Gamble, K.L., Fukuhara, C., Novak, C.M., Tosini, G., Albers, H.E., 2004. Tetrodotoxin administration in the suprachiasmatic nucleus prevents NMDA-induced reductions in pineal melatonin without influencing Per1 and Per2 mRNA levels. Eur. J. Neurosci. 19, 2808–2814. Penev, P.D., Zee, P.C., Wallen, E.P., Turek, F.W., 1995. Aging alters the phase-resetting properties of a serotonin agonist on hamster circadian rhythmicity. Am. J. Physiol. 268, R293–R298. Pennartz, C.M., Bierlaagh, M.A., Geurtsen, A.M., 1997. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. J. Neurophysiol. 78, 1811–1825. Pennartz, C.M., Bos, N.P., Jeu, M.T., Geurtsen, A.M., Mirmiran, M., Sluiter, A.A., Buijs, R.M., 1998. Membrane properties and morphology of vasopressin neurons in slices of rat suprachiasmatic nucleus. J. Neurophysiol. 80, 2710–2717. Pennartz, C.M., de Jeu, M.T., Bos, N.P., Schaap, J., Geurtsen, A.M., 2002. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 416, 286–290. Peterson, B.B., Liao, H.W., Dacey, D.M., Yau, K.-W., Gamlin, P.D.R., Robinson, F.R., Marshak, D.W., 2003. Functional architecture of the photoreceptive ganglion cell in primate retina: morphology, mosaic organization and central targets of melanopsin immunostained cells. ARVO Abst. 5182. 55 Pickard, G.E., 1982. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J. Comp. Neurol. 211, 65–83. Pickard, G.E., 1985. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci. Lett. 55, 211–217. Pickard, G.E., 1994. Intergeniculate leaflet ablation alters circadian rhythms in the mouse. NeuroReport 5, 2186–2188. Pickard, G.E., Rea, M.A., 1997. TFMPP, a 5HT1B receptor agonist, inhibits light-induced phase shifts of the circadian activity rhythm and c-Fos expression in the mouse suprachiasmatic nucleus. Neurosci. Lett. 231, 95–98. Pickard, G.E., Silverman, A.J., 1981. Direct retinal projections to hypothalamus, piriform cortex and accessory optic nuclei in the golden hamster as demonstrated by a sensitive anterograde horseradish peroxidase technique. J. Comp. Neurol. 196, 155–172. Pickard, G.E., Turek, F.W., 1983. The suprachiasmatic nuclei: two circadian clocks? Brain Res. 268, 201–210. Pickard, G.E., Ralph, M.R., Menaker, M., 1987. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J. Biol. Rhythms 2, 35–56. Pickard, G.E., Weber, E.T., Scott, P.A., Riberdy, A.F., Rea, M.A., 1996. 5HT1B receptor agonists inhibit light-induced phase shifts of behavioral circadian rhythms and expression of the immediate-early gene c-fos in the suprachiasmatic nucleus. J. Neurosci. 16, 8208–8220. Pickard, G.E., Smith, B.N., Belenky, M., Rea, M.A., Dudek, F.E., Sollars, P.J., 1999. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J. Neurosci. 19, 4034–4045. Pickard, G.E., Smeraski, C.A., Tomlinson, C.C., Banfield, B.W., Kaufman, J., Wilcox, C.L., Enquist, L.W., Sollars, P.J., 2002. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J. Neurosci. 22, 2701–2710. Piggins, H.D., Rusak, B., 1993. Electrophysiological effects of pressure-ejected bombesin-like peptides on hamster suprachiasmatic nucleus neurons in vitro. J. Neuroendocrinol. 5, 575–581. Piggins, H.D., Rusak, B., 1997. Effects of microinjections of substance P into the suprachiasmatic nucleus region on hamster wheel-running rhythms. Brain Res. Bull. 42, 451–455. Piggins, H.D., Cutler, D.J., Rusak, B., 1994. Effects of ionophoretically applied bombesin-like peptides on hamster suprachiasmatic nucleus neurons in vitro. Eur. J. Pharmacol. 271, 413–419. Piggins, H.D., Cutler, D.J., Rusak, B., 1995a. Ionophoretically applied substance P activates hamster suprachiasmatic nucleus neurons. Brain Res. Bull. 37, 475–479. Piggins, H.D., Antle, M.C., Rusak, B., 1995b. Neuropeptides phase shift the mammalian circadian pacemaker. J. Neurosci. 15, 5612–5622. Piggins, H.D., Stamp, J.A., Burns, J., Rusak, B., Semba, K., 1996. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J. Comp. Neurol. 376, 278–294. Piggins, H.D., Marchant, E.G., Goguen, D., Rusak, B., 2001a. Phase-shifting effects of pituitary adenylate cyclase activating polypeptide on hamster wheel-running rhythms. Neurosci. Lett. 305, 25–28. Piggins, H.D., Samuels, R.E., Coogan, A.N., Cutler, D.J., 2001b. Distribution of substance P and neurokinin-1 receptor immunoreactivity in the suprachiasmatic nuclei and 56 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 intergeniculate leaflet of hamster, mouse, and rat. J. Comp. Neurol. 438, 50–65. Porter, J.T., McCarthy, K.D., 1997. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 51, 439–455. Prosser, R.A., 1998. Neuropeptide Y blocks serotonergic phase shifts of the suprachiasmatic circadian clock in vitro. Brain Res. 808, 31–41. Prosser, R.A., Dean, R.R., Edgar, D.M., Heller, H.C., Miller, J.D., 1993. Serotonin and the mammalian circadian system: I. In vitro phase shifts by serotonergic agonists and antagonists. J. Biol. Rhythms 8, 1–16. Prosser, R.A., Macdonald, E.S., Heller, H.C., 1994. c-fos mRNA in the suprachiasmatic nuclei in vitro shows a circadian rhythm and responds to a serotonergic agonist. Mol. Brain Res. 25, 151–156. Prosser, R.A., Rutishauser, U., Ungers, G., Fedorkova, L., Glass, J.D., 2003. Intrinsic role of polysialylated neural cell adhesion molecule in photic phase resetting of the mammalian circadian clock. J. Neurosci. 23, 652–658. Provencio, I., Foster, R.G., 1995. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 694, 183–190. Provencio, I., Wong, S., Lederman, A.B., Argamaso, S.M., Foster, R.G., 1994. Visual and circadian responses to light in aged retinally degenerate mice. Vision Res. 34, 1799–1806. Provencio, I., Jiang, G., De Grip, W.J., Hayes, W.P., Rollag, M.D., 1998a. Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. U. S. A. 95, 340–345. Provencio, I., Cooper, H.M., Foster, R.G., 1998b. Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. J. Comp. Neurol. 395, 417–439. Provencio, I., Rodriguez, I.R., Jiang, G., Hayes, W.P., Moreira, E.F., Rollag, M.D., 2000. A novel human opsin in the inner retina. J. Neurosci. 20, 600–605. Provencio, I., Rollag, M.D., Castrucci, A.M., 2002. Photoreceptive net in the mammalian retina. Nature 415, 493. Pu, M., 1999. Dendritic morphology of cat retinal ganglion cells projecting to suprachiasmatic nucleus. J. Comp. Neurol. 414, 267–274. Pu, M., 2000. Physiological response properties of cat retinal ganglion cells projecting to suprachiasmatic nucleus. J. Biol. Rhythms 15, 31–36. Pu, M.L., Pickard, G.E., 1996. Ventral lateral geniculate nucleus afferents to the suprachiasmatic nucleus in the cat. Brain Res. 725, 247–251. Qiu, X., Kumbalasiri, T., Carlson, S.M., Wong, K.Y., Krishna, V., Provencio, I., Berson, D.M., 2005. Induction of photosensitivity by heterologous expression of melanopsin. Nature 433, 745–749. Quintero, J.E., Kuhlman, S.J., McMahon, D.G., 2000. Mouse Period1-driven GFP gene expression cycles in SCN slices and in individual SCN cells. Soc. Res. Biol. Rhythms 7, 87. Quintero, J.E., Kuhlman, S.J., McMahon, D.G., 2003. The biological clock nucleus: a multiphasic oscillator network regulated by light. J. Neurosci. 23, 8070–8076. Ralph, M.R., Menaker, M., 1987. Bicuculline blocks circadian phase delays but not advances. Neurochem. Int. 11, 55–62. Ralph, M.R., Menaker, M., 1989. GABA regulation of circadian responses to light: I. Involvement of GABAA-benzodiazepine and GABAB receptors. J. Neurosci. 9, 2858–2865. Ralph, M.R., Mrosovsky, N., 1992. Behavioral inhibition of circadian responses to light. J. Biol. Rhythms 7, 353–359. Ralph, M.R., Foster, R.G., Davis, F.C., Menaker, M., 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978. Rea, M.A., 1989. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res. Bull. 23, 577–581. Rea, M.A., 1992. Different populations of cells in the suprachiasmatic nuclei express c-fos in association with light-induced phase delays and advances of the free-running activity rhythm in hamsters. Brain Res. 579, 107–112. Rea, M.A., Pickard, G.E., 2000a. Serotonergic modulation of photic entrainment in the Syrian hamster. Biol. Rhythm Res. 31, 284–314. Rea, M.A., Pickard, G.E., 2000b. A 5-HT1B receptor agonist inhibits light-induced suppression of pineal melatonin production. Brain Res. 858, 424–428. Rea, M.A., Ferriera, S., Randolph, W., Glass, J.D., 1993a. Daily profile of the extracellular concentration of glutamate in the suprachiasmatic region of the Siberian hamster. Proc. Soc. Exp. Biol. Med. 204, 104–109. Rea, M.A., Buckley, B., Lutton, L.M., 1993b. Local administration of EAA antagonists blocks light-induced phase shifts and c-fos expression in hamster SCN. Am. J. Physiol. 265, R1191–R1198. Rea, M.A., Barrera, J., Glass, J.D., Gannon, R.L., 1995. Serotonergic potentiation of photic phase shifts of the circadian activity rhythm. NeuroReport 6, 1289–1292. Reddy, A.B., Field, M.D., Maywood, E.S., Hastings, M.H., 2002. Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J. Neurosci. 22, 7326–7330. Redlin, U., Mrosovsky, N., 1999a. Masking of locomotor activity in hamsters. J. Comp. Physiol., A 184, 429–437. Redlin, U., Mrosovsky, N., 1999b. Masking by light in hamsters with SCN lesions. J. Comp. Physiol., A 184, 439–448. Redlin, U., Vrang, N., Mrosovsky, N., 1999. Enhanced masking response to light in hamsters with IGL lesions. J. Comp. Physiol., A 184, 449–456. Redlin, U., Cooper, H.M., Mrosovsky, N., 2003. Increased masking response to light after ablation of the visual cortex in mice. Brain Res. 965, 1–8. Reebs, S.G., Lavery, R.J., Mrosovsky, N., 1989. Running activity mediates the phase-advancing effects of dark pulses on hamster circadian rhythms. J. Comp. Physiol., A 165, 811–818. Reed, H.E., Cutler, D.J., Brown, T.M., Brown, J., Coen, C.W., Piggins, H.D., 2002. Effects of vasoactive intestinal polypeptide on neurones of the rat suprachiasmatic nuclei in vitro. J. Neuroendocrinol. 14, 639–646. Reuss, S., 1991. Photoperiod effects on bombesin- and cholecystokinin-like immunoreactivity in the suprachiasmatic nuclei of the Djungarian hamster (Phodopus sungorus). Neurosci. Lett. 128, 13–16. Reuss, S., Bürger, K., 1994. Substance P-like immunoreactivity in the hypothalamic suprachiasmatic nucleus of Phodopus sungorus—Relation to daytime, photoperiod, sex and age. Brain Res. 638, 189–195. Reuss, S., Decker, K., Rosseler, L., Layes, E., Schollmayer, A., Spessert, R., 1995. Nitric oxide synthase in the hypothalamic suprachiasmatic nucleus of rat: evidence from histochemistry, immunohistochemistry and Western blot; and colocalization with VIP. Brain Res. 695, 257–262. Rhoades, R.W., Hsu, L., Parfett, G., 1979. An electronmicroscopic analysis of the optic nerve in the golden hamster. J. Comp. Neurol. 186, 491–504. Roberts, W.M., 1994. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J. Neurosci. 14, 3246–3262. Rosenwasser, A.M., Dwyer, S.M., 2002. Phase shifting the hamster circadian clock by 15-minute dark pulses. J. Biol. Rhythms 17, 238–247. Ruby, N.F., Saran, A., Kang, T., Franken, P., Heller, H.C., 1996. Siberian hamsters free run or become arrhythmic after a phase delay of the photocycle. Am. J. Physiol. 271, R881–R890. Ruby, N.F., Kang, T., Heller, H.C., 1997. Melatonin attenuates photic BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 disruption of circadian rhythms in Siberian hamsters. Am. J. Physiol. 273, R1540–R1549. Ruby, N.F., Joshi, N., Heller, H.C., 1998. Phase shift magnitude and direction determine whether Siberian hamsters reentrain to the photocycle. J. Biol. Rhythms 13, 506–517. Ruby, N.F., Dubocovich, M.L., Heller, H.C., 2000. Siberian hamsters that fail to reentrain to the photocycle have suppressed melatonin levels. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 278, R757–R762. Ruby, N.F., Brennan, T.J., Xie, X., Cao, V., Franken, P., Heller, H.C., 2002a. Role of melanopsin in circadian responses to light. Science 298, 2211–2213. Ruby, N.F., Joshi, N., Heller, H.C., 2002b. Constant darkness restores entrainment to phase-delayed Siberian hamsters. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 283, R1314–R1320. Ruby, N.F., Barakat, M.T., Heller, H.C., 2004. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in Siberian hamsters. J. Biol. Rhythms 19, 530–541. Rusak, B., Morin, L.P., 1976. Testicular responses to photoperiod are blocked by lesions of the suprachiasmatic nuclei in golden hamsters. Biol. Reprod. 15, 366–374. Rutishauser, U., Landmesser, L., 1996. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell–cell interactions. Trends Neurosci. 19, 422–427. Saeb-Parsy, K., Dyball, R.E., 2003. Defined cell groups in the rat suprachiasmatic nucleus have different day/night rhythms of single-unit activity in vivo. J. Biol. Rhythms 18, 26–42. Saper, C.B., Chou, T.C., Scammell, T.E., 2001. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731. Sato, T., Kawamura, H., 1984. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus). Neurosci. Res. 1, 45–52. Schaap, J., Bos, N.P., de Jeu, M.T., Geurtsen, A.M., Meijer, J.H., Pennartz, C.M., 1999. Neurons of the rat suprachiasmatic nucleus show a circadian rhythm in membrane properties that is lost during prolonged whole-cell recording. Brain Res. 815, 154–166. Schaap, J., Albus, H., Eilers, P.H., Detari, L., Meijer, J.H., 2001. Phase differences in electrical discharge rhythms between neuronal populations of the left and right suprachiasmatic nuclei. Neuroscience 108, 359–363. Schaap, J., Albus, H., VanderLeest, H.T., Eilers, P.H., Detari, L., Meijer, J.H., 2003. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: implications for circadian waveform and photoperiodic encoding. Proc. Natl. Acad. Sci. U. S. A. 100, 15994–15999. Schwartz, W.J., Takeuchi, J., Shannon, W., Davis, E.M., Aronin, N., 1994. Temporal regulation of light-induced Fos and Fos-like protein expression in the ventrolateral subdivision of the rat suprachiasmatic nucleus. Neuroscience 58, 573–583. Schwartz, W.J., Peters, R.V., Aronin, N., Bennett, M.R., 1996. Unexpected c-fos gene expression in the suprachiasmatic nucleus of mice entrained to a skeleton photoperiod. J. Biol. Rhythms 11, 35–44. Schwartz, W.J., Carpino Jr., A., De la Iglesia, H.O., Baler, R., Klein, D.C., Nakabeppu, Y., Aronin, N., 2000. Differential regulation of fos family genes in the ventrolateral and dorsomedial subdivisions of the rat suprachiasmatic nucleus. Neuroscience 98, 535–547. Schwartz, M.D., Nunez, A.A., Smale, L., 2004. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience 127, 13–23. Semo, M., Munoz, L.M., Foster, R.G., Jeffery, G., 2005. Melanopsin (Opn4) positive cells in the cat retina are randomly distributed across the ganglion cell layer. Vis. Neurosci. 22, 111–116. Shearman, L.P., Zylka, M.J., Weaver, D.R., Kolakowski Jr., L.F., Reppert, S.M., 1997. Two period homologs: circadian 57 expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269. Shearman, L.P., Sriram, S., Weaver, D.R., Maywood, E.S., Chaves, I., Zheng, B., Kume, K., Lee, C.C., van der Horst, G.T., Hastings, M.H., Reppert, S.M., 2000. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019. Shen, H., Semba, K., 1994. A direct retinal projection to the dorsal raphe nucleus in the rat. Brain Res. 635, 159–168. Shen, H., Watanabe, M., Tomasiewicz, H., Rutishauser, U., Magnuson, T., Glass, J.D., 1997. Role of neural cell adhesion molecule and polysialic acid in mouse circadian clock function. J. Neurosci. 17, 5221–5229. Shen, H., Glass, J.D., Seki, T., Watanabe, M., 1999. Ultrastructural analysis of polysialylated neural cell adhesion molecule in the suprachiasmatic nuclei of the adult mouse. Anat. Rec. 256, 448–457. Shen, S., Spratt, C., Sheward, W.J., Kallo, I., West, K., Morrison, C.F., Coen, C.W., Marston, H.M., Harmar, A.J., 2000. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc. Natl. Acad. Sci. U. S. A. 97, 11575–11580. Shen, H., Watanabe, M., Tomasiewicz, H., Glass, J.D., 2001. Genetic deletions of NCAM and PSA impair circadian function in the mouse. Physiol. Behav. 73, 185–193. Shi, H., Bartness, T.J., 2001. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res. Bull. 54, 375–385. Shibata, S., Shiratsuchi, A., Liou, S.Y., Ueki, S., 1984. The role of calcium ions in circadian rhythm of suprachiasmatic nucleus neuron activity in rat hypothalamic slices. Neurosci. Lett. 52, 181–184. Shibata, S., Liou, S.Y., Ueki, S., 1986. Influence of excitatory amino acid receptor antagonists and of baclofen on synaptic transmission in the optic nerve to the suprachiasmatic nucleus in slices of rat hypothalamus. Neuropharmacology 25, 403–409. Shibata, S., Tsuneyoshi, A., Hamada, T., Tominaga, K., Watanabe, S., 1992. Effect of substance P on circadian rhythms of firing activity and the 2-deoxyglucose uptake in the rat suprachiasmatic nucleus in vitro. Brain Res. 597, 257–263. Shibata, S., Watanabe, A., Hamada, T., Watanabe, S., 1994. Protein-synthesis inhibitor blocks (R,S)-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)- or substance P-induced phase shift of the circadian rhythm of neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci. Lett. 168, 159–162. Shigeyoshi, Y., Taguchi, K., Yamamoto, S., Takekida, S., Yan, L., Tei, H., Moriya, T., Shibata, S., Loros, J.J., Dunlap, J.C., Okamura, H., 1997. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91, 1043–1053. Shimazoe, T., Nakamura, S., Kobayashi, K., Watanabe, S., Miyasaka, K., Kono, A., Funakoshi, A., 2004. Role of 5-HT1B receptors in entrainment disorder of Otsuka Long Evans Tokushima fatty (OLETF) rats. Neuroscience 123, 201–205. Shirakawa, T., Moore, R.Y., 1994. Responses of rat suprachiasmatic nucleus neurons to substance P and glutamate in vitro. Brain Res. 642, 213–220. Shirakawa, T., Honma, S., Katsuno, Y., Oguchi, H., Honma, K., 2000. Synchronization of circadian firing rhythms in cultured rat suprachiasmatic neurons. Eur. J. Neurosci. 12, 2833–2838. Shiromani, P.J., Overstreet, D., Levy, D., Goodrich, C.A., Campbell, S.S., Gillin, J.C., 1988. Increased REM sleep in rats selectively bred for cholinergic hyperactivity. Neuropsychopharmacology 1, 127–133. Shivers, K., Muscat, L., 2004. Relative distribution of crossed and uncrossed retinohypothalamic tract (RHT) projections to the suprachiasmatic nucleus (SCN) in rat and mouse. Soc. Neurosci. 765.14. 58 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 Silver, J., Brand, S., 1979. A route for direct retinal input to the preoptic hypothalamus: dendritic projections into the optic chiasm. Am. J Anat. 155, 391–401. Silver, R., Lehman, M.N., Gibson, M., Gladstone, W.R., Bittman, E.L., 1990. Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN-lesioned adult hamsters. Brain Res. 525, 45–58. Silver, R., Romero, M.T., Besmer, H.R., Leak, R.K., Nunez, J.M., LeSauter, J., 1996a. Calbindin-D28K cells in the hamster SCN express light-induced Fos. NeuroReport 7, 1224–1228. Silver, R., LeSauter, J., Tresco, P.A., Lehman, M.N., 1996b. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813. Silver, R., Sookhoo, A.I., LeSauter, J., Stevens, P., Jansen, H.T., Lehman, M.N., 1999. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. NeuroReport 10, 3165–3174. Smale, L., Boverhof, J., 1999. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. J. Comp. Neurol. 403, 190–208. Smale, L., Michels, K.M., Moore, R.Y., Morin, L.P., 1990. Destruction of the hamster serotonergic system by 5,7-DHT: effects on circadian rhythm phase, entrainment and response to triazolam. Brain Res. 515, 9–19. Smale, L., Blanchard, J.H., Moore, R.Y., Morin, L.P., 1991. Immunocytochemical characterization of the suprachiasmatic nucleus and the intergeniculate leaflet in the diurnal ground squirrel, Spermophilus lateralis. Brain Res. 563, 77–86. Smale, L., Castleberry, C., Nunez, A.A., 2001. Fos rhythms in the hypothalamus of Rattus and Arvicanthis that exhibit nocturnal and diurnal patterns of rhythmicity. Brain Res. 899, 101–105. Smale, L., Lee, T., Nunez, A.A., 2003. Mammalian diurnality: some facts and gaps. J. Biol. Rhythms 18, 356–366. Smart, C.M., Biello, S.M., 2001. WAY-100635, a specific 5-HT1A antagonist, can increase the responsiveness of the mammalian circadian pacemaker to photic stimuli. Neurosci. Lett. 305, 33–36. Smeraski, C.A., Sollars, P.J., Ogilvie, M.D., Enquist, L.W., Pickard, G.E., 2004. Suprachiasmatic nucleus input to autonomic circuits identified by retrograde transsynaptic transport of pseudorabies virus from the eye. J. Comp. Neurol. 471, 298–313. Smith, R.D., Inouye, S.-I.T., Turek, F.W., 1989. Central administration of muscimol phase-shifts the mammalian circadian clock. J. Comp. Physiol. 164, 805–814. Smith, B.N., Sollars, P.J., Dudek, F.E., Pickard, G.E., 2001. Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J. Biol. Rhythms 16, 25–38. Sollars, P.J., Pickard, G.E., 1993. Time course of fiber outgrowth from fetal anterior hypothalamic heterografts. Brain Res. 614, 212–219. Sollars, P.J., Kimble, D.P., Pickard, G.E., 1995. Restoration of circadian behavior by anterior hypothalamic heterografts. J. Neurosci. 15, 2109–2122. Sollars, P.J., Ogilvie, M.D., Rea, M.A., Pickard, G.E., 2002. 5-HT1B receptor knockout mice exhibit an enhanced response to constant light. J. Biol. Rhythms 17, 428–437. Sollars, P.J., Smeraski, C.A., Kaufman, J.D., Ogilvie, M.D., Provencio, I., Pickard, G.E., 2003. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis. Neurosci. 20, 601–610. Speh, J.C., Moore, R.Y., 1990. Synaptogenesis and retinohypothalamic tract (RHT) development in the hamster suprachiasmatic nucleus (SCN). Society for Neuroscience 16, 602 (Ref Type: Abstract). Speh, J.C., Moore, R.Y., 1993. Retinohypothalamic tract development in the hamster and rat. Dev. Brain Res. 76, 171–181. Spoelstra, K., Oklejewicz, M., Daan, S., 2002. Restoration of self-sustained circadian rhythmicity by the mutant clock allele in mice in constant illumination. J. Biol. Rhythms 17, 520–525. Sprouse, J., Reynolds, L., Braselton, J., Schmidt, A., 2004a. Serotonin-induced phase advances of SCN neuronal firing in vitro: a possible role for 5-HT5A receptors? Synapse 54, 111–118. Sprouse, J., Reynolds, L., Li, X., Braselton, J., Schmidt, A., 2004b. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 46, 52–62. Stamp, J.A., Piggins, H.D., Rusak, B., Semba, K., 1997. Distribution of ionotropic glutamate receptor subunit immunoreactivity in the suprachiasmatic nucleus and intergeniculate leaflet of the hamster. Brain Res. 756, 215–224. Starkey, S.J., 1996. Melatonin and 5-hydroxytryptamine phase-advance the rat circadian clock by activation of nitric oxide synthesis. Neurosci. Lett. 211, 199–202. Stephan, F.K., Donaldson, J.A., Gellert, J., 1982. Retinohypothalamic tract symmetry and phase shifts of circadian rhythms in rats and hamsters. Physiol. Behav. 29, 1153–1159. Stetson, M.H., Watson-Whitmyre, M., 1976. Nucleus suprachiasmaticus: the biological clock in the hamster? Science 191, 197–199. Summer, T.L., Ferraro, J.S., McCormack, C.E., 1984. Phase-response and Aschoff illuminance curves for locomotor activity rhythm of the rat. Am. J. Physiol. 246, R299–R304. Sumova, A., Illnerova, H., 1996. Melatonin instantaneously resets intrinsic circadian rhythmicity in the rat suprachiasmatic nucleus. Neurosci. Lett. 218, 181–184. Sumova, A., Travnickova, Z., Mikkelsen, J.D., Illnerova, H., 1998. Spontaneous rhythm in c-Fos immunoreactivity in the dorsomedial part of the rat suprachiasmatic nucleus. Brain Res. 801, 254–258. Sumova, A., Travnickova, Z., Illnerova, H., 2000. Spontaneous c-Fos rhythm in the rat suprachiasmatic nucleus: location and effect of photoperiod. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 279, R2262–R2269. Sumova, A., Bendova, Z., Sladek, M., Kovacikova, Z., Illnerova, H., 2004. Seasonal molecular timekeeping within the rat circadian clock. Physiol. Res. 53 (Suppl. 1), S167–S176. Swann, J.M., Turek, F.W., 1985. Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science 228, 898–900. Takahashi, J.S., DeCoursey, P.J., Bauman, L., Menaker, M., 1984. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature 308, 186–188. Takatsuji, K., Miguel-Hidalgo, J.-J., Tohyama, M., 1991. Substance P-immunoreactive innervation from the retina to the suprachiasmatic nucleus in the rat. Brain Res. 568, 223–229. Takatsuji, K., Senba, E., Mantyh, P.W., Tohyama, M., 1995. A relationship between substance P receptor and retinal fibers in the rat suprachiasmatic nucleus. Brain Res. 698, 53–61. Takeuchi, Y., Takahashima, M., Katoh, Y., Nishikawa, T., Takahashi, K., 1991. N-Methyl-D-aspartate, quisqualate and kainate receptors are all involved in transmission of photic stimulation in the suprachiasmatic nucleus in rats. Brain Res. 563, 127–131. Tang, I.H., Murakami, D.M., Fuller, C.A., 2002. Unilateral optic nerve transection alters light response of suprachiasmatic nucleus and intergeniculate leaflet. Am. J. Physiol. 282, R569–R577. Teclemariam-Mesbah, R., ter Horst, G.J., Postema, F., Wortel, J., Buijs, R.M., 1999. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 406, 171–182. Teshima, K., Kim, S.H., Allen, C.N., 2003. Characterization of an BR A I N R ES E A RC H R EV IE W S 5 1 (2 0 0 6) 1 –60 apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience 120, 65–73. Thomson, A.M., 1984. Slow, regular discharge in suprachiasmatic neurons is calcium dependent, in slices of rat brain. Neuroscience 13, 761–767. Thomson, A.M., West, D.C., 1990. Factors affecting slow regular firing in the suprachiasmatic nucleus in vitro. J. Biol. Rhythms 5, 59–75. Tiao, Y.-C., Blakemore, C., 1976. Regional specialization in the golden hamster's retina. J. Comp. Neurol. 168, 439–458. Tieman, S.B., Tieman, D.G., 1996. N-Acetylaspartylglutamate immunoreactivity in human retina. Vision Res. 36, 941–947. Tieman, S.B., Moffett, J.R., Irtenkauf, S.M., 1991. Effect of eye removal on N-acetylaspartylglutamate immunoreactivity in retinal targets of the cat. Brain Res. 562, 318–322. Tierno, A., Fiore, P., Gannon, R.L., 2002. Delta opioid inhibition of light-induced phase advances in hamster circadian activity rhythms. Brain Res. 937, 66–73. Tischler, R.C., Morin, L.P., 2003. Reciprocal serotonergic connections between the hamster median and dorsal raphe nuclei. Brain Res. 981, 126–132. Tokunaga, A., Ono, K., Kondo, S., Tanaka, H., Kurose, K., Nagai, H., 1992. Retinal projections in the house musk shrew, Suncus murinus, as determined by anterograde transport of WGA-HRP. Brain Behav. Evol. 40, 321–329. Tominaga, K., Shibata, S., Ueki, S., Watanabe, S., 1992. Effects of 5-HT1A receptor agonists on the circadian rhythm of wheel-running activity in hamsters. Eur. J. Pharmacol. 214, 79–84. Tournier, B.B., Menet, J.S., Dardente, H., Poirel, V.J., Malan, A., Masson-Pevet, M., Pevet, P., Vuillez, P., 2003. Photoperiod differentially regulates clock genes' expression in the suprachiasmatic nucleus of Syrian hamster. Neuroscience 118, 317–322. Tousson, E., Meissl, H., 2004. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J. Neurosci. 24, 2983–2988. Trachsel, L., Heller, H.C., Miller, J.D., 1995. Nicotine phase-advances the circadian neuronal activity rhythm in rat suprachiasmatic nuclei explants. Neuroscience 65, 797–803. Treep, J.A., Abe, H., Rusak, B., Goguen, D.M., 1995. Two distinct retinal projections to the hamster suprachiasmatic nucleus. J. Biol. Rhythms 10, 299–307. Ueyama, T., Krout, K.E., Van Nguyen, X., Karpitskiy, V., Kollert, A., Mettenleiter, T.C., Loewy, A.D., 1999. Suprachiasmatic nucleus: a central autonomic clock. Nat. Neurosci. 2, 1051–1053. Underwood, H., Menaker, M., 1970. Extraretinal light perception: entrainment of the biological clock controlling lizard locomotor activity. Science 170, 190–193. Urbanski, H.F., Doan, A., Pierce, M., 1991. Immunocytochemical investigation of luteinizing hormone- releasing hormone neurons in Syrian hamsters maintained under long or short days. Biol. Reprod. 44, 687–692. van den Pol, A.N., 1980. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J. Comp. Neurol. 191, 661–702. van den Pol, A.N., 1991. The suprachiasmatic nucleus: morphological and cytochemical substrates for cellular interaction. In: Klein, D.C., Moore, R.Y., Reppert, S.M. (Eds.), Suprachiasmatic Nucleus: The Mind's Clock. Oxford Univ. Press, New York, pp. 17–50. van den Pol, A.N., Gorcs, T., 1986. Synaptic relationships between neurons containing vasopressin, gastrin-releasing peptide, vasoactive intestinal polypeptide, and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J. Comp. Neurol. 252, 507–521. van den Pol, A.N., Tsujimoto, K.L., 1985. Neurotransmitters of 59 the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15, 1049–1086. van den Pol, A.N., Cao, V., Heller, H.G., 1998. Circadian system of mice integrates brief light stimuli. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 275, R654–R657. Van der Beek, E.M., Horvath, T.L., Wiegant, V.M., Buijs, R.M., 1997. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J. Comp. Neurol. 384, 569–579. Van der Gucht, E., Hof, P.R., Arckens, L., 2003. Neurochemical organization, architectonic subdivision and three-dimensional reconstruction of cat ventral lateral geniculate nucleus and monkey pregeniculate nucleus. Soc. Neurosci. 33, 68.7. Van der Horst, G.T.J., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., De Wit, J., Verkerk, A., Eker, A.P.M., Van Leenen, D., Buijs, R., Bootsma, D., Hoeijmakers, J.H.J., Yasui, A., 1999. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630. Van Reeth, O., Turek, F.W., 1989. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepines. Nature 339, 49–51. Van Reeth, O., Hinch, D., Tecco, J.M., Turek, F.W., 1991. The effects of short periods of immobilization on the hamster circadian clock. Brain Res. 545, 208–214. Vansteensel, M.J., Yamazaki, S., Albus, H., Deboer, T., Block, G.D., Meijer, J.H., 2003a. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr. Biol. 13, 1538–1542. Vansteensel, M.J., Deboer, T., Dahan, A., Meijer, J.H., 2003b. Onset and offset following GABA-ergic and opioid receptor activation. J. Biol. Rhythms 18, 297–306. Varcoe, T.J., Kennaway, D.J., Voultsios, A., 2003. Activation of 5-HT2C receptors acutely induces Per gene expression in the rat suprachiasmatic nucleus at night. Brain Res. Mol. Brain Res. 119, 192–200. Vertes, R.P., 1991. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 313, 643–668. Vidal, L., Morin, L.P., 2005. Hypothalamic and zona incerta neurons expressing hypocretin, but not melanin concentrating hormone, project to the hamster intergeniculate leaflet. Neuroscience 134, 1081–1090. Vincent, S.R., Kimura, H., 1992. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46, 755–784. Vindlacheruvu, R.R., Ebling, F.J.P., Maywood, E.S., Hastings, M.H., 1992. Blockade of glutamatergic neurotransmission in the suprachiasmatic nucleus prevents cellular and behavioural responses of the circadian system to light. Eur. J. Neurosci. 4, 673–679. Vitaterna, M.H., King, D.P., Chang, A.-M., Kornhauser, J.M., Lowrey, P.L, McDonald, J.D., Dove, W.F., Pinto, L.H., Turek, F.W., Takahashi, J.S., 1994. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725. Vrang, N., Larsen, P.J., Moller, M., Mikkelsen, J.D., 1995a. Topographical organization of the rat suprachiasmatic-paraventricular projection. J. Comp. Neurol. 353, 585–603. Vrang, N., Larsen, P.J., Mikkelsen, J.D., 1995b. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 684, 61–69. Vrang, N., Mrosovsky, N., Mikkelsen, J.D., 2003. Afferent projections to the hamster intergeniculate leaflet demonstrated 60 BR A I N R ES E A RC H R EV IE W S 5 1 ( 2 0 0 6) 1 –60 by retrograde and anterograde tracing. Brain Res. Bull. 59, 267–288. Wagner, S., Castel, M., Gainer, H., Yarom, Y., 1997. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387, 598–603. Wagner, S., Sagiv, N., Yarom, Y., 2001. GABA-induced current and circadian regulation of chloride in neurones of the rat suprachiasmatic nucleus. J. Physiol. 537, 853–869. Wang, H., Morris, J.F., 1996. Presence of neuronal nitric oxide synthase in the suprachiasmatic nuclei of mouse and rat. Neuroscience 74, 1059–1068. Warren, E.J., Allen, C.N., Brown, R.L., Robinson, D.W., 2003. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur. J. Neurosci. 17, 1727–1735. Watanabe, A., Ono, M., Shibata, S., Watanabe, S., 1995. Effect of a nitric oxide synthase inhibitor, N-nitro-L-arginine methylester, on light-induced phase delay of circadian rhythm of wheel-running activity in golden hamsters. Neurosci. Lett. 192, 25–28. Watanabe, K., Vanecek, J., Yamaoka, S., 2000. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 877, 361–366. Watts, A.G., Swanson, L.W., 1987. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 258, 230–252. Watts, A.G., Swanson, L.W., Sanchez-Watts, G., 1987. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp. Neurol. 258, 204–229. Weber, E.T., Rea, M.A., 1997. Neruopeptide Y blocks light-induced phase advances but not delays of the circadian activity rhythm in hamsters. Neurosci. Lett. 231, 159–162. Weber, E.T., Gannon, R.L., Michel, A.M., Gillette, M.U., Rea, M.A., 1995. Nitric oxide synthase inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res. 692, 137–142. Welsh, D.K., Logothetis, D.E., Meister, M., Reppert, S.M., 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706. Wheal, H.V., Thomson, A.M., 1984. The electrical properties of neurones of the rat suprachiasmatic nucleus recorded intracellularly in vitro. Neuroscience 13, 97–104. Wickland, C., Turek, F.W., 1994. Lesions of the thalamic intergeniculate leaflet block activity-induced phase shifts in the circadian activity rhythm of the golden hamster. Brain Res. 660, 293–300. Wollnik, F., Brysch, W., Uhlmann, E., Gillardon, F., Bravo, R., Zimmermann, M., Schlingensiepen, K.H., Herdegen, T., 1995. Block of c-Fos and JunB expression by antisense oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. Eur. J. Neurosci. 7, 388–393. Yagita, K., Tamanini, F., van der Horst, G.T., Okamura, H., 2001. Molecular mechanisms of the biological clock in cultured fibroblasts. Science 292, 278–281. Yamaguchi, S., Isejima, H., Matsuo, T., Okura, R., Yagita, K., Kobayashi, M., Okamura, H., 2003. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302, 1408–1412. Yamazaki, S., Goto, M., Menaker, M., 1999. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J. Biol. Rhythms 14, 197–201. Yamazaki, S., Numano, R., Abe, M., Hida, A., Takahashi, R., Ueda, M., Block, G.D., Sakaki, Y., Menaker, M., Tei, H., 2000. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685. Yan, L., Okamura, H., 2002. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 15, 1153–1162. Yan, L., Silver, R., 2004. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur. J. Neurosci. 19, 1105–1109. Yan, L., Takekida, S., Shigeyoshi, Y., Okamura, H., 1999. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience 94, 141–150. Yannielli, P.C., Harrington, M.E., 2000. Neuropeptide Y applied in vitro can block the phase shifts induced by light in vivo. NeuroReport 11, 1587–1591. Yannielli, P.C., Harrington, M.E., 2001. The neuropeptide YY5 receptor mediates the blockade of “photic-like” NMDA-induced phase shifts in the golden hamster. J. Neurosci. 21, 5367–5373. Yannielli, P., Harrington, M.E., 2004. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog. Neurobiol. 74, 59–76. Yannielli, P.C., McKinley, B.J., Harrington, M.E., 2002. Is novel wheel inhibition of per1 and per2 expression linked to phase shift occurrence? Neuroscience 112, 677–685. Yannielli, P.C., Brewer, J.M., Harrington, M.E., 2004. Blockade of the NPY Y5 receptor potentiates circadian responses to light: complementary in vivo and in vitro studies. Eur. J. Neurosci. 19, 891–897. Yokota, S.I., Horikawa, K., Akiyama, M., Moriya, T., Ebihara, S., Komuro, G., Ohta, T., Shibata, S., 2000. Inhibitory action of brotizolam on circadian and light-induced per1 and per2 expression in the hamster suprachiasmatic nucleus. Br. J. Pharmacol. 131, 1739–1747. Yoo, S.H., Yamazaki, S., Lowrey, P.L., Shimomura, K., Ko, C.H., Buhr, E.D., Siepka, S.M., Hong, H.K., Oh, W.J., Yoo, O.J., Menaker, M., Takahashi, J.S., 2004. PERIOD2∷LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 101, 5339–5346. Yoshimura, T., Ebihara, S., 1996. Spectral sensitivity of photoreceptors mediating phase shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N(+/+) mice. J. Comp. Physiol., A 178, 797–802. Yoshimura, T., Nishio, M., Goto, M., Ebihara, S., 1994. Differences in circadian photosensitivity between retinally degenerate CBA/J mice (rd/rd) and normal CBA/N mice (+/+). J. Biol. Rhythms 9, 51–60. Zhang, Y., Zee, P.C., Kirby, J.D., Takahashi, J.S., Turek, F.W., 1993. A cholinergic antagonist, mecamylamine, blocks light-induced Fos immunoreactivity in specific regions of the hamster suprachiasmatic nucleus. Brain Res. 615, 107–112. Zhang, Y., Kornhauser, J.M., Zee, P.C., Mayo, K.E., Takahashi, J.S., Turek, F.W., 1996. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience 70, 951–961. Zylka, M.J., Shearman, L.P., Weaver, D.R., Reppert, S.M., 1998. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20, 1103–1110.