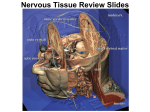
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Glia cells, lipid metabolism and Alzheimer`s disease
Activity-dependent plasticity wikipedia , lookup
Neuroplasticity wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Synaptogenesis wikipedia , lookup
Nervous system network models wikipedia , lookup
Environmental enrichment wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Neuroregeneration wikipedia , lookup
Neuroeconomics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Subventricular zone wikipedia , lookup
Neurogenomics wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Aging brain wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Haemodynamic response wikipedia , lookup
Alzheimer's disease wikipedia , lookup
Glia cells, lipid metabolism and Alzheimer’s disease Student: Debbie Hendrickx Supervisor: Dr. Mark Verheijen 1 Abstract Research on functioning of the central nervous system (CNS) has mainly focused on neurons alone. Glia cells, which far outnumber neurons in the brain, were thought to support those neuronal functions and were considered to be less important. However, intense research has revealed the true role of glia cells in the CNS, which include homeostasis maintenance, providing feedback to neurons and regulation of synaptic plasticity. Astrocytes, the most abundant glia cells, are involved in brain lipid metabolism as they secrete Apolipoprotein E (ApoE), the main lipid carrier in the brain, and express lipoprotein receptors involved in the uptakeof lipids. The human ApoE exists in three isoforms of which the ApoE4 isoform is associated with an increased risk of developing Alzheimer’s disease (AD). Furthermore, epidemiological studies revealed that patients with hypercholesterolemia that were treated with cholesterol lowering drugs also had a reduced risk of developing AD. This implicates that lipid metabolism might contribute to AD pathogenesis. Together with the important role of astrocytes in regulating this lipid metabolism, this suggests that astrocytes might contribute to the pathogenesis of AD, although they are not causative for its onset. 2 Content Glia cells Astrocytes Oligodendrocytes Microglia Satellite cells Schwann cells Dysfunctional glia cells 4 4 7 7 7 7 7 Astrocytes and lipid metabolism 9 Alzheimer’s disease 11 Alzheimer’s disease and lipid metabolism Senile plaques Amyloid precursor protein Apolipoprotein E Neurofibrillary tangles Animal models for Alzheimer’s disease Current and future treatment 13 14 14 17 20 22 25 Astrocytes and Alzheimer’s disease 26 Discussion 29 Conclusion 32 References 33 3 Glia cells Glia cells are non-neuronal cells within the nervous system, where they outnumber neurons by approximately ten-fold. They are non-excitable and communicate with each other as well as with neurons via chemical signals rather than electrical signals. There are several types of glia cells in both the peripheral nervous system (PNS) and central nervous system (CNS), each with their own functions. Astrocytes Astrocytes are the most abundant cell type in the CNS where they serve numerous functions (Fields and Stevens-Graham 2002; Benarroch 2005; Halassa et al. 2006). Astrocytes are interconnected by gap junctions forming a network throughout the brain. They ensheath the nodes of Ranvier and make contacts with oligodendrocytes to maintain their external environment and energy supply for optimal functioning (De Keyser et al. 2008). In case of injury, astrocytes can phagocytose injured cells after which they replace them and form a glial scar. They are slow to react to injury, but stay activated during the late recovery stage (Gehrmann et al. 1995). Astrocytes produce several growth factors and regulate the induction and maintenance of neurite outgrowth, axonal guidance and synapse formation (Gee and Keller 2005). Their most amazing function is probably their role in the tripartite synapse, which consist of the presynaptic neuron, the postsynaptic neuron and the astrocyte itself. This structure is dynamic and important in both development, when the communication between the neurons and the astrocytes mediates synaptogenesis, and throughout adult live when bidirectional signalling can influence the functioning and strength of the synapses, which is also known as synaptic plasticity. (Fields and Stevens-Graham 2002; Benarroch 2005; Halassa et al. 2006; Stevens 2008). Astrocytes act as a physical link between the pre- and postsynaptic neurons on one side and blood capillaries on the other, which is important for maintaining the external environment for optimal functioning of the neurons (Fig. 1) (Parri and Crunelli 2003; Benarroch 2005). 4 Fig. 1. Astrocytes function in a tripartite synapse and form a physical link between neurons and blood vessels. They provide the neurons with energy supplies and maintain their external environment for optimal neuronal functioning (Benarroch 2005). The astrocytic endfeet surround the brain capillary endothelial cells, and as such contribute to the blood-brain barrier (BBB) which protects the brain from ionic variations in the microenvironment and penetrance of toxic substances. Astrocytes can influence BBB permeability when needed. Furthermore, it enables the astrocyte to regulate the microenvironment by transporting ions and other substances in a bidirectional manner from the blood, which is important in the metabolic support of neurons. Astrocytes detect synaptic activity by binding of neurotransmitters to receptors on the astrocytic membrane. By secreting vasoactive substances, astrocytes can regulate the blood flow in reaction to varying levels of neuronal activity, since an active brain region requires more oxygen and energy (Parri and Crunelli 2003; Benarroch 2005). Depolarization of neurons causes the release of neurotransmitters and K+. The excitatory neurotransmitter glutamate and Na+ are co-transported into the astrocytes, thereby terminating the action of glutamate released by the presynaptic neuron and as such prevent continuous post-synaptic activation and desensitization. The accumulating Na+ within the astrocyte triggers activation of the Na+- K+ ATPase. This in turn, causes the clearance of K+ that was also released by the neuron. In addition, K+ is also taken up by astrocytes via inward rectifying K+ channels (Kir channels) (Benarroch 2005; Seifert et al. 2006). Besides terminating the action of glutamate, astrocytes are also responsible for the clearance of γaminobutyric acid (GABA) and glycine, which both are inhibitory neurotransmitters. In all cases, after clearance of the neurotransmitter by the astrocyte, the neurotransmitter is transported back to the presynaptic neuron or first degradated and then re-synthesized in the presynaptic neuron, after which they are stored in synaptic vesicles and ready to be reused. However, maintaining synapses and uptake of neurotransmitter and thereby terminating the presynaptic signals are not the only functions of the astrocyte in the tripartite synapse. Astrocytes have G-protein coupled receptors that bind neurotransmitters, ATP or adenosine. This enables them to monitor the activity of neurons directly (Fields and StevensGraham 2002). Activation of these G-protein coupled receptor results in an intracellular [Ca2+] rise in the astrocyte causing the release of chemical transmitters, including several 5 neurotransmitters, ATP and D-serine (Fields and Stevens-Graham 2002; Halassa et al. 2006). The characteristics of the [Ca2+] increase varies in speed, amplitude and frequency, is depending on the input and may have different functions (Santello and Volterra 2008). The secreted chemical transmitters are also called gliotransmitters and they give feedback to neurons, influencing neuronal excitability and synaptic strength. Different responses might be elicited by secreting different types of gliotransmitters, depending on the characteristics of the [Ca2+] response evoked. One of these gliotransmitters is ATP, which can bind the presynaptic neuron thereby modulating synaptic transmission. ATP also binds receptors on neighbouring astrocytes, inducing inositol triphosphate (IP3) formation that subsequently triggers the release of Ca2+ stored in the endoplasmatic reticulum (ER) (Fig. 2). This way, astrocytes are capable of communicating with each other. A rise in intracellular [Ca2+] could also be elicited by IP3 traveling through the gap junctions between astrocytes. This communication may spread as a Ca2+ wave through a network of astrocytes, and could theoretically alter synaptic transmission of neighbouring neurons as well. Fig. 2. Astrocytes communicate with each other by a traveling Ca2+ signal. Astrocytes secrete ATP, which binds to neighbouring astrocytes and induce the formation of IP3. This in turn will cause the release of stored Ca2+ from the ER (adapted from Benarroch 2005). ATP can be hydrolized to adenosine which inhibits presynaptic neurotransmitter release. Astrocytes can regulate this inhibition by controlling the levels of extracellular adenosine. Astrocytes can also secrete glutamate in a Ca2+ dependent manner, depending on the neurotransmitter released by the presynaptic neuron and the receptors activated on the astrocyte (Santello and Volterra 2008). This will result in a specific Ca2+ reaction, varying in frequency, speed and amplitude either triggering glutamate release or not. This astrocytesecreted glutamate can have different consequences, since it can either target presynaptic axonal terminals or extrasynaptic dendrites. Activation of the N-methyl-D-aspartate (NMDA) receptors on axonal terminals results in strengthening of the synaptic transmission, whereas activation of extrasynaptic NMDA receptors on dendrites could induce synchronous depolarization of groups of neurons that are all innervated by the same astrocyte. Astrocytic glutamate release can increase synaptic transmitter release in excitatory synapses or can alter the strength of inhibitory interneurons by providing excitatory feedback. It remains to be determined whether the same astrocyte can secrete both adenosine (in the form of ATP) and glutamate, which have opposing effects on neurotransmission or that different astrocytes secrete different gliotransmitters. 6 Secretion of D-serine by astrocytes can also regulate the activity of the postsynaptic NMDA receptors and thereby neuronal plasticity by binding to the glycine bindingsite as a co-agonist (Santello and Volterra 2008). Astrocytes are known to secrete ApoE, a lipoprotein involved in transporting cholesterol, which gives them a role in lipid metabolism as well (Hirsch-Reinshagen and Wellington 2007), as will be discussed below. Oligodendrocytes Oligodendrocytes, another subtype of glia cells in the CNS, are responsible for the insulation of axons by providing a myelin sheath (Baumann et al. 2001). One oligodendrocyte can ensheath multiple axons. This results in an increase in conduction speed, provides physical support. As such, myelination is important for the maintenance of axons, however, myelination also inhibits regeneration of axons after neuronal injury. Microglia Microglia are the immune cells of the CNS and are the first cell type to react to infections, injury and/or degeneration (Gehrmann et al. 1995; Kreutzman 1995; Rock et al. 2004). Once activated, they recruit to the site of injury or infection and phagocytose affected cells and secrete cytotoxic and/or inflammatory mediators. Secretion of interleukin-1 (IL-1) by microglia could activate astrocytes, which might explain their late response. Microglia also have antigen presenting properties and are positive for both MHC-I and MHC-II. Satellite cells In the PNS, satellite cells ensheat neuronal cell bodies within ganglions and provide physical support and protection (Hanani 2005). They are capable of receiving and transmitting chemical signals and supply nutrients to the neurons and regulate the external chemical microenvironment. Schwann cells Schwann cells are the PNS counterpart of oligodendrocytes and myelinate the axons of peripheral neurons to increase conduction speed and to provide physical support. One Schwann cell can myelinate only a single segment of one axon in contrary to the oligodendrocytes. They are responsible for the maintenance of axons and even promote axon regeneration in case of injury. In addition, they provide feedback on neuronal activity by regulating the amount of neurotransmitter release by the presynaptic terminal and thereby control synaptic strength (Fields and Stevens-Graham 2002). Dysfunctional glia cells Based on what it described above, it is obvious that glia cells have an important role in neuronal development and maintenance, functioning and plasticity. Dysregulation in the functioning of glia cells could therefore have serious consequences for the functioning of the nervous system, e.g. dysfunctional astrocytes contribute to multiple diseases, as will be discussed below. 7 Astrocytes are responsible for the clearance of glutamate from the synaptic cleft. If they fail to do so, the postsynaptic neuron gets overexcitated and cell death may follow, a process that is called excitotoxity. The postsynaptic neuron degenerates and without any contacts, the presynaptic neuron will form an altered projection creating an excitatory feedback loop, known as mossy fiber sprouting. This is what happens with granule cells in the hippocampus after head injury or epileptic seizures (Santhakumar et al. 2005). Granule cells make new projection to their own dendrites and since their glutamatergic, they’ll create an excitatory feedback loop, enhancing neuronal excitability and increasing seizure probability. Insufficient clearance of glutamate by astrocytes possibly also underlies amyotrophic lateral sclerosis (ALS). Patients with ALS showed a loss of the main glutamate transporter, called excitatory amino acid transporter 2 (EAAT2), which causes insufficient clearance of glutamate and degeneration of cortical and spinal motor neurons (Seifert et al. 2006). Furthermore, astrocytes were found to secrete toxic substances due to a mutation in the antioxidant enzyme Cu/Zn superoxide dismutase 1 (SOD1) (De Keyser et al. 2008). It remains to be determined which and whether one of these mechanisms contributes to the unset of ALS. Schizophrenia could result from an increase in the excitatory amino acid transporter (EAAT) 2 expression by astrocytes in the prefrontal cortex (De Keyser et al. 2008). The EAAT2 is responsible for the clearance of glutamate from the synaptic cleft and an increased expression causes glutamate to be taken up more efficiently and glutamatergic neurotransmission to decrease. This results in a reduced thalamic function, which normally ‘filters’ incoming sensory information. Schizophrenic patients thus receive too many sensory signals, provoking psychosis. Dysfunctional astrocytes may also contribute to the development of multiple sclerosis (MS) (De Keyser et al. 2004; Benarroch 2005). Patients with MS have β2-adrenergic receptordeficient astrocytes in their white matter. These receptors are involved in the formation of cAMP, which normally prevents expression of MHC-II by astrocytes. In MS, astrocytes can therefore transform into antigen-presenting cells by cAMP-expression of MCH-II molecules. This could induce a T cell-mediated immune reaction against a myelin component of the oligodendrocytes, causing demyelination. In addition, astrocytic trophic support to oligodendrocytes is reduced in MS, causing apoptosis of these cells. Also, glycogenolysis in astrocytes is impaired, resulting in an insufficient energy supply to axons and axonal degeneration. Astrocytes are also involved in cerebral edema, which is an excess of fluid in either the intracellular or extracellular brain tissue (Benarroch 2005; De Keyser et al. 2008). The two main types are vasogenic and cytotoxic cerebral edema, which coexist in many neurologic disorders. Vasogenic edema is characterized by a disruption of the endothelial tight junction due to astrocytic secretion of vascular endothelial growth factor (VEGF), which downregulated occludin expression, a tight junction protein. Furthermore, aquaporin-4 (AQP4), a water channel important in osmotic homeostasis, is upregulated in astrocytes. This leads to an increased BBB permeability and osmotic dysregulation, causing an increase in extracellular volume and astrocytic swelling. Several causes may underlie vasogenic edema, including cerebral tumours, head trauma, focal inflammation and some other brain disorders. 8 Cytotoxic edema, also known as cellular edema, is caused by astrocytic swelling probably due to malfunction of the Na+- K+ ATPase, resulting in the uptake of Na+ and water or other substances that increase osmolarity and thereby water uptake. Cytotoxic edema can be caused by hypoxia or brain injury. In both types of edema the astrocytic swelling causes glutamate release by reversal of the glutamate transporter, which leads to excitotoxity and degeneration of normal brain cells. Astrocytes are thought to play a role in the spreading of a wave of cortical inhibition, known as cortical spreading depression (CSD) (Benarroch 2005, Seifert et al. 2006, De Keyser et al. 2008). This CSD is what causes the aura in migraine, but might also play a role in migraine without aura. However, results contradict about the involvement of astrocytes in CSD and migraine. A study done by Peters et al. concluded that different mechanisms underlie astrocytic Ca2+ waves and CSD (Peters et al. 2003). The true role of astrocytes in CSD remains elusive. Astrocytes are not always causative for a disease, but could still contribute to its progression. Hepatic failure results in insufficient clearance of neurotoxic substances from the blood and will lead to hepatic encephalopathy (Benarroch 2005, Seifert et al. 2006, De Keyser et al. 2008). When ammonia is not eliminated by the liver, levels increase in both blood and cerebrospinal fluid (CSF). This hyperammoneamia causes increased diffusion of ammonia into astrocytes, where it is converted together with glutamate to glutamine by the enzyme glutamine synthetase. Glutamine accumulation causes osmotic stress and dysregulation in K+ homeostasis resulting in astrocytic swelling. In addition, ammonia may inhibit glutamate uptake by downregulation of EAAT1 and EAAT2 causing excitotoxity and neuron degeneration (Benarroch 2005). Patients with Alzheimer’s disease (AD), a neurodegenerative disease causing dementia, showed an altered astrocytic phenotype. It remains to be determined whether this astrocytic phenotype is the cause or result of AD, but it could indicate that astrocytes contribute to AD as well. Furthermore, a known risk factor for AD is carrying of the ApoE4 allele, a lipoprotein expressed by astrocytes (Polvikoski et al. 1995). ApoE is involved in cholesterol metabolism and homeostasis and is present in amyloid-β peptide plaques in the cerebral cortex that characterizes AD. Subjects with the ApoE4 allele also show greater plaque formation irrespective of having dementia or not. Cholesterol levels can influence amyloid-β peptide synthesis, but the direct link between astrocytes, ApoE in lipid metabolism and AD remains elusive (Hirsch-Reinshagen and Wellington 2007; Cedazo-Mínguez 2007), and is the focus of this thesis. The molecular functioning op ApoE will be explained in a first attempt to further evaluate the link between astrocytes and AD. Astrocytes and lipid metabolism Cholesterol is a major component of many membranes in the brain, including the plasma membranes of astrocytes and neurons, and of the myelin sheaths formed by oligodendrocytes (Björkhem and Meaney 2004). It has an important function in regulating membrane lipid organization, including membrane or lipid rafts, and membrane fluidity (Reid et al. 2007). Furthermore, it contributes to neuronal repair and remodelling. Almost all cholesterol in the 9 brain is synthesized in situ, since circulating cholesterol and brain cholesterol are separated by the BBB (Dietschy and Turley 2001; Björkhem and Meaney 2004). It is important to maintain cholesterol at constant levels and any alterations could result in dysfunctional cells and even diseases. The brain is unable to degrade excess cholesterol, so it has to be transported over the BBB to be degradated by the liver. This can be accomplished by binding to lipoproteins that facilitates transport of cholesterol over the BBB or by the conversion of cholesterol to 24Shydroxycholesterol to be able to cross the BBB itself (Björkhem and Meaney 2004). Apolipoprotein E (ApoE) is the main lipoprotein in the brain, where it is predominantly secreted by astrocytes, but also by microglia (Fig3A) (Hirsch-Reinshagen and Wellington 2007). Even neurons have been reported to secrete ApoE, under various physiological and pathological conditions, which is regulated by astrocytes (Harris et al. 2004). Three isoforms exist, namely ApoE2, ApoE3 and ApoE4, which are the main components of the primary lipid-carrying particles, similar in size and density as the high density lipoprotein (HDL). These isoforms function differently in normal and abnormal conditions, due to their difference in structure and biophysical properties (Huang et al. 2004). ApoE plays a major role in lipid metabolism and homeostasis in the brain (Björkhem and Meaney 2004). It is important for the maintenance and repair of neurons by delivering cholesterol and other lipids to the target areas. ApoE is lipidated by ATP-binding cassette proteins (ABC transporters), that transports cellular cholesterol and phospholipids from mainly astrocytes on ApoE particles (Wahrle et al. 2004; Hirsch-Reinshagen and Wellington 2007). The ApoE4 isoform has the lowest potency to act as a cholesterol acceptor, making them least efficient in promoting the efflux of cholesterol from plasma membranes (Michikawa et al. 2000). Several classes of ABC transporters exist. ABCA1 is important in initial lipidation of lipid-free or lipid-poor ApoE (Fig. 3B) (Hirsch-Reinshagen and Wellington 2007). Additional lipidation is done by ABCG1 and ABCG4, which only lipidize phospholipids-enriched particles (Fig. 3C) (Wang et al. 2008). After lipidation, the ApoE-cholesterol complex is being transported across the BBB or endocytosed by neurons or glia cells via ApoE receptors, which are all members of the LDL receptor family (Fig 3D) (Michikiwa 2006). These receptors are the LDL receptor, LDL receptor-related protein (LRP), very low-density lipoprotein (VLDL) receptor, ApoE receptor 2 and megalin (Björkhem and Meaney 2004; Cedazo-Mínguez 2007). 10 Fig. 3. Cholesterol metabolism in the brain. A) ApoE is secreted by astrocytes and microglia. B) ApoE is initially lipidated by ABCA1 and C) further lipidated by ABCG1. D) The ApoE-cholesterol complex is either endocytosed or transported across the BBB (Adapted from HirschReinshagen and Wellington 2007) The endocytosed cholesterol-ApoE complexes are then hydrolyzed in lysosomes and either stored as reserve or used as a feedback to regulate cholesterol and lipoprotein receptors expression (Ledesma and Dotti 2006). The oxysterols derived from hydrolyzed cholesterol binds liver X receptors (LXR) in both astrocytes and neurons (Patel and Forman 2004). One of these oxysterols is 24S-hydroxycholesterol and binding of LXRs results in activation or enhanced transcription of the ABCA1 transporters and ApoE, thereby increasing plasma cholesterol and decreasing cellular cholesterol. So, intracellular cholesterol levels are regulated by strict homeostatic mechanisms and any alteration could have serious consequences for the functioning of neurons and glial cells. A dysregulation in lipid metabolism is thought to contribute to some of the pathologies of AD. Since astrocytes regulate the brain’s lipid metabolism it is hypothesized that astrocytes actually underlie the pathogenesis of AD. Alzheimer’s disease AD is the most common form of dementia affecting approximately 5% of people over 65 years of age (Klafki et al. 2006). The risk of developing AD increases even further with aging and nearly half of the people over 85 years of age have AD. AD is a progressive neurodegenerative disorder, resulting in disturbances in cognitive functions including memory, decision making, attention, planning, spatial orientation and language (Klafki et al. 2006). A diagnosis is made after physical and neuropsychological testing to exclude other forms of dementia, such as Parkinson, dementia with lewy bodies or frontotemporal dementia. (Price et al. 1998). Furthermore, clinical history of the patient and a family history of dementia are assessed. However, an ultimate diagnosis can only be made after death, when post-mortem brain analysis can confirm the presence of extracellular deposition of amyloid-β peptides, called senile plaques and intracellular deposition of hyperphosphorylated tau proteins, called neurofibrillary tangles (Holmes 2005). Most patients have a sporadic onset of AD (SAD) (90-95%), but some patients may have familial AD (5-10%) (Harman 2002). Familial AD (FAD) is caused by mutations in 11 genes resulting in an increased production of amyloid-β peptides, especially amyloid-β42. These include mutation in predominantly presenilin 1 (PSEN1) (25-30% of FAD cases) and in some cases APP (5-20%) or presenilin 2 (PSEN2). The age of onset is lower in FAD and the first signs of dementia occur between the age of 40-60 years, whereas the onset for SAD is usually over the age of 60 years. An ApoE4 genotype is associated with an increased risk of developing both SAD and FAD in a dose-dependent manner. This means that subjects with two alleles for ApoE4 have the highest risk of developing AD. ApoE genotype affects both age of unset as well as severity of the pathological hallmarks of AD. In a longitudinal cohort study, 611 previously healthy subjects over the age of 65 years underwent annual clinical evaluation for up to 6 years (Wilson et al. 2002). Of the 611 subjects, 98 developed AD, which was assessed by 19 different cognitive function tests and a post-mortem brain analysis. A total of 5 domains of cognitive function were evaluated, namely episodic memory, semantic memory, working memory, perceptual speed and visuospatial ability. Subjects with an ApoE4 isoform (either one allele or both alleles) had a relative risk of 1.92 of developing AD, compared to subjects carrying the other isoforms. Episodic memory was the most affected cognitive function in subjects with the ApoE4 genotype, but the underlying biological mechanism for this selectivity remains unresolved. Some differences were found between the association of ApoE4 genotype and the risk of developing AD among different racial and ethnic groups. White subjects with an ApoE4 genotype have a 2.73-fold increased risk of developing AD, whereas other racial or ethnic minorities show a much weaker or even a lack of association between an ApoE4 genotype and development of AD (Evans et al. 2003). Black subjects showed an insignificant increased risk of 1.02, indicating that other genetic and/or environmental risk factors might also be important for the development of AD (Lahiri et al. 2004). However, results are controversial since a study of Graff-Radford et al reported that black subjects with one copy of the ApoE4 isoform had an increased risk of 2.6 of developing AD, whereas subjects with two copies had an increased risk of 10.5, thus indicating an association between the ApoE4 genotype and an increased risk of developing AD in African Americans (Graff-Radford et al. 2002). Differences in study results could be due to differences in study design, recruitment of participants, sample size, methods of cognitive function assessments, statistic analysis used and whether or not post-mortem brain analysis was done to confirm the diagnosis. Also, some studies indicate a contribution of gender in the pathogenesis of AD. Androgens seem to be protective against cognitive decline and the detrimental effects of ApoE4 on cognitive functioning in mice and probably also in humans (Raber et al. 2002). Females have lower circulating concentrations of androgens than men, making them more susceptible to cognitive impairments and development of dementia (Raber 2008). Indeed, the majority (68%) of AD patients is female (Brookmeyer et al. 1998). Moreover, the ApoE4 isoform inhibits AR signalling, decreasing the protective effects of androgens even more. Androgen levels decrease with age in both males and females, making aging females with the ApoE4 genotype the most susceptible for cognitive impairments, including AD. 12 Like mentioned earlier, the diagnosis of AD can only be confirmed after death when postmortem brain analysis should reveal senile plaque formation and neurofibrillary tangles (Holmes 2005). The influence of cholesterol metabolism on AD pathogenesis will be discussed in the next paragraph. Alzheimer’s disease and lipid metabolism Patients with AD show high cholesterol levels and people with hyperchcholesterolemia in midlife are at greater risk of developing AD later in life (Kivipelto et al. 2001). Cholesterollowering drugs in human epidemiological studies proved beneficial in reducing that risk, although some results are contradictory (Eckert et al. 2005). Statins lower cholesterol levels by inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), the main regulatory step in the cholesterol biosynthesis pathway. Applied statins to lower brain cholesterol are thought to require lipophilic properties to be able to cross the BBB and affect brain cholesterol metabolism (Thelen et al. 2005). Short-term high-dose treatment of mice with simvastatin, a lipophilic statin, reduced the level of cholesterol precursor lathosterol, whereas treatment with the more hydrophilic statin pravastatin had no effect on lathosterol levels at all. Both drugs had no effect on cholesterol levels itself, which could be due to the long half-life of cholesterol and short-term drug application. The difference in lipophilic and hydrophilic statins on the occurrence of AD was recently also evaluated in a human prospective population-based cohort study (Haag at al. 2008). Participants were followed up for an average of 9 years to study the possible protective effects of statins on AD pathogenesis. Statins significantly reduced the risk of AD, but no differences were observed between lipophilic and hydrophilic statins. Changes in cholesterol in the circulation might cause alterations in brain cholesterol as well, which would make transfer across the BBB needless. The reason for this difference in results remains elusive and other animal or human studies also show inconsistency in the effects of different statins in AD occurrence. This could be due to different animal models and control animals used, different dietary, the different statins used and for how long and in which dosage, and assessment of AD in animal models. In human studies this could be due to different study designs, duration and dosage of different statins used, number and age and physical condition of participants and how AD is diagnosed. To conclude, high cholesterol levels can increase the risk of developing AD. This risk can be reduced by administrating cholesterol-lowering statins, indicating that cholesterol metabolism contributes to AD pathogenesis. Senile plaques, neurofibrillary tangles and neurodegeneration of specific brain areas are the major hallmarks of AD. The formation of senile plaques is considered to be the leading cause of AD pathogenesis, triggering the other pathological features thereby ultimately causing neurodegeneration, but this hypothesis is not unanimously supported (Mudher and Lovestone 2002). Nevertheless, I will discuss the formation of senile plaques in AD and how lipid metabolism can influence this process first. 13 Senile plaques AD is associated with an increased production of amyloid-β peptides. Accumulation and deposition of these peptides cause plaque formation, called senile plaques which predominantly localize in the cortex and hippocampi (Holmes 2005). Two categories of senile plaques exist, namely diffuse plaques and classic neuritic plaques. Diffuse plaques are rather harmless, whereas the classical neuritic plaques are thought to damage surrounding neuropil, which is a network of unmyelinated axons and dendrites and glia cells within the grey matter. Furthermore, they are characterized by activated microglia and reactive astrocytes, which might already indicate that astrocytes themselves are involved in the pathogenesis of AD. These classical neuritic plaques consist of insoluble amyloid-β peptides and might contribute to AD pathogenesis as well as other neurodegenerative diseases. Amyloid-β peptides only become neurotoxic as plaque-associated fibers (Pike et al. 1993). Those amyloid-β peptides containing the hydrophobic β29-35 sequence aggregate well and are capable of forming the neurotoxic fibers constituting the senile plaques. Mutations leading to amino-acid substitution in the APP gene, results in a more hydrophobic protein might therefore also enhance amyloid-β peptide aggregation and consequently neurotoxicity and neurodegeneration in FAD. Walsch et al. evaluated the aggregation properties of different amyloid-β peptides and showed that certain amino-acid substitutions indeed stabilized αhelices and β-strands in fibers by their hydrophobic actions (Walsch et al. 1997). The aggregated amyloid-β peptide than promote neurodegeneration by inducing apoptosis (Loo et al. 1993). It is important to understand the underlying mechanism of the transformation of amyloid peptides to the neurotoxic aggregated form as this could be an interesting target for future therapeutic strategies (Walsch et al. 1997). Increased cholesterol levels influences the generation of the neurotoxic amyloid-β peptides, that accumulate in AD (Zheng and Koo 2006; Reid et al. 2007). To understand the possible influence of cholesterol on amyloid-β peptides synthesis, it is important to know the molecular mechanism behind amyloid-β peptides synthesis. Amyloid precursor protein Amyloid-β peptides are formed by two sequential cleavage steps of the amyloid precursor protein (APP), a transmembrane protein possibly involved in synaptogenesis, neurite outgrowth and synapse remodelling (Zheng and Koo 2006). The initial cleavage in the extracellular domain can be done by either α-secretase or β-secretase, which results in soluble APP derivatives in both cases, called APPsα and APPsβ respectively (Zheng and Koo 2006; Reid et al. 2007). β-secretase is sometimes also referred to as β-site of APP cleaving enzyme (BACE1). The remaining transmembrane APP fragment, CTFα or CTFβ, is further cleaved by γ-secretase, a multiprotein complex which includes presenilin 1 (PSEN1) or presenilin 2 (PSEN2). This cleavage results in production of soluble fragments differing in size depending on initial cleavage by α-secretase or β-secretase. Initial cleavage by β-secretase will result in formation of amyloid-β peptides, whereas initial cleavage by α-secretase results in the formation of the harmless non-amyloidgenic P3 peptide, since it cleaves within the amyloid-β domain (Fig. 4). 14 Fig. 4. Initial cleavage of the APP is done by either α-secretase or βsecretase. Subsequent cleavage of the remaining intracellular part is done by γ-secretase. EC; extracellular, TM; transmembrane; IC; intracellular; red domain is the amyloid-β domain (Zheng and Koo 2006). The precise location of the additional cleavage by γ-secretase could further enhance plaque formation by increasing the production of amyloid-β42, instead of amyloid-β40 (Suh and Checler 2002). The more amyloidogenic amyloid-β42 accumulates easier and is the primary component of amyloid plaques. Some cases of FAD are caused by mutations in the APP gene. Mutations in the APP near the α-secretase site might result in decreased α-secretase cleavage activity and enhanced β-secretase cleavage and thereby enhanced amyloid-β peptides production (Holmes 2005). On the other hand, mutations near the β-secretase site might also enhance production of amyloidβ peptides. Mutations near the γ-secretase site results in an increased amyloid-β42: amyloidβ40 ratio. The amyloid-β42 is more hydrophobic and consequently accumulated much faster (Suh and Checler 2002; Sorrentino and Bonavita 2007). Therefore, it is not surprising that amyloid-β42 is the major component of amyloid plaques. Accumulated amyloid-β42 forms the centre of senile plaques and enables the soluble amyloid-β40 to deposit as well. Initial deposits actually stimulate misfolding of other amyloidogenic peptides (Sorrentino and Bonavita 2007). Mutations in the γ-secretase components PSEN1 and PSEN2 have both been implicated with FAD, since they can influence the location of the γ-secretase cleavage. This results in an increased production of amyloid-β42, instead of amyloid-β40 (Suh and Checler 2002). Studies on animal models have indicated that PSEN1 is particularly important in the proteolysis activity of the γ-secretase, since PSEN1 knock-out mice showed reduced levels of amyloid-β peptides (De Strooper et al 1998). Many mutations are already revealed in the PSEN1 gene, all enhancing production of the amyloid-β42 and thereby promoting senile plaque formation. Less is known about PSEN2, but some mutations have been identified on this genes as well (Suh and Checler 2002). A double knockout mouse (PS cDKO), targeting both PSEN1 and PSEN2, showed a phenotype that was similar to the PSEN1 KO mouse, indicating that PSEN1 is predominantly responsible for the production of amyloid-β peptides (Beglopoulos et al. 2004). The PS cDKO mice had undetectable levels of amyloid-β40 but a small amount of amyloid-β42, suggesting that PSEN1 and/or PSEN2 are exclusively responsible for amyloid-β40 production, whereas amyloid-β42 production might also be regulated by other genes and proteins. 15 APP can both be found within and outside of membrane rafts, which are enriched in cholesterol. Cholesterol levels can influence the synthesis of amyloid-β peptides, by mechanisms that are not yet fully understood. APP is cleaved by membrane-bound enzymes: APP is cleaved by β-secretase within membrane rafts and by α-secretase outside membrane rafts (Ehehalt et al. 2003). Cholesterol depletion resulted in a reduced β-secretase cleavage and an increased α-secretase cleavage, indicating that cholesterol in lipid rafts is indeed involved in regulating the initial cleavage of APP. In addition, cholesterol depletion can decrease the rate of endocytosis and these authors have shown that endocytosis is necessary for β-secretase cleavage, but not for αsecretase cleavage (Ehehalt et al. 2003). They hypothesize that β-secretase and APP only meet each other after endocytosis because lipid rafts are rather small and the β-secretase and APP are localized in different rafts. Inhibiting endocytosis therefore reduces their change to meet. This hypothesis was supported by inducing crosslinks between β-secretase and APP on the cell surface which overcame the need for endocytosis, indicating that clustering of the βsecretase and the APP by endocytosis is needed for cleavage (fig. 5). Fig. 5. (A) Lipid rafts (black) are rather small and APP and β-secretase (BACE) only meet during and/or after endocytosis after which APP is cleaved to form CTFβ, the initial step in producing amyloid-β peptides. (B) Cleavage by β-secretase is reduced when endocytosis is inhibited. Inducing crosslinks between APP and β-secretase with antibodies overcomes this effect, indicating that the clustering of lipid rafts, normally induced by endocytosis, is needed for βsecretase and APP to meet and initial cleavage to occur (Ehehalt et al. 2003). Furthermore, γ-secretase also predominantly localizes within membrane rafts, but it remains to be determined when and where γ-secretase and CTFβ meet for additional cleavage to produce the toxic amyloid-β peptides. Activation of the LXR on astrocytes by oxysterols results in enhanced cholesterol efflux by increasing ABCA1 and ApoE expression, as mentioned earlier (Patel and Forman 16 2004; Koldamova et al. 2004). This results in a reduction of β-secretase cleavage and a subsequent decrease in amyloid-β peptides production. So, LXR reacts to elevated cholesterol levels by activating proteins involved in cholesterol efflux and transport, resulting in reduced plasma cholesterol levels. This promotes cleavage by the α-secretase, thereby preventing plaque formation and AD development. Taken together, the level of cholesterol can influence initial cleavage of APP in two ways: 1) Cholesterol depletion promotes α-secretase cleavage by diminishing lipid rafts in which βsecretase operates, and 2) it reduces the rate of endocytosis and thereby increasing α-secretase cleavage. So, cholesterol is important in regulating APP cleavage, indicating an important role of lipid metabolism in the formation of the neurotoxic amyloid-β peptides. Apolipoprotein E Besides transporting cholesterol for homeostasis maintenance, ApoE can also bind amyloid-β peptides, thereby facilitating their uptake by microglia via receptor mediated endocytosis after which they are degradated (Hirsch-Reinshagen and Wellington 2007). Three ApoE isoforms exist, namely ApoE2, ApoE3 and ApoE4, with the ApoE3 isoform being the most common as it is present in 70% to 80% of the population (Huang et al. 2004; Huang 2005). The ApoE4 isoform is carried by 10% to 15% of the population and is considered a risk factor of AD (Wilson et al. 2002). The ApoE2 isoform is the least common and accounts for only 5% to 10%. The different isoforms differ in their binding properties due to their difference in structure, which could explain their difference in amyloid-β peptide clearance (Huang et al. 2004; Huang 2005). This structural difference results from two polymorphisms at residues 112 and 158, which consists of either arginine or cysteine. ApoE4 has arginine at both locations, whereas ApoE2 has two cysteines. ApoE3 has cysteine on location 112 and arginine on 158. Apo2 and ApoE3 preferentially bind to large high-density lipid protein (HDL), whereas ApoE4 preferentially bind to small very low density lipoprotein (VLDL) (CedazoMínguez 2007). ApoE2 has little affinity for the LDL receptors and circulating ApoE2 levels are therefore highest. ApoE3 has intermediate LDLR binding affinity while ApoE4 the highest (Mann et al. 2004), and which is therefore easily endocytosed, resulting in low steadystate circulating levels. Furthermore, ApoE4 is most susceptible to proteolysis compared with other ApoE isoforms (Huang 2005). Carriers of the ApoE4 isoform also show high circulating cholesterol levels, which could be due to their lower circulating ApoE levels which could promote β-secretase cleavage and enhance amyloid-β peptides production (Huang 2005). ApoE2 seems relatively protective for AD, since it is associated with low cholesterol levels and high steady-state circulating ApoE levels. The level of circulating ApoE could determine the faith of amyloid-β peptides (Wilhelmus et al. 2005). High levels of circulating ApoE should be able to clear the amyloid-β peptides and keep them soluble, whereas low circulating ApoE levels will result in deposition and accumulation of the amyloid-β peptides. Beside, the different circulating ready-state levels of the different ApoE isoforms, promoting cholesterol efflux is also isoform-dependent (Michikawa et al. 2000). The ApoE4 isoform has the lowest potency to act as a cholesterol acceptor, making them least efficient in 17 promoting the efflux of cholesterol from plasma membranes. Furthermore, lipidated ApoE binds with a higher affinity to the amyloid-β peptides than their lipid-poor counterparts (LaDu et al. 1994). The ApoE4 isoform has the lowest potency to promote cholesterol efflux and are therefore least lipidated, resulting in the lowest binding affinity for the amyloid-β peptides among the ApoE isoforms. The structural difference between the isoforms also results in different effects when binding to amyloid-β peptides. Binding of ApoE3 to amyloid-β peptides seems to be protective against neurodegeneration by facilitating clearance of amyloid-β peptides by astrocytes, and thereby to prevent accumulation and subsequent plaque formation. ApoE4 facilitates accumulation of the amyloid-β peptides and is even found in amyloid plaques of patients with AD (Huang et al. 2004). A recent study demonstrates that ApoE promotes degradation of both soluble and fibrillar amyloid-β peptides by the proteolytic enzymes insulin degrading enzyme (IDE) and neprilysin (NEP) in an isoform dependent manner, which explains their difference in clearance efficiency (Jiang et al. 2008). The ApoE4 isoform is impaired in promoting proteolysis, resulting in decreased clearance of the amyloid-β peptide and increased risk of plaque formation. The lipidation status of ApoE is also important in promoting proteolysis, as decreased lipidation was associated with increased amyloid-β peptides production (Jiang et al. 2008). Increased lipidation enhanced amyloid-β peptide clearance. At least a part of the amyloid-β peptides are cleared from the brain by crossing the BBB (Wilhelmus et al. 2005). LRP-1 on the BBB binds free amyloid-β peptides and facilitates their transport by mediating internalisation, resulting in their efflux from the brain. This also accounts for amyloid-β peptides bound to ApoE, since ApoE acts as a ligand for LRP-1 (Deane et al. 2008). The ApoE4 isoform shift the efflux of amyloid-β peptides by the LRP-1 to the VLDLR, which has a much slower endocytosis rate, resulting in less clearance across the BBB. ApoE4 also enhances vulnerability of neurons to the neurotoxicity induced by the amyloid-β peptides (Wilhelmus et al. 2005). Cells that express the ApoE4 isoform on both chromosomes were most vulnerable to the neurotoxity induced by the amyloid-β peptides, compared with cells that have a different ApoE genotype. Changes in cholesterol levels can influence ApoE expression as was shown in an animal model of AD, using double transgenic mice expressing both human APP and PSEN1 (Petanceska et al. 2003). Long-term changes in cholesterol were both induced by high cholesterol diets or pharmacologically with a cholesterol-lowering drug. The high cholesterol diet resulted in hypercholesterolemia and increased levels of CTFβ and amyloid-β peptides. In addition, ApoE expression was enhanced in these animals. Hypocholesterolemia resulted in a decrease of amyloidogenic APP processing and was associated with reduced levels of ApoE expression. These authors suggest that increased ApoE expression might have a toxic effect thereby contributing to amyloid-β peptide deposition. However, they did not mention the expression levels for the different ApoE isoforms and whether these mice even express human ApoE or not. Human ApoE exist in three isoforms, with their own binding properties, while mice have only one ApoE isoform. The best model to study the characteristics of human 18 ApoE would therefore be an ApoE-/- mouse expressing on of the human ApoE isoforms and compare them with mice expressing the other isoforms. ApoE4 is indeed associated with increased plaque formation, but ApoE2 is rather protective in AD pathogenesis. Therefore, increased ApoE expression could also be protective, contributing to amyloid-β peptide clearance rather than destructive contributing to amyloid-β deposition. So, the outcome of this enhanced ApoE expression is isoform dependent. Taken together, ApoE is the main lipoprotein in the brain and is involved in maintaining cholesterol levels constant. ApoE can bind the amyloid-β peptides and is responsible for their clearance. The ApoE-amyloid-β peptide complex can be processed in different ways. The complex can be endocytosed via ApoE receptors by both neurons and glia cells or may cross the BBB via the same receptors. These clearance mechanisms may not always work as efficiently resulting in accumulation of the amyloid-β peptides, forming toxic plaques (fig. 6). Fig. 6. ApoE binds amyloid-β peptides, thereby facilitating their clearance by either promoting endocytosis by neurons and glia, or by facilitating transport across the BBB. Insufficient clearance causes deposition and plaque formation. The ApoE4 isoform is associated with the least readystate ApoE levels, lowest amyloid-β peptides binding affinity and the least efficient clearance, making carriers of the ApoE4 genotype more prone to develop senile plaques and AD High cholesterol levels can enhance ApoE expression, but the outcome is isoform dependent, since the different isoforms have different binding properties to lipoproteins, their lipoprotein receptors and amyloid-β peptides, resulting in differences in amyloid-β peptide clearance and plaque formation. Furthermore, the level of circulating ready-state ApoE as well as the neurotoxicity induced by amyloid-β peptides is isoform-dependent, with the ApoE4 isoform being the most disadvantuous. An ApoE4 genotype has the least circulating ApoE levels due to less expression as well as increased susceptibility to proteolysis, resulting in less lipid transport. Furthermore, the ApoE4 isoform has the lowest binding affinity for the amyloid-β peptides. This causes cholesterol levels to increase and amyloid-β peptide clearance to decrease both contributing to the development of plaque formation, which is a main characteristic of AD. 19 Lipid metabolism can influence the formation of senile plaques (fig. 7). High cholesterol levels promote cleavage by the β-secretase, thereby enhancing the production of the neurotoxic amyloid-β peptides. Furthermore, hypercholesterolemia causes an increase in ApoE expression. This should be protective, since ApoE transports cholesterol and decreases its plasma membrane levels, which promotes cleavage by the α-secretase resulting production of the non-amyloidgenic P3 proteins. Moreover, ApoE facilitates the clearance of the amyloid-β peptides. However, this does not account for the ApoE4 isoform, which is impaired in promoting amyloid-β peptides clearance and is associated with a higher vulnerability to amyloid-β peptides- induced neurotoxicity. The ApoE4 isoform has the lowest circulating ready-state ApoE levels, which might be protective seen the detrimental effects of ApoE4. So, increased ApoE4 expression induced by high cholesterol levels prevents the clearance of amyloid-β peptides and enhances the vulnerability of the neurotoxicity induced by the amyloid-β peptides. Fig. 7 Increased cholesterol levels promote cleavage by the β-secretase, resulting in the production of amyloid-β peptides. Also, high cholesterol levels enhance ApoE expression. The amyloid-β peptides bind ApoE and can be processed in three ways. First, ApoE promotes the endocytosis and ultimate clearance of amyloid-β peptides by proteolytic enzymes. Second, ApoE facilitates the transport across the BBB to clear it form the brain. Third, when these clearance mechanisms fail, the amyloid-β peptides aggregate and form senile plaques. Neurofibrillary tangles Neurofibrillary tau accumulations are the other major pathological hallmark seen in AD, besides amyloid plaques (Götz et al. 2004). The microtubule-associated tau protein localizes in axons and is involved in microtubule dynamics, axonal transport and neurite outgrowth (Johnson and Stoothoff 2004). Normal phosphorylation modulates these functions by negatively regulating microtubule assembly. Hyperphosphorylation of the tau protein lowers its binding affinity to microtubules, resulting in dissociation otherwise known as a toxic loss of function. However, not all phosphorylations lead to aggregation of the tau protein (Johnson and Stoothoff 2004). Some phosphorylation of specific locations on the tau protein results in dissociation of the microtubules, but do not promote tau aggregation. It might be possible that other locations of phosphorylations on the tau protein are responsible for inducing tau aggregation and subsequent tangle formation. Free dissociated tau transfers to somatodendritic compartments where they accumulate to form tau filaments, also known as a toxic gain of function. The intracellular accumulated tau proteins are called neurofibrillary 20 tangles, due to their appearance. Besides hyperphosphorylation, aggregation is also promoted by cleavage of tau by caspases-3, which probably precedes hyperphosphorylation. Hyperphosphorylation of Tau can result from excess phosphorylation by yet unknown kinases, resulting in dissociation of the microtubules and self assembly to form neurofibrillary tangles (Johnson and Stoothoff 2004). However, hyperphosphorylation of the tau protein could also result from decreased dephosphorylation by protein phosphatase 2A (PP2A) and reduced activity and expression of PP2A is associated with AD (Kins et al. 2003). Hypercholesterolemia can affect tangle formation indirectly by promoting production of the amyloid-β peptides (Fig. 8). It is hypothesized that production of fibrillar amyloid-β peptides results in increased tau phosphorylation possibly by increasing GSK3β activity (Busciglio et al. 1995; Johnson and Stoothoff 2004). Activation of LDL receptors by ApoE inhibits phosphorylation of the potential tau kinase GSK3β in neurons, thereby decreasing levels of phosphorylated tau and possibly prevents aggregation and subsequent tangle formation (Hoe et al. 2006). So, enhanced expression of ApoE can be protective by inhibition of kinase activation, thereby reducing tau phosphorylation and preventing subsequent neurofibrillary tangle formation. This effect, however, is dependent on ApoE isoform. The ApoE4 isoform is less effective in inhibiting activation of the potential tau kinases, resulting in relatively increased phosphorylated tau proteins. PSEN1, a component of the γ-secretase, binds tau and GSK3β directly (Takashima et al. 1998). PSEN1 might mediate interaction of the GSK3β with its substrate tau. Mutations in the PSEN1 gene that are associated with FAD, cause increased binding of GSK3β. This enhances the ability of GSK3β to phosphorylate tau and increase tangle formation besides promoting amyloidgenic cleavage of APP. Furthermore, amyloid-β42 oligomers can induce cleavage of tau by activation of caspase-3, which is believed to precede hyperphosphorylation of tau in the pathogenesis of AD (Chong et al. 2006). Amyloid-β42 oligomers activated the caspases-3 via the ERK1/2 signalling cascade, which is also involved in inducing apoptosis. Amyloid-β deposits probably promote neurodegeneration by activating this pathway as well. Fig. 8 Increased cholesterol levels promote cleavage by the β-secretase, resulting in the production of amyloid-β peptides. These amyloid-β peptides can promote activation of the potential tau kinase GSK3β, thereby triggering hyperphosphorylation of the tau protein. Furthermore, amyloid-β peptides activates the caspases-3 cascade. Both hyperphosphorylation and cleavage of the tau protein promote its aggregation, resulting in tangle formation. Also, the ApoE4 isoform is least efficient in preventing GSK3β activation, AB and tau hyperphosphorylated enhance tau phosphorylation! therebyInteraction also contributing to increased tau levels. 21 Animal models for Alzheimer´s disease Various animal models were develop to study the molecular mechanisms underlying the pathologies of AD and to test new therapeutic strategies (Emilien et al 2000; Price et al. 1998). Mostly mice models are used, since they are relatively easy to genetically manipulate. However, a lot remains unclear about the precise mechanism leading to all the pathologies and pathogenesis of AD, making is difficult to generate a mouse model with the exact phenotype. Because of this, an animal model that is fully authentic to human AD has not been developed yet. The different and mostly studied mouse AD models used are summarized in the next table and will be discussed below. APP A, B, C D E PSEN1 F G APP and PSEN1 H ApoE I Oxidative stress J Tau K APP and tau L Overexpressing mutant APP; games mouse, Hsiao mouse, Novartis mouse Overexpress APP fragments; CT100 mouse APP knock-out mouse Overexpressing mutant PSEN1 PSEN1 knock-out mouse Overexpressing both mutant APP and PSEN1 Expressing human ApoE isoforms and mutant APP Oxidative stress Overexpressing (mutant) tau; WT, G272V, P301L Overexpressing both mutant tau and P301L and the Swedish tau mutation Most AD animal models overexpress the wild-type APP, the mutant APP as seen in FAD, or just the C-terminal fragments of the APP. A) The “games mouse” overexpresses a variant of the APP gene that is associated with FAD (Emilien et al 2000; Price et al. 1998). This codon V717I mutation, also known as the ‘London mutation’, causes an increase in amyloid-β42 production. These animals develop amyloid-β plaques and synaptic loss resulting in astrocytosis and microgliosis. However, cognitive and behavioural changes have not been reported so far and intracellular tangle formation, the other major hallmark of AD, have not been observed. Cognitive and behavioural changes might not have been noted, due to the difficulties of assessing these phenotypes in animals. The absence of neurofibrillary tangles could be due to the different level of action of the amyloid-β peptides and the tau protein. B) The “Hsiao mouse” overexpresses a different APP variant associated with FAD, known as the ‘Swedish mutation’, resulting in overproduction of both amyloid-β40 and amyloid-β42 (Schwab et al. 2004). These mice show some amyloid plaque formation, activated astrocytes and impaired cognitive functioning in an age-dependent manner. It remains to be determined if these cognitive changes are the result of amyloid-β peptide production or that it might be the result of overexpression of the APP and/or accumulation of amyloid-β peptide precursors. In addition, amyloid deposition seems to be dependent of gender since female mice are the most affected, similar as for human AD (Callahan et al. 22 2001). However, these mice showed no intracellular neurofibrillary tangles and only a weak inflammatory response. C) The “Novartis mouse” overexpresses both these APP mutations, resulting in amyloid plaque formation and neuritis, but no intracellular neurofibrillary tangles (Emilien et al 2000; Price et al. 1998). Furthermore, no cognitive or behavioural changes have been described in these mice so far. D) The CT100 mice overexpress the C-terminal fragments of the APP and are used to evaluate γ-secretase functioning and the effect of its inhibitors (Emilien et al 2000; Suh and Checler 2002). These mice show intracellular and extracellular amyloid-β peptide depositions, but extracellular deposition is not at the same level as with the full length APP. In addition, they show gliosis and neuronal cell loss, but phenotype is variable. E) APP knock-out mice show astrocytosis, gliosis, impaired neurological function and had reduced locomotor activity, but no neuronal cell loss or even damage was observed (Emilien et al 2000; Price et al. 1998). The lack of a substantial AD phenotype can be explained by compensation of homologous APP-like proteins 1 and 2 (APLP1 and APLP2). Indeed, double knock-out mice, targeted at both APP and APLP2, are not viable. Although this model does not mimic the phenotype of AD, it can contribute to the understanding of the underlying mechanism by assessing the normal functions of APP by comparing the KO mice with the WT mice. Also, the presence of some pathologies indicates that mutant APP cannot be causative for AD pathogenesis. Other animal models have focused on the PSEN1 gene, since this gene is associated with FAD. F) A mouse model expressing the human mutant PSEN1 gene was developed (Emilien et al 2000; Price et al. 1998). Production of amyloid-β42 is enhanced in these mice, but AD pathologic lesions remain absent. G) Double transgenic mice expressing both mutant PSEN1 and human APP show accelerated amyloid-β deposition, compared with age-matched controls, indicating that overexpression of the rodent amyloid-β peptide alone is insufficient to cause amyloid deposition (Emilien et al 2000; Price et al. 1998). H) PSEN1 knock-out mice die shortly after birth, but have provides some insight into the role of PSEN1 in development and amyloid-β peptide production (Emilien et al 2000; Price et al. 1998). Besides axial skeletal defects in these mouse embryos, the C-terminal of the APP accumulates in fibroblast cell cultures obtained from these embryos and consequently, amyloid-β peptide production is reduced (De Strooper et al 1998). Cleavage by β-secretase and/or α-secretase was not affected. This indicates that PSEN1 is normally involved in γ-secretase activity, which is reasonable considering the fact that PSEN1 is part of the γ-secretase complex. Other animal models involved in AD research: I) ApoE4 is considered a risk factor for the development of both FAD and SAD and an animal model was created to evaluate the contribution of ApoE on the formation of amyloid-β deposits (Emilien et al 2000; Price et al. 1998). ApoE-/- and ApoE+/+ mice were crossed with 23 the “games mice” and compared for amyloid deposition. The V717I × ApoE+/+ mice showed significantly more amyloid deposits than the V717I × ApoE-/- mice, indicating that ApoE contributes to the development of amyloid deposits by influencing amyloid-β peptide clearance or accumulation. Differences in cognitive function or behaviour have not yet described in this animal model. J) Oxidative stress responses are also seen in the pathogenesis of AD, but developing a suitable animal model to evaluate the precise role of these responses in developing amyloid deposits is still a work in progress (Emilien et al 2000). K) Most of these animal models have focussed on amyloid plaques formation and not on intracellular tangles, the other major hallmark of AD. Some tau transgenic animal models were developed in which a human (mutated) tau protein is expressed, although these mutation are not associated with human AD, but rather with other forms of dementia that show tau pathology (Götz et al. 2004). The tau protein was hyperphosphorylated in these mice, but no tangle formation was observed. This model thus only represents the early AD phenotype before neurofibrillary tangles are formed. Mice expressing the human G272V mutant tau show hyperphosphorylated tau protein and tangle formation, but show only a subtle AD phenotype, like impaired spatial learning and memory decline. The human tau P301L mutation was also expressed in transgenic mice. This mutation causes accelerated tangle formation and prevents the tau protein to bind microtubules in humans. Mice expressing this mutated tau protein showed tangle formation, reduced number of motor neurons and severe behavioural disturbances. Administration of intracerebral amyloid-β42 enhanced tangle formation, suggesting that the tau protein acts downstream of amyloid-β peptides and any alterations in amyloid-β peptides processing can therefore have an influence on tau signalling. It remains to be determined if this also accounts in human AD pathogenesis, since results are sometimes controversial. L) Considering that both amyloid plaques as well as tangle formation are important in the pathogenesis of AD, double transgenic mice were created by crossing mice with the ‘Swedish mutation’ with the VLW line, which is a triple mutation in the tau gene including the P301L, the G272V and the R406W mutation (Ribé et al. 2005). These mice overexpress both human mutant APP and human mutant tau, resulting in enhanced amyloid depositions and tau hyperphosphorylation, causing tangle formation, neurofibrillary degeneration and neuronal cell loss in an age- and gender-dependent manner, with female mice being the most susceptible, showing increased amyloid depositions. This might suggest that the tau protein does not act downstream of amyloid-β peptides, but that they can influence each other to enhance both amyloid deposition and tangle formation. These differences in results could be due to the different transgenic animals, the promoter to drive protein expression, levels of protein expression and the genetic background used in each study. More research needs to be done to determine the exact relationship between the amyloid-β peptides and tau in AD pathogenesis. However, none had all the pathological features as seen in AD. This might be because neither deposition of extracellular amyloid-β peptides or intracellular tau proteins is causative for AD, so the precise phenotype cannot be mimicked by targeting only these pathologies. 24 Furthermore, the lack of cognitive and behavioural changes could be because of the difficulties assessing these functions in animals. This could imply that another pathology contributes to both senile plaque and tangle formation as well as the other pathologies seen in AD. Nevertheless, these animal models have already revealed some of the underlying molecular mechanisms that contributes to amyloid plaque formation and development of AD. These include the contribution of normal and mutant APP, PSEN1 and PSEN2, the isoformspecific contribution of ApoE and the influence of cholesterol metabolism in AD pathogenesis. These animal models can also be used to test new therapeutic strategies or to further evaluate the underlying molecular mechanism contributing to the all the pathologies in AD. Current and future treatment Current treatments are unable to prevent, cure or control AD pathology, but are aimed to relieve patients from their symptoms (Klafki et al. 2006; Van Marum 2008). Deposition of the amyloid-β peptides is one of the major hallmarks of AD pathology. Therefore, treatment to prevent or control AD pathogenesis can either be aimed at decreasing production, enhancing clearance or preventing aggregation of the amyloid-β peptides (Klafki et al. 2006; Van Marum 2008). In addition, therapeutic drugs can also be targeted at the neurofibrillary tangles, the other major hallmark of AD (Klafki et al. 2006). A lot of potential therapies are currently developed and tested, but I will only explain those that are relevant for this thesis. As mentioned earlier, statins are able to reduce the risk of developing AD in both animal and human studies. Cholesterol regulated lipid rafts structure and distribution, which could have an negative impact on APP processing, resulting in reduced amyloid-β40 and amyloid-β42 production. Indeed, cholesterol depletion promotes APP cleavage by the αsecretase, resulting in the non-amyloidgenic P3 peptide and reduces β-secretase cleavage which would otherwise result in the production of the neurotoxic amyloid-β peptides. Furthermore, cholesterol depletion reduces endocytosis rate, which is necessary for βsecretase but not for α-secretase activity. Without endocytosis, the β-secretase is unable to meet the APP protein. The γ-secretase is responsible for additional cleavage and also localizes within lipid rafts. It remains to be determined if cholesterol has an influence on the additional cleavage of APP by γ-secretase as well. Statins must cross the BBB to inhibit cholesterol synthesis in the brain. However, some studies also found beneficial effects of hydrophilic statins, which are unable to cross the BBB (Haag at al. 2008). This might be because statins could also affect other physiological processes involved in AD pathogenesis besides cholesterol synthesis by their anti-oxidant and anti-inflammatory properties (Reid et al. 2007). This is supported by the fact that some studies find an association between elevated amyloid-β peptide production and a decreased plasma cholesterol level (Abad-Rodriguez et al. 2004). This eliminates the possibility that the beneficial effects of statins are caused by a decrease in cholesterol levels. Statins that do cross the BBB might actually be harmful in patients with low cholesterol levels. This was also indicated by studying the Niemann-Pick type C1 (NPC1) protein. Mutation in the NPC1 protein, a protein involved in intracellular cholesterol transport, causes cholesterol retention in 25 late endosomes/lysosomes and thus reduced plasma cholesterol (Patel et al. 1999). However, mice with this mutation show enhanced amyloid-β peptide production, also indicating that a decrease in plasma cholesterol could enhance amyloid-β peptide production (Burns et al. 2003). These results also suggest that statins could potentially be harmful by reducing plasma cholesterol levels and enhancing amyloid-β peptide production and subsequent senile plaque formation. However, another study using NPC1 deficient cell cultures, observed an increase in amyloid-β peptide production, so results are controversial (Runz et al. 2002). Statins could also indirectly target amyloid-β peptide transport across the BBB to clear it from the brain (Van Marum 2008). The receptor for advanced glycolation end products (RAGE) is responsible for transporting amyloid-β peptides from the systemic regulation into the brain, whereas LRP-1 transfers amyloid-β peptides from the brain into the systemic regulation. An upregulation of RAGE and a downregulation of LRP-1 are associated with AD and could thus be a potential therapeutic target. However, drugs that downregulate RAGE and/or upregulate LRP-1 have not been tested in AD patients yet. Hypercholesterolemia was associated with increased levels of RAGE and reduced levels of LRP-1, resulting in increased amyloid-β peptide production, which might contribute to the increased risk of developing AD (Prasanthi et al. 2008). These results suggest that statins might be beneficial in restoring the normal distribution of RAGE and LRP-1. Results indicate that statins are able to upregulate LRP-1, thereby promoting efflux of the amyloid-β peptides (Deane et al. 2004). Decreasing amyloid-β peptides levels, reduces plaque formation and subsequent development of AD. To conclude, it is without a doubt that altered cholesterol metabolism can contribute to the pathogenesis of AD. Altered cholesterol levels can enhance senile plaque and neurofibrillary tangle formation. Also, patients with hypercholesterolemia have an increased the risk of developing AD. Statins are beneficial to reduce this risk for AD pathogenesis. However, it remains to be determined by what mechanisms they accomplish this effect, since results are controversial. They might reduce the risk of AD directly by reducing brain cholesterol levels thereby influencing APP processing, reducing oxidative stress and/or reducing inflammation. They may also act indirectly by affecting the balance of amyloid-β peptide transport across the BBB. Astrocytes en Alzheimer’s disease A mentioned above, cholesterol metabolism can contribute to the development of AD. Astrocytes are the regulators of this cholesterol metabolism, which might indicate that astrocytes are involved in the development of AD. This would be logical, since AD is characterized by neurodegeneration and astrocytes are involved in providing the lipid necessary for regeneration. Furthermore, astrocytes have phagocytotic properties, enabling them to clear the brain from degenerative tissues. Like mentioned earlier, senile plaques are characterized by accumulation of activated astrocytes, already indicating the involvement of astrocytes in the pathogenesis of AD (Holmes 2005). It remains questionable if this astrocytosis promotes or inhibits AD pathogenesis (Pihlaja et al. 2008). Activated astrocytes could promote AD pathogenesis by 26 secreting cytokines, thereby enhancing neuroinflammation and subsequent neurodegeneration. On the other hand, astrocytes might be involved in the clearance of amyloid-β peptides by their phagocytotic property. Astrocytes could contribute to the pathogenesis of AD by insufficient clearance of the amyloid-β peptides. Astrocytes from adult mouse cultures are able to internalize and degradate amyloid-β peptides, which might be mediated by the LDLR in an ApoE dependent manner. This is confirmed in a study using ApoE KO astrocytes, which fail to internalize and degradate amyloid-β peptides (Koistinaho et al. 2004). This could lead to the hypothesis that the increased secretion of ApoE by the activated astrocytes enhances ApoE dependent astrocytic clearance of the amyloid-β peptides. The ApoE4 isoform would be least efficient in promoting this astrocytic clearance, due to their lower ready-state levels and lower binding affinity to the amyloid-β peptides. In addition, increased cholesterol transport can enhance regeneration by providing the lipids necessary for generating new plasma membranes for neurons. In addition, activated astrocytes show an upregulation of the LRP, which is associated with senile plaques (Arélin et al. 2002). This LRP upregulation is dependent on the presence of its ligand ApoE and is thought to act as a clearance mechanism for amyloid-β peptides. ApoE binds the amyloid-β peptides and act as a ligand for the LRP to promote astrocytic clearance of this ApoE-amyloid-β peptide complex. So, activated astrocytes should prevent senile plaque formation by reducing amyloid-β peptide levels. Dysfunctional astrocytes could therefore result in an enhanced amyloid-β peptide production and senile plaque formation and thereby contributing to AD pathogenesis. However, it remains questionable if astrocytes would be causative for AD. Another dysfunctional mechanism could have triggered the activation of astrocytes and subsequent LRP expression. This could be the increased amyloidβ peptide production, which would then obviously precede astrocytes activation, rather then being its consequence. Dysfunctional astrocytes could also indirectly contribute to inefficient amyloid-β peptide clearance. Astrocytes could contribute to enhanced amyloid-β peptide levels, by inefficient or insufficient expression of the ABC transporters, which are involved in the lipidation of ApoE. Both dysfunctional ABCA for initial lipidation (Hirsch-Reinshagen and Wellington 2007), or ABCG1 or ABCG4 for additional lipidation (Wang et al. 2008) would result in low-lipidated ApoE. Low-lipidated ApoE particles have a lower binding affinity for amyloid-β peptides (LaDu et al. 1994), which could result in an increase in amyloid-β peptides, because this would reduce clearance efficiency. This could ultimately lead senile plaque formation, thereby contributing the AD pathogenesis. Also, astrocytes could possibly contribute to the pathology of AD by insufficient expression of ApoE. This would also lead to insufficient clearance of amyloid-β peptides, since ApoE bind amyloid-β peptides, thereby facilitating its clearance (Hirsch-Reinshagen and Wellington 2007). Moreover, low ApoE levels would increase cholesterol levels, which is considered a risk factor for AD (Kivipelto et al. 2001). Also, a reduced cholesterol metabolism would result in an insufficient delivery of new lipids for regeneration. 27 Astrocytes might also contribute to the risk factors of AD. Astrocytes are known to express the LXR, which is activated by binding of oxysterols (Patel and Forman 2004). An increase in cholesterol levels results in an increase of oxysterols and enhanced LXR activation on astrocytes. This in turn, will enhance expression of the ABCA1 transporters on astrocytes and enhanced ApoE expression, which should normalize cholesterol levels. In theory, it could be possible that astrocyte fail to decrease membrane cholesterol by insufficient or inefficient ABCA1 transporters. This would lead to hypercholesterolemia, which is considered a risk factor for AD. Astrocytes might also be directly involved in the production of amyloid-β peptides. Astrocytes are able to down-regulate neuronal APP by cell-cell contact (Vincent and Smith 2000). This also accounted for Apo KO astrocytes, indicating that astrocytes themselves are responsible for this effect. Nevertheless, ApoE isoform could still influence astrocytic APP processing and ApoE4-expressing astrocytes were associated with enhanced amyloid-β peptide production. So, astrocytes are involved in APP expression and amyloid-β peptide production and might therefore directly contribute to AD pathogenesis. Taken together, astrocytes could contribute to AD by insufficient clearance of the amyloid-β peptides, contributing to the risk factors of AD, or directly by affecting APP levels. This would lead to an increased amyloid-β peptide production and subsequent senile plaque formation. However, it is still doubtable that dysfunctional astrocytes are causative for AD by the mechanisms pointed out above, since senile plaques are not the only hallmark of AD. Neurofibrillary tangles are the other important hallmark in AD pathogenesis (Götz et al. 2004). However, some believe that the tau pathologies of AD are the result of the so called ‘amyloid cascade’, which believes that amyloid depositions are the trigger for AD onset, resulting in the other hallmarks of AD. (Michikiwa 2006). If this is true, than any dysregulation causing senile plaque formation would also result in tangle formation. 28 Discussion Astrocytes are involved in lipid metabolism by secreting ApoE and expressing ABC transporters and LXRs (Hirsch-Reinshagen and Wellington 2007; Patel and Forman 2004). Three ApoE isoforms exist in humans, namely ApoE2, ApoE3 and ApoE4 (Huang et al. 2004; Huang 2005). An ApoE4 genotype is associated with an increased risk of developing AD, which is characterized by extracellular accumulation of amyloid-β peptides and intracellular neurofibrillary tangles of polymerized hyperphosphorylated tau protein. Epidemiological studies revealed that treatment with statins, a cholesterol-lowering drug, in patients with hypercholesterolemia also reduced the risk of developing AD. This implies that alterations in lipid metabolism, which lead to increased plasma cholesterol levels, could contribute to AD pathogenesis. Indeed, hypercholesterolemia promotes cleavage by the βsecretase and is associated with enhanced amyloid-β peptides production and deposition. ApoE can bind amyloid-β peptides and promote its clearance in an isoform dependent manner (Hirsch-Reinshagen and Wellington 2007; Jiang et al. 2008). The ApoE4 isoform is impaired in promoting amyloid-β peptide proteolysis and is associated with enhanced susceptibility to amyloid-β peptide induced neurotoxicity (Jiang et al. 2008; Wilhelmus et al. 2005). Furthermore, ApoE4 is associated with low circulating ApoE levels and high cholesterol levels, resulting in less efficiency in promoting amyloid-β peptides clearance (Wilhelmus et al. 2005). It remains to be determined if an altered lipid metabolism is sufficient to trigger AD pathogenesis. Long-term hypercholesterolemia can increase ApoE expression (Petanceska et al. 2003). These authors found that an increase in ApoE levels is associated with an increase in amyloid-β peptide production and deposition and concluded that ApoE might have a neurotoxic effect, contributing to enhanced senile plaque formation and neurodegeneration. However, they do not mention the ApoE isoforms studied and whether their mouse model even carried the human ApoE genes. Their conclusion may therefore be invalid. An ApoE4 genotype would indeed enhance plaque formation compared to the other isoforms, but this could also be due to its inability to prevent amyloid-β peptide aggregation or neurodegeneration rather than promoting senile plaque formation or neurodegeneration directly. An increase in ApoE levels could even be protective by supplying the lipids that are necessary for neuronal repair (Mahley et al. 2006). Indeed, ApoE expression is enhanced after neuronal injury, which might indicate that ApoE has a protective function and that the ApoE4 isoform is less efficient in preventing neurodegeneration. However, ApoE can have a pathological conformation that is neurotoxic and induces degeneration. The ApoE4 isoform is more prone to assume this conformation, suggesting that ApoE4 can easier induce neurodegeneration directly than the other ApoE isoforms. The precise role of the ApoE4 isoform in promoting senile plaque formation and/or neurodegeneration still needs to be determined. Since cholesterol levels are proven to influence amyloid-β peptide production, statins were suggested as a possible treatment for AD (Eckert et al. 2005). Indeed, statins could reduce the production of amyloid-β peptides and subsequent plaque formation both in vitro and in vivo (Fassbender et al. 2001). It needs to be determined if administration of statins 29 could also be beneficial in humans with normal cholesterol levels. This could potentially be dangerous, since cholesterol is a major component of plasma membranes of both astrocytes and neurons and of myelin sheaths (Björkhem and Meaney 2004). Depletion of cholesterol in humans with normal cholesterol levels may therefore have an effect on the normal functioning of the CNS. Indeed, a reduction in cholesterol levels can also enhance amyloid-β peptide production (Abad-Rodriguez et al. 2004). Also, mutations in the NPC1 result in reduced plasma cholesterol levels, but enhanced amyloid-β peptide production. This indicates that statins might be potentially harmful when they cross the BBB and decrease brain cholesterol levels in patients with normal cholesterol levels. Further research should evaluate this potential danger before statins are generally used to prevent AD. Hypercholesterolemia can also affect tau phosphorylation indirectly dependent on ApoE isoform. Like mentioned before, hypercholesterolemia increased ApoE expression (Petanceska et al. 2003). ApoE is able to inhibit phosphorylation of GSK3β and decreased expression of Cdk5 and p35 in an isoform dependent manner (Hoe et al. 2006). This prevents hyperphosphorylation of tau and subsequent neurofibrillary tangle formation. The ApoE4 isoform is impaired in inhibiting kinase activation, resulting in relatively increased phosphorylated tau proteins. So, unlike other ApoE isoforms, ApoE4 might not be protective by preventing neurofibrillary tangle formation in cases of hypercholesterolemia. Most hypotheses for the pathology of AD are tested in mice, since they are easy to modify genetically. Transgenic mice (over)express human genes and can be used to evaluate the role of the corresponding proteins in health and disease. ApoE is the major lipid carrier in the brain and the human ApoE4 isoform is associated with an increased risk of AD development. However, the human ApoE gene is unique and might therefore have different properties as rodent ApoE (Huang et al. 2004). Most animal models studying the pathology and pathogenesis of AD do not take the human ApoE into account, which make them less realistic to the human situation. This could be overcome by using transgenic mouse models that also express the human ApoE gene. In an optimal mouse model, different groups are used that each express a different human ApoE isoform to evaluate the effects of these isoforms on AD pathogenesis. The ApoE gene might not be the only difference between human and mice in AD pathogenesis. As cholesterol metabolism seems to be involved in AD pathology, it is noteworthy that mice have a much faster cholesterol turnover than humans (Dietschy and Turley 2002). As a consequence, mouse cholesterol synthesis is greater than in humans. The effects of statin treatment in mice might therefore overestimate the results of human statin administration. Statins lower cholesterol levels by inhibiting HMG-CoA and since cholesterol synthesis is greater in mice, the effects of statins that interfere with this cholesterol synthesis might be more pronounced in mice than in humans. Therefore, positive results of statin treatment in mice might not be reproducible in humans, or to a much lesser extent. Most AD animal models overexpress mutant genes that are associated with FAD (Emilien et al 2000; Price et al. 1998). None of these models, however, develop the full clinical pathology as seen in human AD patients, indicating that these mutations are not solely causative for AD pathogenesis or that underlying molecular mechanism might be different between mice and humans and results gained from mouse models cannot be extrapolated to humans. 30 Intracellular neurofibrillary tangles of polymerized hyperphosphorylated tau protein, called neurofibrillary tangles, are the other major hallmark of AD (Götz et al. 2004). Tau is associated with microtubules and regulates its assembly (Johnson and Stoothoff 2004). Hyperphosphorylation causes dissociation by lowering tau’s binding affinity for the microtubules. The free dissociated tau proteins can accumulate to form tau filaments that are associated with neurodegeneration by unknown mechanisms. The phosphorylation status of the tau protein is regulated by kinases and phosphatases and mutations in the tau gene can alter this regulation. Therefore, animal models overexpressing the human mutated tau gene are used as an animal model for AD (Götz et al. 2004). However, no mutations of the tau protein are found in human AD. The mutations used in these models are tau mutations found in other forms of dementia that also show tau pathology, questioning the relevance of these mouse model for studying AD pathology and pathogenesis. So, a lot about the pathogenesis and pathology of AD remains unknown. Generating better animal models should provide a more detailed insight into the underlying molecular mechanisms triggering AD. Furthermore, these animal models should take the human ApoE into account and should also consider the higher cholesterol turn-over in mice compared to humans. On the other hand, the relation between amyloid-β peptides and tau proteins should be known to be able to generate such a perfect animal model. The effects of potential therapeutic targets could be tested once a perfect animal model is created that shows all pathologies of human AD. This might reduce the inconsistency found on the effects of certain drugs between different studies that use different AD animal models. This indicates that, although much is already known about the pathology of AD, more efforts should be taken to unravel the true mechanisms of AD. This will enable scientists to design better therapeutic strategies to help those people that are suffering from AD. Altered lipid metabolism that leads to increased plasma cholesterol levels contributes to AD pathogenesis. Astrocytes regulate lipid metabolism in the brain, suggesting that astrocytes themselves might contribute to AD. Activated astrocytes are found in senile plaques of patients with AD (Holmes 2005), but it remains a topic of discussion whether these astrocytes are protective against AD progression or contribute to the unset of AD (Pihlaja et al. 2008). On one hand, astrocytes secrete cytokines that might trigger and/or enhance the destructive immune reaction that characterizes AD. On the other hand, they might be involved in the clearance of amyloid-β peptides. Some studies found that astrocytes are able to clear amyloid-β peptides, which indicate that activated astrocytes are protective (Koistinaho et al. 2004; Arélin et al. 2002). Inefficient astrocytes could result in insufficient amyloid-β peptides clearance, thereby possibly contributing to senile plaque formation and AD unset and progression. However, it remains questionable if this could be causative for AD, since something most have triggered the activation of astrocytes. Furthermore, it is questionable that increased amyloid-β peptides levels alone can trigger AD onset. Dysfunctional astrocytes could increase the cholesterol levels, which is a risk factor for AD (Eckert et al. 2005). This is still not causative for the unset of AD, since not all people with hypercholesterolemia will develop AD. Furthermore, decreases in plasma cholesterol 31 have also been associated with enhanced amyloid-β peptide production (Abad-Rodriguez et al. 2004; Burns et al. 2003). Astrocytes can also downregulate APP expression, thereby reducing amyloid-β peptide production (Vincent and Smith 2000). However, senile plaque formation is not the only hallmark of AD, suggesting that an astrocytic dysregulation of APP expression is not causative for AD. The ‘amyloid cascade’ hypothesis believes that senile plaque formation is the trigger for all the other pathologies in AD, making it plausible that dysregulated astrocytes contribute to both senile plaque and tangle formation leading to neurodegeneration. This amyloid cascade theory is not unanimously supported, since some studies suggest that the amyloid-β peptides and the tau proteins act at the same level (Ribé et al. 2005). Taken together, is it without a doubt that astrocytes can contribute to AD, but it is highly unlikely that they are causative for AD pathogenesis. They might contribute to an earlier onset or worse pathology by insufficient amyloid-β peptide clearance and increased plasma cholesterol levels. They are probably not causative for triggering the unset of AD by mechanisms described above. Further research should evaluate the true trigger of AD pathogenesis and the precise role of lipid metabolism and astrocytes. Conclusion Astrocytes contribute to the functioning of the CNS by not only supporting neurons, but also by providing feedback to neurons, regulating synaptic and maintaining homeostasis. Furthermore, they are involved in the brain’s lipid metabolism as they secrete ApoE, the major lipid carrier of the brain and express ABC transporters and LXRs. Three ApoE isoforms exist in humans, and the ApoE4 isoform is associated with an increased risk of developing AD. Furthermore, studies revealed that statin treatment can reduce the risk of AD, suggesting that cholesterol metabolism might contribute to the pathogenesis of AD. AD is characterized by extracellular senile plaque formation and intracellular neurofibrillary tangle formation. Senile plaques are formed by accumulation of amyloid-β peptides, which are produced by the β-secretase cleavage of the APP. Increased cholesterol levels can negatively influence APP processing and can enhance cleavage by the β-secretase. ApoE can promote clearance of the amyloid-β peptides in an isoform dependent manner. The ApoE4 isoform is the least sufficient in promoting clearance and enhances neuronal vulnerability to amyloid-β peptide induced neurotoxicity. Neurofibrillary tangles are formed by hyperphosphorylated tau filaments. ApoE inhibits the activation of a potential tau kinase GSK3β and decreases expression of another potential tau kinase Cdk5, preventing hyperphosphorylation. Again, the ApoE4 isoform is least efficient in preventing this hyperphosphorylation. Furthermore, the ApoE4 isoform is associated with low ready-state ApoE levels and high cholesterol levels, which reduces their ability to prevent senile plaque and neurofibrillary tangle formation even further. It remains uncertain which pathology precedes the other. Revealing the order of pathologies is important for designing novel therapeutic strategies. Targeting the amyloid-β peptide alone would not be sufficient if amyloid-β peptide production is not solely causative 32 for AD pathogenesis. This needs to be evaluated, so better animal models can be developed and potential therapeutic interventions can be tested. Lipid metabolism contributes to AD pathogenesis, by mechanisms that are not entirely clear, but may include the amyloidogenic processing of the APP, decreased amyloid-β peptide clearance and enhanced neurotoxicity of the amyloid-β peptides. Astrocytes regulate the lipid metabolism in the brain. Furthermore, activated astrocytes are associated with senile plaques, indicating that astrocytes contribute to AD pathogenesis. However, astrocytes are probably not causative to AD, since something must have activated the astrocytes. Furthermore, they are mostly associated with senile plaque formation and not with other hallmarks of AD such as neurofibrillary tangle formation. References Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, De Strooper B, Dotti CG. (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol. Dec 6;167(5):953-60. Arélin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. (2002) LRP and senile plaques in Alzheimer's disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. Jul 15;104(1):38-46. Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, Garceau D. (2006) A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006 Nov 28;67(10):1757-63. Baumann N, Pham-Dinh D. (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. Apr;81(2):871-927. Review. Beglopoulos V, Sun X, Saura CA, Lemere CA, Kim RD, Shen J. (2004) Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J Biol Chem. Nov 5;279(45):4690714. Benarroch EE. (2005) Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. Oct;80(10):1326-38. Review. Björkhem I, Meaney S. (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. May;24(5):806-15. Review. Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. (2004) Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloidbeta deposition. J Biol Chem. May 7;279(19):20539-45.. Brookmeyer R, Gray S, Kawas C. (1998) Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. Sep;88(9):1337-42. Burns M, Gaynor K, Olm V, Mercken M, LaFrancois J, Wang L, Mathews PM, Noble W, Matsuoka Y, Duff K. (2003) Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J Neurosci. Jul 2;23(13):5645-9. Busciglio J, Lorenzo A, Yeh J, Yankner BA (1995). Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. Apr;14(4):879-88. 33 Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. (2001) Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. Mar;158(3):1173-7. Canevari L, Clark JB. (2007) Alzheimer's disease and cholesterol: the fat connection. Neurochem Res. AprMay;32(4-5):739-50. Review. Cedazo-Mínguez A. (2007) Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med. Nov-Dec;11(6):1227-38. Review. Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. (2006) ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. Jul 21;281(29):20315-25. De Keyser J, Zeinstra E, Wilczak N. (2004) Astrocytic beta2-adrenergic receptors and multiple sclerosis. Neurobiol Dis. Mar;15(2):331-9. Review. De Keyser J, Mostert JP, Koch MW. (2008) Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. Apr 15;267(1-2):3-16. Review. De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. Jan 22;391(6665):387-90 Deane R, Wu Z, Zlokovic BV. (2004) RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid betapeptide clearance through transport across the blood-brain barrier. Stroke. 2004 Nov;35(11 Suppl 1):2628-31. Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. (2008) ApoE isoformspecific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008 Nov 13. [Epub ahead of print] Dietschy JM, Turley SD. (2001) Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001 Apr;12(2):10512. Review Dietschy JM, Turley SD. (2002) Control of cholesterol turnover in the mouse. J Biol Chem. 2002 Feb 8;277(6):3801-4. Review. No abstract available Eckert GP, Wood WG, Müller WE. (2005) Statins: drugs for Alzheimer's disease? J Neural Transm. Aug;112(8):1057-71. Review. Ehehalt R, Keller P, Haass C, Thiele C, Simons K. (2003) Amyloidogenic processing of the Alzheimer betaamyloid precursor protein depends on lipid rafts. J Cell Biol. Jan 6;160(1):113-23. Emilien G, Maloteaux JM, Beyreuther K, Masters CL. (2000) Alzheimer disease: mouse models pave the way for therapeutic opportunities. Arch Neurol. 2000 Feb;57(2):176-81. Review. Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Hebert LE, Aggarwal N, Beckett LA, Joglekar R, Berry-Kravis E, Schneider J. (2003) Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. Feb;60(2):185-9. 34 Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. (2001) Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. May 8;98(10):5856-61. Fields RD, Stevens-Graham B. (2002) New insights into neuron-glia communication. Science. Oct 18;298(5593):556-62. Review. Gee JR, Keller JN. (2005) Astrocytes: regulation of brain homeostasis via apolipoprotein E. Int J Biochem Cell Biol. Jun;37(6):1145-50. Review. Gehrmann J, Matsumoto Y, Kreutzberg GW. (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. Mar;20(3):269-87. Review. Götz J, Schild A, Hoerndli F, Pennanen L. (2004) Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci. Nov;22(7):453-65. Review Graff-Radford NR, Green RC, Go RC, Hutton ML, Edeki T, Bachman D, Adamson JL, Griffith P, Willis FB, Williams M, Hipps Y, Haines JL, Cupples LA, Farrer LA. (2002) Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. Apr;59(4):594-600. Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. (2008) Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. Oct 17. [Epub ahead of print] Halassa MM, Fellin T, Haydon PG. (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. Feb;13(2):54-63. Hanani M. (2005) Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. Jun;48(3):457-76. Review. Harman D. (2002) Alzheimer's disease: role of aging in pathogenesis. Ann N Y Acad Sci. Apr;959:384-95; discussion 463-5. Review. Harris FM, Tesseur I, Brecht WJ, Xu Q, Mullendorff K, Chang S, Wyss-Coray T, Mahley RW, Huang Y. (2004) Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer's disease. J Biol Chem. Jan 30;279(5):3862-8. Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ. (2007) Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease. PLoS Med. Aug;4(8):e262. Hirsch-Reinshagen V, Wellington CL. (2007) Cholesterol metabolism, apolipoprotein E, adenosine triphosphatebinding cassette transporters, and Alzheimer's disease. Curr Opin Lipidol. Jun;18(3):325-32. Review. Hoe HS, Freeman J, Rebeck GW. (2006) Apolipoprotein E decreases tau kinases and phospho-tau levels in primary neurons. Mol Neurodegener. Dec 13;1:18 Holmes C.(2005) Molecular pathology of Alzheimer's Disease. PSYCHIATRY;4:1:37-40. 35 Hooper NM. (2005) Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans. Apr;33(Pt 2):335-8. Review. Huang Y, Weisgraber KH, Mucke L, Mahley RW. (2004) Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer's disease. J Mol Neurosci.;23(3):189-204. Review. Huang Y. (2006) Apolipoprotein E and Alzheimer disease.Neurology. Jan 24;66(2 Suppl 1):S79-85. Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. (2008) ApoE promotes the proteolytic degradation of Abeta. Neuron. Jun 12;58(5):681-93. Johnson GV, Stoothoff WH. (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. Nov 15;117(Pt 24):5721-9. Review. Lahiri DK, Sambamurti K, Bennett DA. (2004) Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer's disease. Neurobiol Aging. May-Jun;25(5):651-60. Ledesma MD, Dotti CG. (2006) Amyloid excess in Alzheimer's disease: what is cholesterol to be blamed for? FEBS Lett. Oct 9;580(23):5525-32. Review. Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. (1993) Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci U S A. Sep 1;90(17):7951-5. LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. (1994) Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. Sep 23;269(38):23403-6. Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. (2005) Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol Med. Apr;11(4):164-9. Review. Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N Jr, Frautschy SA, Cole GM. (2005) A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. Mar 23;25(12):3032-40. Kins S, Kurosinski P, Nitsch RM, Götz J. (2003) Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am J Pathol. Sep;163(3):833-43. Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. (2001) Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. Jun 16;322(7300):1447-51. Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J. (2006) Therapeutic approaches to Alzheimer's disease. Brain. Nov;129(Pt 11):2840-55. Review Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. Jul;10(7):719-26. 36 Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, Walter M, Roth MG, Lazo JS. (2005) The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J Biol Chem. Feb 11;280(6):4079-88. Kreutzberg GW. (1995) Microglia, the first line of defence in brain pathologies. Arzneimittelforschung. Mar;45(3A):357-60. Review. Mann KM, Thorngate FE, Katoh-Fukui Y, Hamanaka H, Williams DL, Fujita S, Lamb BT. (2004) Independent effects of APOE on cholesterol metabolism and brain Abeta levels in an Alzheimer disease mouse model. Hum Mol Genet. Sep 1;13(17):1959-68. Mahley RW, Weisgraber KH, Huang Y. (2006) Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. Apr 11;103(15):5644-51. Review. Masliah E, Iwai A, Mallory M, Uéda K, Saitoh T. (1996) Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer's disease. Am J Pathol. Jan;148(1):201-10. Michikawa M, Fan QW, Isobe I, Yanagisawa K. (2000) Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. Mar;74(3):1008-16. Michikawa M. (2006) Role of cholesterol in amyloid cascade: cholesterol-dependent modulation of tau phosphorylation and mitochondrial function. Acta Neurol Scand Suppl.;185:21-6. Review. Mudher A, Lovestone S. (2002) Alzheimer's disease-do tauists and baptists finally shake hands?Trends Neurosci. Jan;25(1):22-6. Review. Parri R, Crunelli V. (2003) An astrocyte bridge from synapse to blood flow. Nat Neurosci. Jan;6(1):5-6. Review. No abstract available. Patel SC, Suresh S, Kumar U, Hu CY, Cooney A, Blanchette-Mackie EJ, Neufeld EB, Patel RC, Brady RO, Patel YC, Pentchev PG, Ong WY. (1999) Localization of Niemann-Pick C1 protein in astrocytes: implications for neuronal degeneration in Niemann- Pick type C disease. Proc Natl Acad Sci U S A. Feb 16;96(4):1657-62 Patel NV, Forman BM. (2004) Linking lipids, Alzheimer's and LXRs? Nucl Recept Signal.;2:e001. Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. Dec 9;402(6762):615-22. Petanceska SS, DeRosa S, Sharma A, Diaz N, Duff K, Tint SG, Refolo LM, Pappolla M. (2003) Changes in apolipoprotein E expression in response to dietary and pharmacological modulation of cholesterol. J Mol Neurosci.;20(3):395-406. Peters O, Schipke CG, Hashimoto Y, Kettenmann H. (2003) Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci. Oct 29;23(30):9888-96. Phiel CJ, Wilson CA, Lee VM, Klein PS. (2003) GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. May 22;423(6938):435-9. Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. (1993) Neurodegeneration induced by betaamyloid peptides in vitro: the role of peptide assembly state. J Neurosci. Apr;13(4):1676-87. 37 Jaya Prasanthi RP, Schommer E, Thomasson S, Thompson A, Feist G, Ghribi O. (2008) Regulation of betaamyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer's disease. Mech Ageing Dev. Nov;129(11):649-55. Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinistö L, Halonen P, Kontula K. (1995) Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. Nov 9;333(19):1242-7. Price DL, Tanzi RE, Borchelt DR, Sisodia SS. (1998) Alzheimer's disease: genetic studies and transgenic models. Annu Rev Genet.32:461-93. Review. Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. (2002) Androgens protect against apolipoprotein E4induced cognitive deficits. J Neurosci. Jun 15;22(12):5204-9. Raber J. (2008) AR, apoE, and cognitive function. Horm Behav. May;53(5):706-15. Review. Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. (2002) Tau is essential to beta -amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. Apr 30;99(9):6364-9. Reid PC, Urano Y, Kodama T, Hamakubo T. (2007) Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. J Cell Mol Med. May-Jun;11(3):383-92. Review. Ribé EM, Pérez M, Puig B, Gich I, Lim F, Cuadrado M, Sesma T, Catena S, Sánchez B, Nieto M, GómezRamos P, Morán MA, Cabodevilla F, Samaranch L, Ortiz L, Pérez A, Ferrer I, Avila J, Gómez-Isla T. (2005) Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiol Dis. Dec;20(3):814-22. Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. (2004) Role of microglia in central nervous system infections. Clin Microbiol Rev. Oct;17(4):942-64. Review. Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R, Hartmann T. (2002) Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci. Mar 1;22(5):1679-89. Santhakumar V, Aradi I, Soltesz I. (2005) Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol. Jan;93(1):437-53 Schwab C, Hosokawa M, McGeer PL. (2004) Transgenic mice overexpressing amyloid beta protein are an incomplete model of Alzheimer disease. Exp Neurol. Jul;188(1):52-64. Seifert G, Schilling K, Steinhäuser C. (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. Mar;7(3):194-206. Review. Sorrentino G, Bonavita V. (2007) Neurodegeneration and Alzheimer's disease: the lesson from tauopathies. Neurol Sci. Apr;28(2):63-71. Review. Stevens B. (2008) Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals.16(4):278-88. 38 Suh YH, Checler F. (2002) Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev. Sep;54(3):469-525. Review. Erratum in: Pharmacol Rev. 2006 Jun;58(2):280. Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B. (1998) Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc Natl Acad Sci U S A. Aug 4;95(16):9637-41. Thelen KM, Rentsch KM, Gutteck U, Heverin M, Olin M, Andersson U, von Eckardstein A, Björkhem I, Lütjohann D. (2006) Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J Pharmacol Exp Ther. Mar;316(3):1146-52. Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. (1993) Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. Dec 1;90(23):11282-6. van Marum RJ. (2008) Current and future therapy in Alzheimer's disease. Fundam Clin Pharmacol. Jun;22(3):265-74. Review. Vincent B, Smith JD. (2001) Astrocytes down-regulate neuronal beta-amyloid precursor protein expression and modify its processing in an apolipoprotein E isoform-specific manner. Eur J Neurosci. Jul;14(2):256-66. Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. (2004) ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. Sep 24;279(39):40987-93. Wang N, Yvan-Charvet L, Lütjohann D, Mulder M, Vanmierlo T, Kim TW, Tall AR. (2008) ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J. Apr;22(4):1073-82. Wilhelmus MM, Otte-Höller I, Davis J, Van Nostrand WE, de Waal RM, Verbeek MM. (2005) Apolipoprotein E genotype regulates amyloid-beta cytotoxicity. J Neurosci. Apr 6;25(14):3621-7. Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. (2002) The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. Jul;59(7):1154-60. Zheng H, Koo EH. (2006) The amyloid precursor protein: beyond amyloid Mol Neurodegener. Jul 3;1:5. 39