* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Phosphatases - Georgia Institute of Technology
Survey
Document related concepts
Extracellular matrix wikipedia , lookup
Endomembrane system wikipedia , lookup
P-type ATPase wikipedia , lookup
Chemical synapse wikipedia , lookup
Cell growth wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
NMDA receptor wikipedia , lookup
List of types of proteins wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Transcript
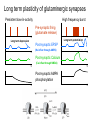
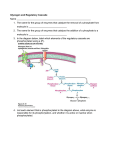
Phosphatases • Structure & Biochemistry – PTP – PPP – PPM Protein Tyrosine Phosphatases Parallel active and inactive phosphatase domains PTP • Diverse family • Receptor/Nonreceptor • Tyrosine specific/Dual specificity – MAPK phosphatases • Catalytic cysteine residue – Reactive sulfur – Oxidizable Cysteine PDB: 3eu0 PTP regulation • Regulatory domains – Targeting to compartments or proteins – Allosteric sensors – Autoinhibition • • • • Proteolysis Phosphorylation Oxidation Diversity – Overlapping substrates among members – Opposing functions – MKPs, SHPs Cell adhesion • IntegrinFAKp130cascrkracad hesion • PTP-PEST – Paxillin binding – Targets FAK, p130cas, others – Focal adhesion turnover • Constitutive • Negative feedback Mitra, et al 2005 Yersinia • Tuberculosis • Bubonic plague • YopH PTP – IgGFcyR (RTK) Fyn Syk dynamin phagocytosis – YopH--|FyB • Highest activity PTP • Many targets related to phagocytosis • Blocks inflammatory phagocytosis of bacteria Yersinia Pestis colony Rocky Mountain Laboratories, NIAID Ser/Thr phosphatases • Common ancestor, common catalytic domain • Regulation by binding partners – Specificity – Localization – Activity Catalytic Mn++ PP1a • Catalytic metals – Fe/Mn – Fe/Zn – Oxidizable • PP1 – Glycogen metabolism (GS) – Cell cycle progression – Glutaminergic transmission (AMPA, NMDA) PDB: 1FJM PPP Phosphatases • PP2A – Cell cycle & division – Cyclin G partner – Suppression of migration • PP2B/calcineurin – Calcium dependent – Immunomodulation – NFAT PPP regulation of glycogen breakdown • adrenaline β-AR AC PKA – PKAPhK phosphorylaseglycogen breakdown into glucose • PhK--|GSsynthesis of glycogen from glucose – PKA--|GM-PP1--|phosphorylase • GM-PP1GS • GM glycogen targeting subunit of PP1 – GM binds ER, near muscle glycogen stores – GM binds PP1; PP1 near glycogen inactivates phosphorylase, activates GS – Phospho-GM does not bind PP1 • Phosphorylation of PP1 itself will also decrease activity PPM Phosphatases • • • • PP2C relatives Mn2+/Mn2+ catalytic center Transcriptional regulation Eg: wip1 – p53wip1--|p38p53apoptosis – Negative feedback on cell death subsequent to DNA damage, UV stress… Long term plasticity of glutaminergic synapses Persistent low-/in-activity High frequency burst Pre-synaptic firing (glutamate release) t Long term potentiation Long term depression t Post-synaptic EPSP t (Na influx through AMPA) t Post-synaptic Calcium t (Ca influx through NMDA) t Post-synaptic AMPA phosphorylation t t