* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download bio98a_l04
Theories of general anaesthetic action wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Expanded genetic code wikipedia , lookup
Gel electrophoresis wikipedia , lookup
Genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Gene expression wikipedia , lookup
Endomembrane system wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Expression vector wikipedia , lookup
Protein domain wikipedia , lookup
Magnesium transporter wikipedia , lookup
Biochemistry wikipedia , lookup
Protein folding wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Interactome wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein–protein interaction wikipedia , lookup
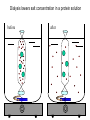
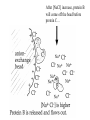
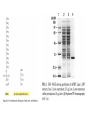
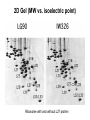
Bio 98 - Lecture 4 Amino acids, proteins & purification Tryptophan and Tyrosine absorb UV light at longer wavelengths than other amino acids 100% 97.2% Lambert-Beer Law: OD or Abs = log10 Io/I = -log10 I/Io = e c l e, the molar extinction coefficient, is the optical density (OD) of a material at a given concentration, c, and l is the pathlength of the cuvette. The molar extinction coefficient for Trp is 5,500 M-1 cm-1 at 280 nm, which means a 1 M solution of Trp has an optical density of 5,500 at 280 nm (OD280) when using a pathlength of 1 cm. Suppose we know that a protein contains 3 Trp and 4 Tyr residues. What is the extinction coefficient at 280 nm of the protein? Extinction coeff. (1 M of substance) per Trp is 5,500 M-1cm-1 while that per Tyr is 1,400 M-1cm-1. Total extinction coefficient of protein is then nTrp x eTrp + nTyr x eTyr = eTotal 3 x 5,500 M-1cm-1 + 4 x 1,400 M-1cm-1 = 22,100 M-1cm-1 = e So a 1 M solution of this protein has an absorbance (Abs or OD) of 22,100 at 280 nm (OD280). What is the OD280 of a 10 mM solution of this protein if we are using a cuvette with a pathlength of 1 cm? e x c x l = Abs280 22,100 M-1 cm-1 x 10-5 M x 1 cm = 0.221. What fraction of the 280 nm photons have not been absorbed after passing through 1 cm of this sample? Abs = log10 Io/I = -log10 I/Io or I/Io = 10-0.221 = 0.60 Isoelectric point (pI) The isoelectric point (pI) is the pH at which a particular molecule or surface carries no net electrical charge. At a pH above their pI, proteins or amino acids carry a net negative ______ charge. At a pH below their pI, they carry a net positive charge. AA with non-ionizable side chain (Gly) AA with ionizable side chain (Glu) pI = 1/2 (pK1 + pK2) = 1/2 (2.34 + 9.60) = 5.97 pI = 1/2 (pK1 + pKR) = 1/2 (2.19 + 4.25) = 3.22 Peptide: A-N-G-E-L-I-A Ionizable group pK Charge of group protonated / deprotonated Charge at pH 9.69 Charge at pH 0 Ala (A) C-term 2.34 0 / -1 -1 0 Glu (E) side chain 4.25 0 / -1 -1 0 Ala (A) N-term 9.69 +1 / 0 +0.5 1 -1.5 1 Net charge pI = (2.34+4.25) / 2 = 3.30 I. WHY PURIFY PROTEINS? A. Research - academic • to study function & regulation • to determine sequence & 3D structure • to clone and study the gene B. Commercial ($$$) – practical • research tools (restriction enzymes) • diagnostic reagents (prostate-specific antigen) • therapeutics (insulin, growth hormones, vaccines, antibodies [biologics]) II. SOLUBLE vs MEMBRANE PROTEINS • Proteins must be free in solution for purification. Not an issue for “soluble proteins.” • Integral membrane proteins (those imbedded in the lipid bilayer) must be solubilized with a detergent first. Membrane Protein Extraction Using Detergents III. STEPS IN THE PURIFICATION OF A TYPICAL SOLUBLE PROTEIN 1. Homogenization: Prepare cell-free extract (rupture cell walls/membranes). 2. Centrifugation: Remove membranes, nuclei, large organelles (mitochondria) etc., keep supernatant. 3. Ammonium sulfate precipitation: (1) Proteins often become less soluble when the ionic strength is increased to very high levels. Precipitation points vary by protein, so purification can be achieved. (2) Precipitates can be redissolved in a small volume, so concentration can be achieved. (3) Dialyze the redissolved proteins against a low salt buffer. 4. Final purification with one or more rounds of Column Chromatography Salt fractionation Add salt to 20% saturation Centrifuge & remove supernatant Centrifuge & remove supernatant Add salt to 40% saturation Cell free extract Ammonium sulfate (NH4)2SO4 2NH4+ SO4-2 Dialysis lowers salt concentration in a protein solution before after General Chromatography Protocol OD280 Three Main Types of Chromatography Ion (anion) exchange Size exclusion Know Thy pI aka gel filtration Specific affinity After [NaCl] increase, protein B will come off the bead before protein C… Affinity purification of a genetically engineered (recombinant) protein containing an engineered purification tag H Ni +5 H H H His-tagged protein: Engineered, usually 6-His (HHHHHH) at N- or C-terminus Affinity column or beads: immobilized Ni2+ or Co2+ How does one monitor the purification of a protein? SDS-PAGE (sodium dodecylsulfate - polyacrylamide gel electrophoresis) About 1 SDS molecule binds per 2 amino acids Activity vs. Specific Activity A B Both beakers have the same activity (units) of ‘red’, though B has a higher specific activity as the ratio of red to other has increased. Specific activity = units/mg 2D Gel (MW vs. isoelectric point) Ribosome with and without L27 protein