* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cortical inputs to the CA1 field of the monkey hippocampus originate
Affective neuroscience wikipedia , lookup
Limbic system wikipedia , lookup
Neuroesthetics wikipedia , lookup
Optogenetics wikipedia , lookup
Synaptic gating wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroplasticity wikipedia , lookup
Environmental enrichment wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
Aging brain wikipedia , lookup
Human brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Cortical cooling wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Time perception wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Motor cortex wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
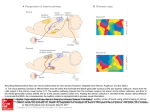
Neuroscience Letters, 115 (1990) 43-48 Elsevier Scientific Publishers Ireland Ltd. 43 NSL 06983 Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE W e n d y A. Suzuki 2 and David G. Amaral 1 1The Sail( Institute for Biological Studies and 2The Group in Neurosciences, University of California at San Diego, San Diego, CA (U.S.A.) (Received 6 February 1990; Accepted 5 March 1990) Key words." Hippocampal formation; CAI, Connections; Primate; Area TE; Perirhinal cortex; Parahippocampal cortex We determined the cortical regions that project directly to the CAI field of the monkey hippocampus by injecting the retrograde tracers Fast blue, Diamidino yellow or WGA-HRP into CAI and examining the distribution of labeled cells. In the temporal lobe, large numbers of retrogradely labeled cells were observed in the perirhinal and parahippocampal cortices. Only an occasional labeled cell, however, was observed in the unimodal visual area TE. Additional projections to CA1 arose in the dorsal bank of the superior temporal sulcus, in the rostral and retrosplenial portions of the cingulate cortex, in the agranular insular cortex, and in the caudal orbitofrontal cortex. In recent years, a number of reports have emphasized that the primate hippocampal formation* receives direct inputs from several neocortical regions. Van Hoesen and colleagues [6-8] used anterograde tracing techniques to demonstrate direct projections to the entorhinal cortex and subiculum from the medial temporal lobe and frontal cortex. Insausti et al. [3] used retrograde tracing techniques to demonstrate that the strongest cortical projections to the monkey entorhinal cortex originate in a band of cortex that lies laterally adjacent to the hippocampal formation and comprises the perirhinal cortex (areas 35 and 36) and the parahippocampal cortex (areas TF and TH). Other projections originated from the dorsal bank of the superior temporal sulcus, the insular cortex, the orbitofrontal cortex and the cingulate cortex, especially the retrosplenial region. The major unifying characteristic of the cortical areas that project to the entorhinal and subicular cortices is that, on anatomical grounds, they are regions of polymodal sensory convergence [5]. "In the term hippocampal formation we include the dentate gyrus, the hippocampus proper, the subicular complex and the entorhinal cortex. Correspondence: D.G. Amaral, The Salk Institute, P.O. Box 85800, San Diego, CA 92138, U.S.A. 0304-3940/90/$ 03.50 © 1990 Elsevier Scientific Publishers Ireland Ltd. 44 More recently, Yukie and Iwai [9,4] reported that the unimodal visual area TE is also directly interconnected with CAI of the monkey hippocampus. They placed injections of W G A - H R P into a ventromedial portion of the medial temporal lobe, referred to as the ventral portion of area TE (TEv), and observed retrogradely labeled cells in the CAI field. Fine granular particulate staining in the stratum lacunosum-moleculare of CA I was interpreted as anterogradely transported label. Since area TE is considered to be a unimodal visual associational area [2,5] that does not project to the entorhinal cortex [3], a projection from TE to CA I would indicate that the hippocampus receives a different type of sensory innervation than the entorhinal cortex. The most direct way of evaluating the origin of temporal lobe projections to CA I is by placing a retrograde tracer into CA I and determining the distribution of retrogradely labeled cells. We report the results of experiments of this type in this paper. A library of 5 experiments with injections of the retrograde tracers Fast blue (FB) or Diamidino yellow (DY) into various fields of the hippocampal formation were available from a previous study [10]. The two tracers were injected on both sides of the brain at different rostrocaudal levels of the hippocampal formation and thus 20 injections were available for analysis. In three additional experiments, discrete injections of the retrograde tracers FB, DY or W G A - H R P were placed into different rostrocaudal levels of the medial portion of the CAi field of the hippocampus. After a survival period of two weeks (or 2 days in the case of W G A - H R P injections), the animals were sacrificed and the brains processed for histological examination. In experiments M-2-87 and M-7-89, at least one of the injections exclusively involved the CAI field. The FB injection in M-7-89 consisted of 800 nl of a 3% FB solution. A l-in-8 series of 30/tm sections through the entire brain was analyzed and the cells located in every other of these sections was plotted and counted using a computer-aided digitizing system. The DY injection on the left side of M-2-87 consisted of 200 nl of 2% DY solution and the sections were analyzed as described above. In our library of cortical [3H]amino acid injections, one experiment (M-4-88) consisted of a 50 nl injection of a 100/~Ci//tl solution of [3H]leucine and [3H]proline into area TF of the parahippocampal cortex. This brain was prepared autoradiographically for the demonstration of anterogradely transported label and will be used to illustrate the terminal field of CA 1 cortical afferents. The cytoarchitectonic organization of the perirhinal and parahippocampal cortices has been described previously [I, 3]. The border between areas 36 or TF and the laterally adjacent area TE is not sharp. The transition between areas 36 or TF and area TE appears to have characteristics intermediate between them and we have labeled this area (36 or TF)/TE in Figs. 1 and 2. The injection in experiment M-7-89 was confined to CA 1 of the caudal hippocampus and was focused in stratum lacunosum-moleculare (Fig. 1). This injection was located at approximately the location that Yukie and Iwai [9] observed the strongest projection from their injections. Thus, based on their report, one might expect to observe large numbers of labeled cells in area TE. However, in the analyzed sections, only 4 retrogradely labeled cells from a total of 4095 cells plotted in the inferior temporal lobe were observed in area TE. Moreover, only an additional 84 cells were 45 M7"89/ CELLS CELL 2 CELLS TE:lC T E : I CELL 0 CELLSIII;I~I!I: INJECTION SITE Fig. 1. Line drawings of representative coronal section through the temporal lobe in experiment M-7-89 showing the distribution and number of retrogradely labeled cells in the inferior temporal lobe. The boundaries of areas 35, 36, TH, TF, and the (36 or TF)/TE transition region are indicated in each section by different shades of gray (see legend at top right). Indicated above each section is the number of retrogradely labeled cells observed in each of the outlined areas. 46 iiiii0 1 0 CELLS 100 CELLS INJECTION SITE Fig. 2. Line drawings of representative coronal sections through the temporal lobe in experiment M-2-87 indicating the distribution and number of retrogradely labeled cells in the inferior temporal lobe. Definitions of areas are the same as in Fig. 1. Indicated above each section is the number of retrogradely labeled cells observed in each of the outlined areas. 47 found in the transitional region between areas 36 or T F and TE. Taken together these cells account for only 2.1% of the retrogradely labeled cells in the inferior temporal cortex. The greatest number of labeled cells was observed in the perirhinal and parahippocampal cortices. There were 2914 labeled cells (or 72%) observed in area 36 and 615 labeled cells (or 12%) in area 35. Approximately 12% of the cells were observed in areas TF and TH. Labeled cells were also observed in the cingulate cortex (205 cells), the dorsal bank of the rostral superior temporal sulcus (122 cells), the agranular insular cortex (26 cells) and caudal area 13a of the orbitofrontal cortex (24 cells). In M-2-87, (Fig. 2) the injection was somewhat smaller than in M-7-89 and was located rostrally in CA 1. There were no labeled cells in area TE and only a few cells found in the transition region. As in M-7-89, the largest number of retrogradely labeled cells was observed in area 36. We did not observe any larger number of labeled cells in area TE in any of the other retrograde cases available for analysis. In all cases in which [3H]amino acid injections involved areas 36 or TF, anterogradely labeled fibers and terminals were observed in the deep portion of the stratum lacunosum-moleculare of C A l . In experiment M-4-88, the injection was confined to area TF and the anterogradely transported label was distributed mainly to the medial portion of CA1 (Fig. 3) and also extended into the transition region between CAI Eq TH INJECTION SITE TF Fig. 3. Line drawings of representative coronal sections through the hippocampal formation arranged from rostral (A) to candal (C), in experiment M-4-88 indicating the distribution of anterograde labeling resulting from an injection of [3H]amino acids into area TF of the parabippocampal cortex. The levels of shading directly correspond to the observed density of autoradiographic grains. Abbreviations: CAI, CA3, fields of the hippocampus; DG, dentate gyrus; EC, entorhinal cortex; PaS, parasubiculum; PrS, presubiculum; S, subiculum; TF, TH, fields of the parahippocampal gyrus. 48 and the subiculum. The density of labeling in CAI appeared to be relatively light in comparison to the heavy innervation of the entorhinal cortex (Fig. 3). We conclude from these studies that the same regions of the ventral temporal lobe that project to the entorhinal cortex also project to CA1. These projections originate mainly from areas 35 and 36 of the perirhinal cortex, and areas TF and TH of the parahippocampal cortex. We observed a sharp drop in the number of labeled cells in the transitional region between these areas and area TE and there was only an occasional retrogradely labeled cell in cortex that clearly met the cytoarchitectonic definition of area TE. It would appear, therefore, that the CA 1 field of the hippocampus does not receive a strong unimodal visual input via area TE but, like the entorhinal cortex, receives multimodal sensory information from the perirhinal and parahippocampal cortices. Based on our interpretation of the illustrations in Yukie and lwai [9] it appears that the injections yielding anterograde transport to the CA I field partially involved the lateral aspects of areas 36 or TF according to our delimitation of these fields. We would suggest, therefore, that the CA 1 projections that they demonstrated arise primarily from the perirhinal and parahippocampal cortices rather than from area TE. We would like to thank Dr. Ricardo Insausti for help with some of the preparations used in this study and to Ms. Janet Weber and Ms. Mary Ann Lawrence for histological assistance. This work was supported by NIH Grant NS 16980. I Amaral, D.G, Insausti, R. and Cowan, W.M., The entorhinal cortex of the monkey: 1. Cytoarchitectonic organization, J. Comp. Neurol., 264 (1987) 32(y 355. 2 Desimone, R. and Gross, C.G., Visual areas in the temporal cortex of the macaque, Brain Res., 178 (1979) 363 -380. 3 Insausti, R., Amaral, D.G. and Cowan, W.M., The entorhinal cortex of the monkey: II. Cortical afterents, J. Comp. Neurol., 264 (1987) 356- 395. 4 Iwai, E. and Yukie, M., A direct projection from hippocampal field CAI to ventral area TE ofinferotemporal cortex in the monkey, Brain Res., 444 (1988) 397401. 5 Pandya, D. N. and Yeterian, E. H., Architecture and connections of cortical association areas. In A. Peters and E.G. Jones (Eds.), Cerebral Cortex, Association and Auditory Cortices, Vol. 4, Plenum Press, New York, 1984, pp. 3-61. 6 Van Hoesen, G.W. and Pandya, D.N., Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents, Brain Res., 95 (1975) I 24. 7 Van Hoesen, G.W., Pandya, D.N. and Butters, N., Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents, Brain Res., 95 (1975) 25 38. 8 Van Hoesen, G.W., Rosene, D.L. and Mesulam, M.M., Subicular input from temporal cortex in the rhesus monkey, Science, 205 (1979) 608~610. 9 Yukie, M. and Iwai, E., Direct projections from ventral TE area of the inferotemporal cortex to hippocampal field CAI in the monkey, Neurosci. Lett., 88 (1988) 6- 10. 10 Witter, M.P., Van Hoesen, G. W. and Amaral, D. G., Topographical organization of the entorhinal projection to the dentate gyrus of the monkey, J. Neurosci., 9 (1989) 216-228.