* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The main points that you should learn from the problems in øvelse 2
Magnesium transporter wikipedia , lookup
SNARE (protein) wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Lipid bilayer wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Cell nucleus wikipedia , lookup
Cytokinesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Cell membrane wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Western blot wikipedia , lookup
Proteolysis wikipedia , lookup
List of types of proteins wikipedia , lookup
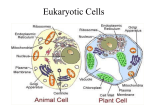
The main points that you should learn from the problems in øvelse 2 and 4 are summarized below. Pages mentioned refer to “essential cell biology”. Øvelse 2 1) Proteins that make up water-filled pores in the membrane (e.g. Porins) require a hydrophilic surface facing the lumen of the channel and a hydrophobic surface facing the hydrophobic core of the lipid bilayer. In a beta-strand the side chains alternate between pointing above and below the sheet (see page 128). Distinguish between hydrophobic and hydrophilic aminoacids. 2) The molecular structure of phosphatidylcholine, the most common phospholipid in the cell membrane (page 368). Importance of the amphipathic structure of the lipid for bilayer stability (see page 370). Removal of most of the hydrophilic head from a lipid leads to instability of the bilayer structure. As a consequence the cell lyses. Proteases will digest the extracellular part of transmembrane proteins. Even if the protein would be removed from the membranecompletely there will be no whole because of the fluidity of the lipid layer (“two dimensional fluid”). 3) The cell surface is coated with carbohydrates (page 382). The lumen of membrane-enclosed organelles is topologically equivalent to the cell exterior (page 374) which is why the sugars point towards the lumen on internal membranes (and not to the cytosol). Enzymes that add sugars to proteins are present in the lumen of the ER (page 517) and in the Golgi (page 518). Lipids are glycosylated in the Golgi. 4) FRAP technique is used to measure the mobility of molecules in a plasma membrane (page 384). The movement of membrane proteins can be restricted in different ways e.g by anchoring to the cortical cytoskeleton (page 383). The cortical cytoskeleton (cell cortex) of a red blood cell is made up mainly of spectrin and gives the cell its shape (page 380). 5) Know at the structure of three types of phospholipids and their differences. Phospholipids (and glycolipids see problem 3) are asymmetrically distributed between the two monolayers of the plasma membrane (page 373). Phospholipids are made at the cytosolic side of the ER membrane (page 373). Flipases are required to flip lipids to the luminal monolayer. Note: The book states that asymmetry is established in the ER. That is not true. In fact the ER membrane does not appear to be very asymmetric. Asymmetry of the phospholipids is established and actively maintained by flippase-like enzymes in the plasma membrane. Øvelse 4 A) All protein synthesis starts in the cytosol. If the protein has a ER import signal (hydrophobic stretch of amino acids at the N-terminus, page 504) the ribosome docks onto the ER membrane and the rest of the protein is synthesized into the lumen of the ER (unless a transfer stop signal is present) (page 510). Proteins with a nuclear import signal are recognized by a receptor in the cytosol (page 505) and enter the nucleus through the nuclear pores. Proteins that shuttle between the nucleus and the cytosol e.g. nuclear transport receptors have both a nuclear import and a nuclear export signal. The protein a) will be synthesized into the lumen of the ER. The nuclear localization signal will have no effect since the nuclear transport receptors are in the cytosol and the protein is in the ER interior. Protein b) will be imported into the mitochondria. There ER retention signal has no effect because the protein is not imported into the ER (there is no signal for import into the ER). Protein c) will shuttle between the nucleus and the cytosol. B) Some proteins that are made in the ER have a function in the ER and should therefore stay in the ER (ER resident proteins) e.g. PDI. There is a constant flow of vesicles from the ER to the Golgi and ER resident proteins (like PDI) can escape to the Golgi. The ER retention signal (ER retrieval signal) is a four amino acid sequence (KDEL) at the C-terminus of ER resident proteins (page 517). The KDEL receptor, which is present in the Golgi and in the ER recognizes the KDEL sequence and binds to it in the Golgi but does not bind to it in the ER (due to the different pH in ER and Golgi). The KDEL receptor shuttles in vesicles between the ER and the Golgi returning proteins with a KDEL signal from the Golgi to the ER. If the KDEL sequence would be missing the protein would travel along the constitutive secretory pathway to the plasma membrane. Retrieval is better than retention because the KDEL signal does not prevent to protein from leaving the ER- it ‘returns’ the protein from the Golgi. C) Acid hydrolases are lysosomal enzymes required for digestion of macromolecules (page 527) Acid hydrolases require a tag (mannose-6-phosphat) for their sorting to lysosomes (via late endosomes, page 527). In I-cell disease the tag or the receptor that recogizes the tag are defect. Acid hydrolases can not be sorted to the lysosomes, but are secreted from the cell (constitutive secretory pathway). The dark inclusions are made up from organic material that could not be digested in the lysosomes due to the lack of acid hydrolases. D) LDL enters the cell via receptor-mediated endocytosis of clathrin coated vesicles (page 526). Clathrin binds to adaptin which binds to the cytoplasmic part of the receptor (page 514). The assembly of clathrin drives vesicle formation (page 513). The mutation in the cytoplasmic part of the LDL receptor impairs binding to adaptin which prevents the assembly of clathrin. E) See answer 15-13 at page A:42 in ‘essential cell biology’ and the ‘synaptic recycling slide’ in the lecture from Wednesday, 28th November.