* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Taylor`s Organic Reactions Summary Sheet
Discodermolide wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Marcus theory wikipedia , lookup
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
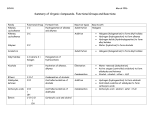
Organic Chemistry Reactions Alkanes Undergo substitution reactions, and combustion reactions (clean) Substitution Reaction: A reaction in which a hydrogen atom is replaced by another atom or group of atoms; reaction of alkanes or aromatics with halogens to produce organic halides and hydrogen halides. Combustion Reaction: The reaction of a substance with oxygen, producing oxides and energy. Alkenes and Alkynes Primarily undergo addition reactions. Addition Reaction: A reaction of alkenes and alkynes in which a molecule, such as hydrogen or a halogen, is added to a double or triple bond. With H2: Hydrogenation With halogens or hydrogen halides: Halogenation With water: Hydration Aromatic Hydrocarbons Undergo substitution reactions like alkanes do, and do not undergo addition reactions like one would usually predict. With X2: Halogenation With HNO3: Nitration With RX: Alkylation Organic Halides Alkyl halides are produced in halogenation reactions with hydrocarbons. Recall Markovnikov’s rule “the rich get richer” applies when hydrogen halides are reactants, and must be considered in designing the synthesis of specific alkyl halides. These alkyl halides can then be transformed into other organic compounds. Preparing Organic Halides: Halogenation Preparing Alkenes from Alkyl Halides: Elimination Reactions Elimination Reaction: A type of organic reaction that results in the loss of a small molecule from a larger molecule. Ex: the removal of H2 from an alkane. Alcohols Alcohols are formed by addition reactions between an Alkene/Alkyne and water this is called a hydration reaction. The hydrogen attaches to the carbon atom that already has more hydrogen atoms; the –OH group attaches to the other carbon atom in the double bond. Hydration Reaction: A reaction that results in the addition of a water molecule. Preparing Alcohols: Hydration Reactions The combustion of alcohols: Like Hydrocarbons, alcohols undergo complete combustion to produce only carbon dioxide and water. From Alcohols to Alkenes: Elimination Reactions (resulting in the removal of water therefore also called a dehydration reaction) Dehydration Reaction: A reaction that results in the removal of water. Ethers Ethers are formed when two alcohol molecules react. A molecule of water is eliminated and the remaining portions of the two alcohol molecules combine to form an ether. This involves a condensation reaction. Condensation Reaction: A reaction in which two molecules combine to form a larger product, with the elimination of a small molecule such as water or an alcohol. Aldehydes Prepared by oxidizing a primary alcohol. Oxidation Reaction: A chemical transformation involving a loss of electrons; historically used in organic chemistry to describe any reaction involving the addition of oxygen atoms or the loss of hydrogen atoms. When a primary alcohol is oxidized, an H atom remains on the C atom. From Aldehyde to Alcohol: Hydrogenation Reactions Ketones Prepared by oxidizing a secondary alcohol. When a secondary alcohol is similarly oxidized, the carbonyl group formed is necessarily attached to two alkyl groups, forming a ketone. From Ketone to Alcohol: Hydrogenation Reaction Carboxylic Acids When an alcohol is mildly oxidized, an aldehyde is produced. Further Controlled oxidation of the aldehyde results in the formation of a carboxylic acid, containing a carboxyl group. Preparing a carboxylic acid: oxidation reactions Esters Esters are organic “salts” formed from the reaction of a carboxylic acid and an alcohol. Esterification: A condensation reaction in which a carboxylic acid and an alcohol combine to produce an ester and Water. From Carboxylic Acids to Organic “Salts”: Esterification Reversal of Esterificaion, the products include a carboxylic acid and the alcohol. Hydrolysis Reaction: A reaction in which a bond is broken by the addition of the components of water, with the formation of two or more products. Amines Amines can be prepared by the reaction of ammonia (a weak base) and an alkyle halide. Primary Amine Secondary Amine Tertiary Amine The final product is a mixture of primary, secondary, and tertiary amines. Amides Carboxylic acids react with ammonia or with 1° and 2° amines to produce amides which are another type of organic salt. Carboxylic acid and ammonia: Condensation Reaction Carboxylic acid and an amine: Condensation Reaction Amides can be hydrolyzed in acidic or basic conditions to produce a carboxylic acid and an amine. Basically it is the reverse of the above reaction.