technical bulletin reaction solvent dimethyl sulfoxide (dmso)
... could expect a loss of between 0.1 and 1.0%. Retention times even in batch stills are usually considerably less than this, and therefore, losses would be correspondingly less.. It has been reported that only 3.7% of volatile materials are produced during 72 hours at the boiling point (189° C) of DMS ...
... could expect a loss of between 0.1 and 1.0%. Retention times even in batch stills are usually considerably less than this, and therefore, losses would be correspondingly less.. It has been reported that only 3.7% of volatile materials are produced during 72 hours at the boiling point (189° C) of DMS ...
Alkenes 4 - ChemWeb (UCC)
... In the transition state for the SN2 attack of a nucleophile on a cyclic mercurinium or bromonium cation bond-breaking of the C-Hg or C-Br bond is more advanced than bond-formation with the incoming nucleophile. Hence the SN2 transition state for this reaction has partial carbocationic (i.e. SN1) cha ...
... In the transition state for the SN2 attack of a nucleophile on a cyclic mercurinium or bromonium cation bond-breaking of the C-Hg or C-Br bond is more advanced than bond-formation with the incoming nucleophile. Hence the SN2 transition state for this reaction has partial carbocationic (i.e. SN1) cha ...
Chapter 16 Aldehydes and Ketones
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
SUPPORTED LIGANDS FOR METAL CATALYZED REACTIONS Rocío Marcos Escartín ISBN:
... alkyl aryl ketones under essentially solvent-free conditions. The best results for ATH have been obtained using immobilized version of TsDPEN and this resin could be recycled with virtually no limits. ...
... alkyl aryl ketones under essentially solvent-free conditions. The best results for ATH have been obtained using immobilized version of TsDPEN and this resin could be recycled with virtually no limits. ...
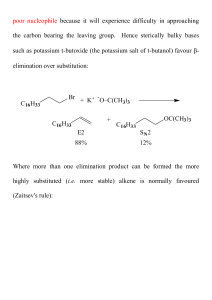
Elimination Reactions
... the more branched position, you are forming a more highly substituted alkene. ...
... the more branched position, you are forming a more highly substituted alkene. ...
OC 2/e Ch 11
... • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 CH 2 OH + N a+ NH 2 4-Pentyn-1-ol HC CCH2 CH2 CH 2 O- Na + + N H3 ...
... • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 CH 2 OH + N a+ NH 2 4-Pentyn-1-ol HC CCH2 CH2 CH 2 O- Na + + N H3 ...
Organic Chemistry
... Use of Carboxylate Anion Nucleophile to form Esters . 87 Hydrolysis of Acid Halides . . . . . . . . . . . . . . . . . . . . . 88 Reaction of Acyl Halide with Ammonia or Amine . . . 89 Esterification of Acid Halides . . . . . . . . . . . . . . . . . . . 90 Esterification of Acid Anhydrides . . . . . . ...
... Use of Carboxylate Anion Nucleophile to form Esters . 87 Hydrolysis of Acid Halides . . . . . . . . . . . . . . . . . . . . . 88 Reaction of Acyl Halide with Ammonia or Amine . . . 89 Esterification of Acid Halides . . . . . . . . . . . . . . . . . . . 90 Esterification of Acid Anhydrides . . . . . . ...
Chapter 8 PowerPoint - Southeast Online
... More Making Pancakes • Let’s now assume that as we are making pancakes, we spill some of the batter, burn a pancake, drop one on the floor, or other uncontrollable events happen so that we only make 11 pancakes. The actual amount of product made in a chemical reaction is called the actual yield. • ...
... More Making Pancakes • Let’s now assume that as we are making pancakes, we spill some of the batter, burn a pancake, drop one on the floor, or other uncontrollable events happen so that we only make 11 pancakes. The actual amount of product made in a chemical reaction is called the actual yield. • ...
Ch. 6 - Department of Chemistry and Biochemistry
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
- Article One Partners
... channel-antagonistic and anticonvulsive properties. They are prepared by a relatively expensive method generating enantiomer mixtures which may be split into the individual optical antipodes. The present invention is aimed at preparing and employing compounds which can be chemically generated by sim ...
... channel-antagonistic and anticonvulsive properties. They are prepared by a relatively expensive method generating enantiomer mixtures which may be split into the individual optical antipodes. The present invention is aimed at preparing and employing compounds which can be chemically generated by sim ...
Polimeri - Polymer Technology Group
... made from crude oil, a nonrenewable raw material. For example, nylon 6,6 is prepared industrially from adipic acid and 1,6-diaminohexane, both of which originate from benzene, a product of petroleum refining. Figure 30.8 Synthesis of adipic acid and ...
... made from crude oil, a nonrenewable raw material. For example, nylon 6,6 is prepared industrially from adipic acid and 1,6-diaminohexane, both of which originate from benzene, a product of petroleum refining. Figure 30.8 Synthesis of adipic acid and ...
Document
... It is important to know what nucleophiles will add to carbonyl groups. • Cl¯, Br¯ and I¯ are good nucleophiles in substitution reactions at sp3 hybridized carbons, but they are ineffective nucleophiles in addition. • When these nucleophiles add to a carbonyl, they cleave the C—O bond, forming an a ...
... It is important to know what nucleophiles will add to carbonyl groups. • Cl¯, Br¯ and I¯ are good nucleophiles in substitution reactions at sp3 hybridized carbons, but they are ineffective nucleophiles in addition. • When these nucleophiles add to a carbonyl, they cleave the C—O bond, forming an a ...
Stoichiometric Calculations
... Remember: Do not use coefficients when converting between moles and mass. The molar mass is for only ONE mole! ...
... Remember: Do not use coefficients when converting between moles and mass. The molar mass is for only ONE mole! ...
Full-Text PDF
... All the DFT static calculations were performed with the Gaussian09 set of programs [69]. For geometry optimization, the well-established and computationally fast GGA functional BP86 was used [70,71]. Geometry optimizations were performed without symmetry constraints, while the located stationary poi ...
... All the DFT static calculations were performed with the Gaussian09 set of programs [69]. For geometry optimization, the well-established and computationally fast GGA functional BP86 was used [70,71]. Geometry optimizations were performed without symmetry constraints, while the located stationary poi ...
Nucleophilic Acyl Substitution
... compound responsible for the burning sensation felt in muscles during anaerobic exercise, and it is also found in sour milk. Spinach and other leafy green vegetables are rich in oxalic acid. Succinic acid and citric acid are important intermediates in the citric acid cycle (Section 25.1), a series o ...
... compound responsible for the burning sensation felt in muscles during anaerobic exercise, and it is also found in sour milk. Spinach and other leafy green vegetables are rich in oxalic acid. Succinic acid and citric acid are important intermediates in the citric acid cycle (Section 25.1), a series o ...
LINALOOL BIOTRANSFORMATION WITH FUNGI
... the liquid cultures were determined using 1-octanol as internal standard. The stereoisomeric distribution of linalool oxides was determined by enantioselective GC. The dry biomass was determined with an infrared moisture analyzer (Sartorius, Germany). Glucose was analyzed enzymatically (Yellow Sprin ...
... the liquid cultures were determined using 1-octanol as internal standard. The stereoisomeric distribution of linalool oxides was determined by enantioselective GC. The dry biomass was determined with an infrared moisture analyzer (Sartorius, Germany). Glucose was analyzed enzymatically (Yellow Sprin ...
Acid-Catalyzed Hydration of Alkenes
... Optically inactive reactants produce optically inactive products. (Racemic mixtures are optically inactive) The correlary is that an optically active starting material MAY produce an optically active product depending on the mechanism. ...
... Optically inactive reactants produce optically inactive products. (Racemic mixtures are optically inactive) The correlary is that an optically active starting material MAY produce an optically active product depending on the mechanism. ...
Reactions
... H2O H2SO4 The regiochemistry is determined by the relative stability of the intermediate carbocation. But what if you want 1-propyl alcohol? What could you do to “trick” the regiochemistry? ...
... H2O H2SO4 The regiochemistry is determined by the relative stability of the intermediate carbocation. But what if you want 1-propyl alcohol? What could you do to “trick” the regiochemistry? ...
amine
... • In the case of N-methyl-2-phenylethylamine there are two combinations that can lead to the product: ...
... • In the case of N-methyl-2-phenylethylamine there are two combinations that can lead to the product: ...
Document
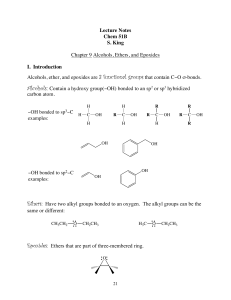
... Alcohols, Ethers and Epoxides • More complex ethers are named using the IUPAC system. One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl ...
... Alcohols, Ethers and Epoxides • More complex ethers are named using the IUPAC system. One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.