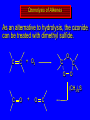* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Reactions of Alkenes
Enantioselective synthesis wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Discodermolide wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Asymmetric induction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Polythiophene wikipedia , lookup
George S. Hammond wikipedia , lookup
Stille reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydrogenation wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
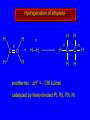
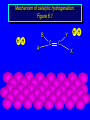
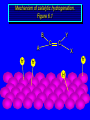
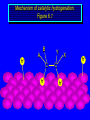
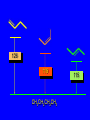
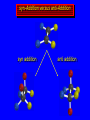
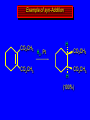
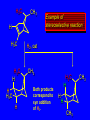
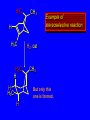
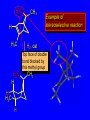
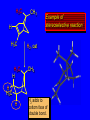
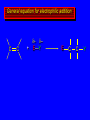
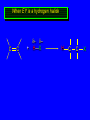
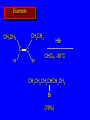
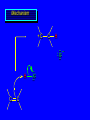
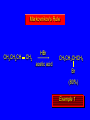
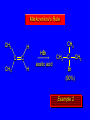
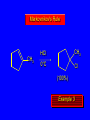
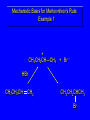
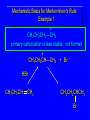
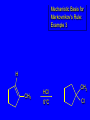
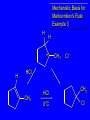
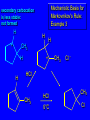
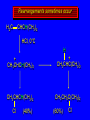
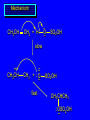
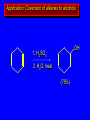
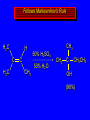
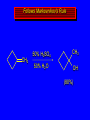
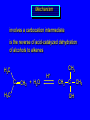
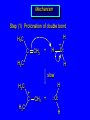
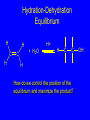
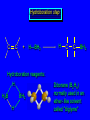
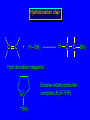
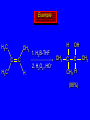
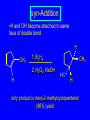
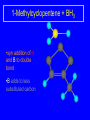
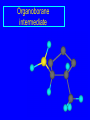
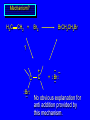
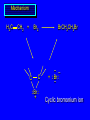
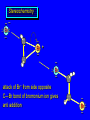
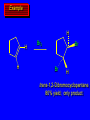
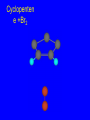
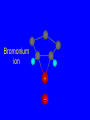
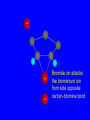
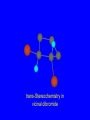
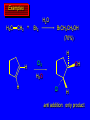
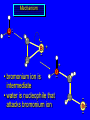
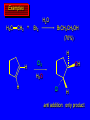
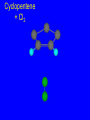
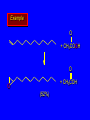
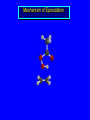
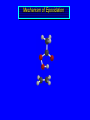
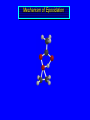
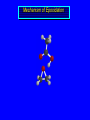
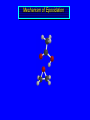
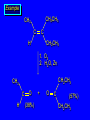
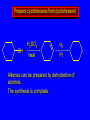
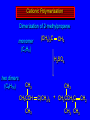
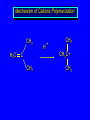
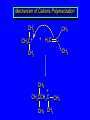
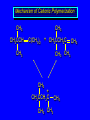
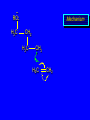
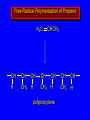
Reactions of Alkenes: Addition Reactions Reactions of Alkenes The characteristic reaction of alkenes is addition to the double bond. C C + A—B A C C B Hydrogenation of Alkenes Hydrogenation of ethylene H C H + C H H H—H H H H C C H H exothermic H° = –136 kJ/mol catalyzed by finely divided Pt, Pd, Rh, Ni H Example H3C CH2 H3C H2, Pt CH3 H3C H H3C (73%) Problem 6.1 What three alkenes yield 2-methylbutane on catalytic hydrogenation? Problem 6.1 What three alkenes yield 2-methylbutane on catalytic hydrogenation? H2, Pt Mechanism of catalytic hydrogenation. Figure 6.1 B H H A H H Y C C X Mechanism of catalytic hydrogenation. Figure 6.1 B A H Y C C X H H H Mechanism of catalytic hydrogenation. Figure 6.1 B H Y A H C X C H H Mechanism of catalytic hydrogenation. Figure 6.1 B H Y A H C X C H H Mechanism of catalytic hydrogenation. Figure 6.1 B H Y A C H X C H H Mechanism of catalytic hydrogenation. Figure 6.1 B Y A C H H X C H H Heats of Hydrogenation can be used to measure relative stability of isomeric alkenes correlation with structure is same as when heats of combustion are measured 126 119 CH3CH2CH2CH3 115 Heats of Hydrogenation (kJ/mol) Ethylene 136 Monosubstituted 125-126 cis-Disubstituted 117-119 trans-Disubstituted 114-115 Terminally disubstituted 116-117 Trisubstituted 112 Tetrasubstituted 110 Problem 6.2 Match each alkene of Problem 6.1 with its correct heat of hydrogenation. 126 kJ/mol 118 kJ/mol 112 kJ/mol Problem 6.2 Match each alkene of Problem 6.1 with its correct heat of hydrogenation. 126 kJ/mol highest heat of hydrogenation; least stable isomer 118 kJ/mol 112 kJ/mol lowest heat of hydrogenation; most stable isomer Stereochemistry of Alkene Hydrogenation Two spatial (stereochemical) aspects of alkene hydrogenation: • syn addition of both H atoms to double bond • hydrogenation is stereoselective, corresponding to addition to less crowded face of double bond syn-Addition versus anti-Addition syn addition anti addition Example of syn-Addition CO2CH3 CO2CH3 H CO2CH3 H2, Pt CO2CH3 H (100%) Stereoselectivity A reaction in which a single starting material can give two or more stereoisomeric products but yields one of them in greater amounts than the other (or even to the exclusion of the other) is said to be stereoselective. H3C CH3 H H3C H3C H H H3C H Example of stereoselective reaction H2, cat CH3 Both products correspond to syn addition of H2. H3C H H H CH3 CH3 H3C CH3 H H3C H3C H H H3C Example of stereoselective reaction H2, cat CH3 But only this one is formed. H H3C CH3 H H3C Example of stereoselective reaction H2, cat Top face of double bond blocked by this methyl group H3C H H H3C H CH3 H3C CH3 H H3C H3C H Example of stereoselective reaction H2, cat CH3 H H3C H H2 adds to bottom face of double bond. Electrophilic Addition of Hydrogen Halides to Alkenes General equation for electrophilic addition C C – + E—Y E C C Y When EY is a hydrogen halide C C – + H—X H C C X Example CH2CH3 CH3CH2 C H HBr C H CHCl3, -30°C CH3CH2CH2CHCH2CH3 Br (76%) Mechanism Electrophilic addition of hydrogen halides to alkenes proceeds by rate-determining formation of a carbocation intermediate. Electrons flow from the system of the alkene (electron rich) toward the positively polarized proton of the hydrogen halide. Mechanism +C C H .. – : :X .. H C C .. X: .. Mechanism +C C H .. – : :X .. H C C .. X: .. .. :X .. C C H Regioselectivity of Hydrogen Halide Addition: Markovnikov's Rule Markovnikov's Rule When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogen substituents, and the halogen adds to the carbon that has the fewer hydrogen substituents. Markovnikov's Rule CH3CH2CH CH2 HBr acetic acid CH3CH2CHCH3 Br (80%) Example 1 Markovnikov's Rule CH3 C CH3 CH3 H HBr C H acetic acid CH3 C Br (90%) Example 2 CH3 Markovnikov's Rule CH3 HCl CH3 0°C Cl (100%) Example 3 Mechanistic Basis for Markovnikov's Rule Protonation of double bond occurs in direction that gives more stable of two possible carbocations. Mechanistic Basis for Markovnikov's Rule: Example 1 CH3CH2CH CH2 HBr CH3CH2CHCH3 acetic acid Br Mechanistic Basis for Markovnikov's Rule: Example 1 + CH3CH2CH—CH3 + Br – HBr CH3CH2CH CH2 CH3CH2CHCH3 Br Mechanistic Basis for Markovnikov's Rule: Example 1 + CH3CH2CH2—CH2 primary carbocation is less stable: not formed + CH3CH2CH—CH3 + Br – HBr CH3CH2CH CH2 CH3CH2CHCH3 Br Mechanistic Basis for Markovnikov's Rule: Example 3 H CH3 HCl CH3 0°C Cl Mechanistic Basis for Markovnikov's Rule: Example 3 H H + H CH3 Cl – HCl CH3 HCl CH3 0°C Cl Mechanistic Basis for Markovnikov's Rule: Example 3 secondary carbocation is less stable: not formed H + H H CH3 + H H CH3 Cl – HCl CH3 HCl CH3 0°C Cl Carbocation Rearrangements in Hydrogen Halide Addition to Alkenes Rearrangements sometimes occur H2C CHCH(CH3)2 HCl, 0°C H + CH3CHCH(CH3)2 + CH3CHC(CH3)2 CH3CHCH(CH3)2 CH3CH2C(CH3)2 Cl (40%) (60%) Cl Addition of Sulfuric Acid to Alkenes Addition of H2SO4 CH3CH CH2 HOSO2OH CH3CHCH3 OSO2OH Isopropyl hydrogen sulfate follows Markovnikov's rule: yields an alkyl hydrogen sulfate Mechanism CH3CH CH2 + H .. O .. SO2OH slow + CH3CH CH3 ..– + :O .. fast SO2OH CH3CHCH3 : OSO .. 2OH Alkyl hydrogen sulfates undergo hydrolysis in hot water CH3CHCH3 O + H—OH SO2OH heat CH3CHCH3 O H + HO—SO2OH Application: Coversion of alkenes to alcohols OH 1. H2SO4 2. H2O, heat (75%) But... not all alkenes yield alkyl hydrogen sulfates on reaction with sulfuric acid these do: H2C=CH2, RCH=CH2, and RCH=CHR' these don't: R2C=CH2, R2C=CHR, and R2C=CR2 (These form polymers, sec 6.21) Acid-Catalyzed Hydration of Alkenes Acid-Catalyzed Hydration of Alkenes C + C H—OH reaction is acid catalyzed; typical hydration medium is 50% H2SO4-50% H2O H C C OH Follows Markovnikov's Rule H3C C H3C CH3 H 50% H2SO4 C CH3 50% H2O CH3 C CH2CH3 OH (90%) Follows Markovnikov's Rule CH2 50% H2SO4 CH3 50% H2O OH (80%) Mechanism involves a carbocation intermediate is the reverse of acid-catalyzed dehydration of alcohols to alkenes CH3 H3C H+ C H3C CH2 + H2O CH3 C OH CH3 Mechanism Step (1) Protonation of double bond H H3C C CH2 + + O: H H3C H slow H3C H3C H + C CH3 + : O: H Mechanism Step (2) Capture of carbocation by water H3C H + C : O: CH3 + H3C fast CH3 CH3 C CH3 H + O: H H Mechanism Step (3) Deprotonation of oxonium ion CH3 CH3 C H : +O CH3 H + : O: H H fast CH3 CH3 C CH3 .. O: H H + H + O: H Relative Rates Acid-catalyzed hydration ethylene CH2=CH2 1.0 propene CH3CH=CH2 1.6 x 106 2-methylpropene (CH3)2C=CH2 2.5 x 1011 The more stable the carbocation, the faster it is formed, and the faster the reaction rate. Principle of microscopic reversibility CH3 H3C H+ C H3C CH2 + H2O CH3 C CH3 OH In an equilibrium process, the same intermediates and transition states are encountered in the forward direction and the reverse, but in the opposite order. Thermodynamics of Addition-Elimination Equilibria Hydration-Dehydration Equilibrium H C H H+ H + H2O C H C C H How do we control the position of the equilibrium and maximize the product? OH Le Chatelier’s Principle A system at equilibrium adjusts so to minimize any stress applies to it. For the hydration-dehydration equilibria, the key stress is water. Adding water pushes the equilibrium toward more product (alcohol). Removing water pushes the equilibrium toward more reactant (alkene). Hydroboration-Oxidation of Alkenes Synthesis Suppose you wanted to prepare 1-decanol from 1-decene? OH Synthesis Suppose you wanted to prepare 1-decanol from 1-decene? Needed: a method for hydration of alkenes with a regioselectivity opposite to Markovnikov's rule. OH Synthesis Two-step reaction sequence called hydroborationoxidation converts alkenes to alcohols with a regiochemistry opposite to Markovnikov's rule. 1. hydroboration 2. oxidation OH Hydroboration step C C + H—BH2 H C C BH2 Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. Hydroboration step C C + H—BH2 H C C BH2 Hydroboration reagents: H BH2 H2B H Diborane (B2H6) normally used in an ether- like solvent called "diglyme" Hydroboration step C C + H—BH2 H C C Hydroboration reagents: Borane-tetrahydrofuran complex (H3B-THF) .. +O – BH 3 BH2 Oxidation step H2O2, HO– H C C BH2 H C C Organoborane formed in the hydroboration step is oxidized with hydrogen peroxide. OH Example 1. B2H6, diglyme 2. H2O2, HO– OH (93%) Example H3C CH3 C H3C C H 1. H3B-THF 2. H2O2, HO– CH3 H OH C C CH3 H (98%) CH3 Example OH 1. B2H6, diglyme 2. H2O2, HO– (no rearrangement) (82%) Stereochemistry of Hydroboration-Oxidation Features of HydroborationOxidation • hydration of alkenes • regioselectivity opposite to Markovnikov's rule • no rearrangement • stereospecific syn addition syn-Addition •H and OH become attached to same face of double bond H CH3 1. B2H6 2. H2O2, NaOH H CH3 HO H only product is trans-2-methylcyclopentanol (86%) yield Mechanism of Hydroboration-Oxidation Hydroboration-oxidation electrons from alkene flow into 2P orbital of boron Hydroboration-oxidation H atom with 2 electrons migrates to C with greatest (+) charge (most highly substituted). Syn add. Hydroboration-oxidation Anion of hydrogen peroxide attacks boron Hydroboration-oxidation Carbon with pair of electrons migrates to oxygen, displacing hydroxide. Oxygen on same side as boron Hydroboration-oxidation Hydrolysis cleaves boron-oxygen bond resulting in anti-Markovnikov addition 1-Methylcyclopentene + BH3 •syn addition of H and B to double bond •B adds to less substituted carbon Organoborane intermediate Add hydrogen peroxide •OH replaces B on same side trans-2Methylcyclopentanol Addition of Halogens to Alkenes C C + X2 X C C X electrophilic addition to double bond forms a vicinal dihalide Example CH3CH CHCH(CH3)2 Br2 CHCl3 0°C CH3CHCHCH(CH3)2 Br Br (100%) Scope Limited to Cl2 and Br2 F2 addition proceeds with explosive violence I2 addition is endothermic: vicinal diiodides dissociate to an alkene and I2 Stereochemistry of Halogen Addition Anti-addition Example H H H Br2 Br Br H trans-1,2-Dibromocyclopentane 80% yield; only product Example H H Cl Cl2 H H Cl trans-1,2-Dichlorocyclooctane 73% yield; only product Mechanism of Halogen Addition to Alkenes: Halonium Ions Mechanism is electrophilic addition Br2 is not polar, but it is polarizable two steps involved (1) formation of bromonium ion (2) nucleophilic attack on bromonium ion by bromide Relative Rates Bromination ethylene H2C=CH2 1 propene CH3CH=CH2 2-methylpropene (CH3)2C=CH2 2,3-dimethyl-2-butene (CH3)2C=C(CH3)2 920,000 61 5400 More highly substituted double bonds react faster. Alkyl groups on the double bond make it more “electron rich.” Mechanism? H2C CH2 + Br2 BrCH2CH2Br ? C : Br : .. + C .. – + : Br : .. No obvious explanation for anti addition provided by this mechanism. Mechanism H2C CH2 + Br2 C C : Br : + BrCH2CH2Br .. – + : Br : .. Cyclic bromonium ion Formation of bromonium ion Br Mutual polarization of electron distributions of Br2 and alkene Br Formation of bromonium ion Electrons flow from alkene toward Br2 Br – Br + + Formation of bromonium ion – Br electrons of alkene displace Br– from Br + Br Stereochemistry .. – : Br : .. .. Br + .. .. : Br .. attack of Br– from side opposite C—Br bond of bromonium ion gives anti addition .. Br : .. Example H H H Br2 Br Br H trans-1,2-Dibromocyclopentane 80% yield; only product Cyclopenten e +Br2 Bromonium ion – – – Bromide ion attacks the bromonium ion from side opposite carbon-bromine bond trans-Stereochemistry in vicinal dibromide Conversion of Alkenes to Vicinal Halohydrins C C + X2 X C C X alkenes react with X2 to form vicinal dihalides C C + X2 X C C X alkenes react with X2 to form vicinal dihalides alkenes react with X2 in water to give vicinal halohydrins C C + X2 + H2O X C C OH + H—X Examples H2O H2C CH2 + Br2 BrCH2CH2OH (70%) H H Cl2 OH H2O H Cl H anti addition: only product Mechanism O: .. .. Br + .. + • bromonium ion is intermediate • water is nucleophile that attacks bromonium ion O .. .. Br : .. Examples H2O H2C CH2 + Br2 BrCH2CH2OH (70%) H H Cl2 OH H2O H Cl H anti addition: only product Cyclopentene + Cl2 Chloronium ion Water attacks chloronium ion from side opposite carbon-chlorine bond transStereochemistry in oxonium ion trans-2-Chlorocyclopentanol Regioselectivity CH3 H3C C H3C CH2 Br2 H2O CH3 C CH2Br OH (77%) Markovnikov's rule applied to halohydrin formation: the halogen adds to the carbon having the greater number of hydrogens. Explanation H H H .. O .. O H3C H3C C CH2 : Br : H3C H3C H CH2 C : Br : transition state for attack of water on bromonium ion has carbocation character; more stable transition state (left) has positive charge on more highly substituted carbon Free-radical Addition of HBr to Alkenes The "peroxide effect" Markovnikov's Rule CH3CH2CH CH2 HBr acetic acid CH3CH2CHCH3 Br (80%) Example 1 Addition of HBr to 1-Butene CH3CH2CH CH2 HBr CH3CH2CHCH3 Br only product in absence of peroxides CH3CH2CH2CH2Br only product when peroxides added to reaction mixture Addition of HBr to 1-Butene CH3CH2CH CH2 HBr addition opposite to Markovnikov's rule occurs with HBr (not HCl or HI) CH3CH2CH2CH2Br only product when peroxides added to reaction mixture CH2 + HBr h CH2Br H (60%) Addition of HBr with a regiochemistry opposite to Markovnikov's rule can also occur when initiated with light with or without added peroxides. Mechanism Addition of HBr opposite to Markovnikov's rule proceeds by a free-radical chain mechanism. Initiation steps: R .. O .. .. O .. R R .. .. . + .O O .. .. R Mechanism Addition of HBr opposite to Markovnikov's rule proceeds by a free-radical chain mechanism. Initiation steps: R R .. O .. .. O .. .. . + H O .. R .. : Br .. R R .. .. . + .O O .. .. R .. .. . + : H O Br .. .. Propagation steps: .. CH2 + . Br : .. CH3CH2CH . CH3CH2CH CH2 .. : Br .. Gives most stable free radical CH3CH2CH . H CH2 .. : Br .. .. Br .. : .. : CH3CH2CH2CH2 Br .. + .. . Br : .. Epoxidation of Alkenes Epoxides • are examples of heterocyclic compounds • three-membered rings that contain oxygen ethylene oxide CH2 H2C O propylene oxide CHCH3 H2C O Epoxide Nomenclature Substitutive nomenclature: •named as epoxy-substituted alkanes. •“epoxy” precedes name of alkane 1,2-epoxypropane 2-methyl-2,3-epoxybutane H3C 1 2 O H3C 4 CHCH3 C CHCH3 H2C 3 O Problem 6.17 Give the IUPAC name, including stereochemistry, for disparlure. H O H cis-2-Methyl-7,8-epoxyoctadecane Epoxidation of Alkenes O C C + RCOOH peroxy acid O C C O + RCOH Example O + CH3COOH O + CH3COH O (52%) Epoxidation of Alkenes O C C + RCOOH syn addition O C C O + RCOH Relative Rates Epoxidation ethylene H2C=CH2 1 propene CH3CH=CH2 22 2-methylpropene (CH3)2C=CH2 484 2-methyl-2-butene (CH3)2C=CHCH3 6526 More highly substituted double bonds react faster. Alkyl groups on the double bond make it more “electron rich.” Mechanism of Epoxidation Mechanism of Epoxidation Mechanism of Epoxidation Mechanism of Epoxidation Mechanism of Epoxidation Ozonolysis of Alkenes Ozonolyis has both synthetic and analytical applications. • synthesis of aldehydes and ketones • identification of substituents on the double bond of an alkene Ozonolysis of Alkenes First step is the reaction of the alkene with ozone. The product is an ozonide. C C + O3 O C O C O Ozonolysis of Alkenes Second step is hydrolysis of the ozonide. Two aldehydes, two ketones, or an aldehyde and a ketone are formed. C C O + O3 C O C O H2O, Zn C O + O C Ozonolysis of Alkenes As an alternative to hydrolysis, the ozonide can be treated with dimethyl sulfide. C C O + O3 C O C O (CH3)2S C O + O C Example CH2CH3 CH3 C C H CH2CH3 1. O3 2. H2O, Zn CH2CH3 CH3 C H O (38%) + O C (57%) CH2CH3 Introduction to Organic Chemical Synthesis Prepare cyclohexane from cyclohexanol OH devise a synthetic plan reason backward from the target molecule always use reactions that you are sure will work Prepare cyclohexane from cyclohexanol OH ask yourself the key question "Starting with anything, how can I make cyclohexane in a single step by a reaction I am sure will work?" Prepare cyclohexane from cyclohexanol OH H2 Pt The only reaction covered so far for preparing alkanes is catalytic hydrogenation of alkenes. This leads to a new question. "Starting with anything, how can I prepare cyclohexene in a single step by a reaction I am sure will work?" Prepare cyclohexane from cyclohexanol OH H2SO4 H2 heat Pt Alkenes can be prepared by dehydration of alcohols. The synthesis is complete. Prepare 1-bromo-2-methyl-2-propanol from tert-butyl alcohol (CH3)3COH (CH3)2CCH2Br OH "Starting with anything, how can I make the desired compound in a single step by a reaction I am sure will work?" The desired compound is a vicinal bromohydrin. How are vicinal bromohydrins prepared? Prepare 1-bromo-2-methyl-2-propanol from tert-butyl alcohol (CH3)2C CH2 Br2 H2O (CH3)2CCH2Br OH Vicinal bromohydrins are prepared by treatment of alkenes with Br2 in water. How is the necessary alkene prepared? Prepare 1-bromo-2-methyl-2-propanol from tert-butyl alcohol (CH3)3COH H2SO4 heat (CH3)2C CH2 Br2 H2O (CH3)2CCH2Br OH 2-Methylpropene is prepared from tert-butyl alcohol by acid-catalyzed dehydration. The synthesis is complete. Reactions of Alkenes with Alkenes: Polymerization Polymerization of alkenes cationic polymerization free-radical polymerization coordination polymerization Cationic Polymerization Dimerization of 2-methylpropene monomer (C4H8) (CH3)2C CH2 H2SO4 two dimers (C8H16) CH3 CH3CCH CH3 CH3 C(CH3)2 + CH3CCH2C CH3 CH3 CH2 Mechanism of Cationic Polymerization CH3 H2C H+ CH3 CH3C + C CH3 CH3 Mechanism of Cationic Polymerization CH3 CH3C + CH3 + H2C C CH3 CH3 CH3 + CH3CCH2C CH3 CH3 CH3 Mechanism of Cationic Polymerization CH3 CH3CCH CH3 C(CH3)2 + CH3CCH2C CH3 CH3 CH3 CH3 + CH3CCH2C CH3 CH3 CH3 CH2 Free-Radical Polymerization of Ethylene H2C CH2 200 °C 2000 atm CH2 CH2 CH2 CH2 O2 peroxides CH2 polyethylene CH2 CH2 .. • RO .. H2C Mechanism CH2 .. RO: H2C Mechanism CH2 • .. RO: H2C Mechanism CH2 • H2C CH2 .. RO: H2C Mechanism CH2 H2C CH2 • .. RO: H2C Mechanism CH2 H2C CH2 • H2C CH2 .. RO: H2C Mechanism CH2 H2C CH2 H2C CH2 • .. RO: H2C Mechanism CH2 H2C CH2 H2C CH2 • H2C CH2 Free-Radical Polymerization of Propene H2C CHCH3 CH CH CH CH CH CH CH H CH3 H CH3 H CH3 H polypropylene .. • RO .. H2C Mechanism CHCH3 .. RO: H2C Mechanism CHCH3 • .. RO: H2C Mechanism CHCH3 • H2C CHCH3 .. RO: H2C Mechanism CHCH3 H2C CHCH3 • .. RO: H2C Mechanism CHCH3 H2C CHCH3 • H2C CHCH3 .. RO: H2C Mechanism CHCH3 H2C CHCH3 H2C CHCH3 • .. RO: H2C Mechanism CHCH3 H2C CHCH3 H2C CHCH3 • H2C CHCH3 H2C=CHCl polyvinyl chloride H2C=CHC6H5 polystyrene F2C=CF2 Teflon