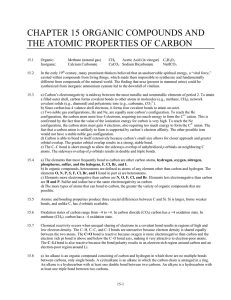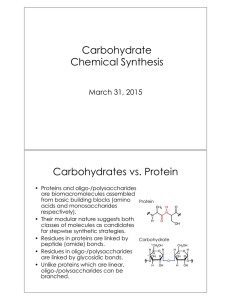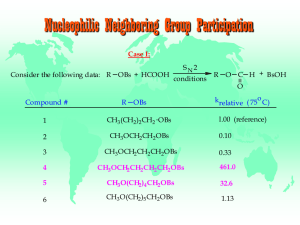
Nucleophilic Neighboring Group Participation Case I
... cyclic oxonium ion In addition to the available S N 2 pathway, a more favorable alternate pathway for displacement of brosylate is possible for compounds 4 & 5. The internal displacement pathway leads to the formation of relatively stable 5- and 6membered cyclic oxonium salts. As the chain length in ...
... cyclic oxonium ion In addition to the available S N 2 pathway, a more favorable alternate pathway for displacement of brosylate is possible for compounds 4 & 5. The internal displacement pathway leads to the formation of relatively stable 5- and 6membered cyclic oxonium salts. As the chain length in ...
ORGANIC CHEMISTRY 4th ed Solution Manual
... of one class of compounds using reactions of other classes of compounds studied earlier in the year. How to keep track of everything? It might be possible to memorize every bit of information presented to you, but you would still lack a fundamental understanding of the subject. It is far better to g ...
... of one class of compounds using reactions of other classes of compounds studied earlier in the year. How to keep track of everything? It might be possible to memorize every bit of information presented to you, but you would still lack a fundamental understanding of the subject. It is far better to g ...
Study Guide and Solutions Manual
... of one class of compounds using reactions of other classes of compounds studied earlier in the year. How to keep track of everything? It might be possible to memorize every bit of information presented to you, but you would still lack a fundamental understanding of the subject. It is far better to g ...
... of one class of compounds using reactions of other classes of compounds studied earlier in the year. How to keep track of everything? It might be possible to memorize every bit of information presented to you, but you would still lack a fundamental understanding of the subject. It is far better to g ...
Chapter15 odd probs
... For an alkene, assuming only one double bond, the general formula is CnH2n. When a double bond is formed in an alkane, two hydrogen atoms are removed. For an alkyne, assuming only one triple bond, the general formula is CnH2n–2. Forming a triple bond from a double bond causes the loss of two hydroge ...
... For an alkene, assuming only one double bond, the general formula is CnH2n. When a double bond is formed in an alkane, two hydrogen atoms are removed. For an alkyne, assuming only one triple bond, the general formula is CnH2n–2. Forming a triple bond from a double bond causes the loss of two hydroge ...
Organic Chemistry - Zanichelli online per la scuola
... Atoms with similar electronegativities form covalent bonds by sharing electrons. A single bond is the sharing of one electron pair between two atoms. A covalent bond has specific bond length and bond energy. Carbon, with four valence electrons, mainly forms covalent bonds. It usually forms four such ...
... Atoms with similar electronegativities form covalent bonds by sharing electrons. A single bond is the sharing of one electron pair between two atoms. A covalent bond has specific bond length and bond energy. Carbon, with four valence electrons, mainly forms covalent bonds. It usually forms four such ...
irm_ch14
... Carbon has 4 valence electrons and so forms 4 covalent bonds. A halogen atom has 7 valence electrons and so forms 1 covalent bond. ...
... Carbon has 4 valence electrons and so forms 4 covalent bonds. A halogen atom has 7 valence electrons and so forms 1 covalent bond. ...
technical bulletin reaction solvent dimethyl sulfoxide (dmso)
... cause slow decomposition of DMSO. If this occurs, it can be readily detected by the odor of trace amounts of methyl mercaptan and bis(methylthio)methane. The rate of decomposition is a timetemperature function that can be accelerated by the addition of acids and retarded by some bases. DMSO is a ver ...
... cause slow decomposition of DMSO. If this occurs, it can be readily detected by the odor of trace amounts of methyl mercaptan and bis(methylthio)methane. The rate of decomposition is a timetemperature function that can be accelerated by the addition of acids and retarded by some bases. DMSO is a ver ...
Chapter 16 Aldehydes and Ketones
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
irm_ch15
... 15.29 Common names for ketones are similar to the common names used for ethers with the carbonyl group taking the place of the oxygen atom. The alkyl groups are named, and the word ketone is added as a separate word: alkyl alkyl ketone or dialkyl ketone. Three ketones have additional common names; a ...
... 15.29 Common names for ketones are similar to the common names used for ethers with the carbonyl group taking the place of the oxygen atom. The alkyl groups are named, and the word ketone is added as a separate word: alkyl alkyl ketone or dialkyl ketone. Three ketones have additional common names; a ...
organic chemistry - carey - problems solutions
... A neutral nitrogen atom has 5 valence electrons: therefore, each atom is electrically neutral in molecular nitrogen. Nitrogen, as before, is electrically neutral. A neutral carbon has 4 valence electrons, and so carbon in this species, with an electron count of 5, has a unit negative charge. The spe ...
... A neutral nitrogen atom has 5 valence electrons: therefore, each atom is electrically neutral in molecular nitrogen. Nitrogen, as before, is electrically neutral. A neutral carbon has 4 valence electrons, and so carbon in this species, with an electron count of 5, has a unit negative charge. The spe ...
Chapter 12 Organic Compounds with Oxygen and Sulfur Alcohols
... Oxidation of Primary (1°) Alcohols When a primary alcohol is oxidized, [O], • one H is removed from the –OH. • another H is removed from the carbon bonded to the OH. • an aldehyde is produced. [O] Primary alcohol ...
... Oxidation of Primary (1°) Alcohols When a primary alcohol is oxidized, [O], • one H is removed from the –OH. • another H is removed from the carbon bonded to the OH. • an aldehyde is produced. [O] Primary alcohol ...
oxazolines. their preparation, reactions, and
... the lower molecular weight amides can be distilled without instead of the more common procedure of using an azeocyclizing. tropic agent. This is claimed to reduce the reaction time to When amino alcohols having complete substitution on the less than 12 hr.4 Oxazoline esters or diesters are also form ...
... the lower molecular weight amides can be distilled without instead of the more common procedure of using an azeocyclizing. tropic agent. This is claimed to reduce the reaction time to When amino alcohols having complete substitution on the less than 12 hr.4 Oxazoline esters or diesters are also form ...
Active Learning in Chemical Education
... systems and which are thought to be involved in the depletion of ozone in the upper atmosphere. This class of organic compounds is known as the halogenated hydrocarbons. In addition to their use in refrigerants they are used as solvents, aerosol sprays, antiseptics, dry cleaning fluids, insecticides ...
... systems and which are thought to be involved in the depletion of ozone in the upper atmosphere. This class of organic compounds is known as the halogenated hydrocarbons. In addition to their use in refrigerants they are used as solvents, aerosol sprays, antiseptics, dry cleaning fluids, insecticides ...
Lecture 8
... • At lower reaction temperatures, primary and equatorial OH groups are usually benzoylated before axial ones. • Order of reactivity of the -OH groups on a pyranose ring will vary depending on the sugar. ...
... • At lower reaction temperatures, primary and equatorial OH groups are usually benzoylated before axial ones. • Order of reactivity of the -OH groups on a pyranose ring will vary depending on the sugar. ...
PowerPoint - Naming Hydrocarbons
... • A hydrocarbon is an organic molecule composed of carbon and hydrogen (duh). • There are 3 main classes of hydrocarbons – Alkanes: Since there are only single bonds throughout these molecules, they are referred to as saturated hydrocarbons. The general formula of an alkane is CnH2n + 2. – Alkenes: ...
... • A hydrocarbon is an organic molecule composed of carbon and hydrogen (duh). • There are 3 main classes of hydrocarbons – Alkanes: Since there are only single bonds throughout these molecules, they are referred to as saturated hydrocarbons. The general formula of an alkane is CnH2n + 2. – Alkenes: ...
irm_ch17
... possible between amine molecules but not between alkane molecules. 17.28 Hydrogen bonds involving oxygen (the alcohol) are stronger than hydrogen bonds involving nitrogen (the amine). 17.29 a. CH3–CH2–NH2 is more soluble in water because it has a shorter carbon chain (less nonpolar character than th ...
... possible between amine molecules but not between alkane molecules. 17.28 Hydrogen bonds involving oxygen (the alcohol) are stronger than hydrogen bonds involving nitrogen (the amine). 17.29 a. CH3–CH2–NH2 is more soluble in water because it has a shorter carbon chain (less nonpolar character than th ...
Chapter 14
... yes, alcohols with 1-4 carbon atoms hydrogen bond with water yes; the water can hydrogen bond to the O in ether no; a carbon chain longer than 4 carbon atoms diminishes the effect of the OH group. no; alkanes are nonpolar and do not hydrogen bond yes; the OH in phenol ionizes in water, which makes ...
... yes, alcohols with 1-4 carbon atoms hydrogen bond with water yes; the water can hydrogen bond to the O in ether no; a carbon chain longer than 4 carbon atoms diminishes the effect of the OH group. no; alkanes are nonpolar and do not hydrogen bond yes; the OH in phenol ionizes in water, which makes ...
infrared spectroscopy
... pressed between plates made of sodium chloride Sodium chloride is used because it has no IR absorptions; glass or plastic plates would have IR absorptions of their own Sodium chloride plates are good from 4000 to 650 cm-1; below 650 cm-1 they begin to absorb Potassium bromide plates can be used in p ...
... pressed between plates made of sodium chloride Sodium chloride is used because it has no IR absorptions; glass or plastic plates would have IR absorptions of their own Sodium chloride plates are good from 4000 to 650 cm-1; below 650 cm-1 they begin to absorb Potassium bromide plates can be used in p ...
Alcohols, Phenols, Thiols, & Ethers
... 1-propanol would have the higher boiling point because an alcohol can form hydrogen bonds, but the ether cannot. ...
... 1-propanol would have the higher boiling point because an alcohol can form hydrogen bonds, but the ether cannot. ...
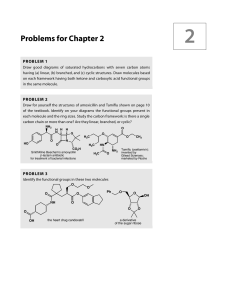
Problems for Chapter 2
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
Lorell Thesis Final Version in PDF S
... finished. To my husband, Tito, who has given me the happiest days of my life and stood beside me in all the happy and stressful moments. To my daughter Lorelys Maia, she is my whole life and is always inspiring me how to be a better person overall. To my parents José and Betzaida, who always support ...
... finished. To my husband, Tito, who has given me the happiest days of my life and stood beside me in all the happy and stressful moments. To my daughter Lorelys Maia, she is my whole life and is always inspiring me how to be a better person overall. To my parents José and Betzaida, who always support ...
CH 2
... 1-propanol would have the higher boiling point because an alcohol can form hydrogen bonds, but the ether cannot. ...
... 1-propanol would have the higher boiling point because an alcohol can form hydrogen bonds, but the ether cannot. ...
barker_rg
... molecule on the carbonyl carbon, as shown in Reaction (9), followed by the formation of a new covalent bond is affected by the presence of substituents. ...
... molecule on the carbonyl carbon, as shown in Reaction (9), followed by the formation of a new covalent bond is affected by the presence of substituents. ...
CH 3 - bYTEBoss
... That are primary (−NH2) or secondary (−NH−) form hydrogen bonds. That are primary have higher melting points than secondary. That are tertiary (no H on N) do not form hydrogen bonds and have lower melting points. All form hydrogen bonds with water. With 1-5 carbon atoms are soluble in wate ...
... That are primary (−NH2) or secondary (−NH−) form hydrogen bonds. That are primary have higher melting points than secondary. That are tertiary (no H on N) do not form hydrogen bonds and have lower melting points. All form hydrogen bonds with water. With 1-5 carbon atoms are soluble in wate ...
OC 2/e 9 Alcohols
... interact with themselves and with other polar compounds by dipole-dipole interactions Dipole-dipole interaction: the attraction between the positive end of one dipole and the negative ...
... interact with themselves and with other polar compounds by dipole-dipole interactions Dipole-dipole interaction: the attraction between the positive end of one dipole and the negative ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.