ORGANIC CHEMISTRY 4th ed Solution Manual
... The reverse of the card would show the reagents necessary for this reaction: H2, Pt or Pd catalyst The card can actually be studied in two ways. You may ask yourself: What reagents will convert alkenes into alkanes? Or, using the back of the card: What chemical reaction is carried out with hydrogen ...
... The reverse of the card would show the reagents necessary for this reaction: H2, Pt or Pd catalyst The card can actually be studied in two ways. You may ask yourself: What reagents will convert alkenes into alkanes? Or, using the back of the card: What chemical reaction is carried out with hydrogen ...
Study Guide and Solutions Manual
... The reverse of the card would show the reagents necessary for this reaction: H2, Pt or Pd catalyst The card can actually be studied in two ways. You may ask yourself: What reagents will convert alkenes into alkanes? Or, using the back of the card: What chemical reaction is carried out with hydrogen ...
... The reverse of the card would show the reagents necessary for this reaction: H2, Pt or Pd catalyst The card can actually be studied in two ways. You may ask yourself: What reagents will convert alkenes into alkanes? Or, using the back of the card: What chemical reaction is carried out with hydrogen ...
technical bulletin reaction solvent dimethyl sulfoxide (dmso)
... DMSO is a versatile and powerful solvent that will dissolve most aromatic and unsaturated hydrocarbons, organic nitrogen compounds, organo-sulfur compounds and many inorganic salts. It is miscible with most of the common organic solvents such as alcohols, esters, ketones, lower ethers, chlorinated s ...
... DMSO is a versatile and powerful solvent that will dissolve most aromatic and unsaturated hydrocarbons, organic nitrogen compounds, organo-sulfur compounds and many inorganic salts. It is miscible with most of the common organic solvents such as alcohols, esters, ketones, lower ethers, chlorinated s ...
irm_ch17
... forms one hydrogen bond to a water molecule. The nitrogen atom’s non-bonding electron pair forms the third hydrogen bond with a water molecule, shown in Figure 17.6.) 17.26 a. 2 ...
... forms one hydrogen bond to a water molecule. The nitrogen atom’s non-bonding electron pair forms the third hydrogen bond with a water molecule, shown in Figure 17.6.) 17.26 a. 2 ...
Introductory Chemistry
... Energy is also an important topic in chemistry, so now that atoms and molecules, chemical reactions, and stoichiometry have been introduced, I include energy as a quantitative property. With this, the basic topics of chemistry are introduced; the remaining chapters discuss either more applied topic ...
... Energy is also an important topic in chemistry, so now that atoms and molecules, chemical reactions, and stoichiometry have been introduced, I include energy as a quantitative property. With this, the basic topics of chemistry are introduced; the remaining chapters discuss either more applied topic ...
irm_ch14
... Glycerol is a thick liquid that has the consistency of honey. Ethanol is often produced by a fermentation process. Methanol is used as a race car fuel. Methanol can be industrially produced from CO and H2. ...
... Glycerol is a thick liquid that has the consistency of honey. Ethanol is often produced by a fermentation process. Methanol is used as a race car fuel. Methanol can be industrially produced from CO and H2. ...
Organic Chemistry - Zanichelli online per la scuola
... do this—you should have no difficulty with examinations. Furthermore, you should be very well prepared for further courses that require this course as a prerequisite. Near the end of this study guide you will find additional sections that may help you to study for the final examination in the course ...
... do this—you should have no difficulty with examinations. Furthermore, you should be very well prepared for further courses that require this course as a prerequisite. Near the end of this study guide you will find additional sections that may help you to study for the final examination in the course ...
Chapter 4 MATERIAL BALANCES AND APPLICATIONS
... i. Minimize the symbols assigned to unknown quantities by utilizing all the given process specifications and using laws of physics. ii. After doing calculations on certain basis, you may scale up or scale down (convert to new basis) while keeping the process balanced. This is done by multiplying all ...
... i. Minimize the symbols assigned to unknown quantities by utilizing all the given process specifications and using laws of physics. ii. After doing calculations on certain basis, you may scale up or scale down (convert to new basis) while keeping the process balanced. This is done by multiplying all ...
organic chemistry - carey - problems solutions
... A neutral nitrogen atom has 5 valence electrons: therefore, each atom is electrically neutral in molecular nitrogen. Nitrogen, as before, is electrically neutral. A neutral carbon has 4 valence electrons, and so carbon in this species, with an electron count of 5, has a unit negative charge. The spe ...
... A neutral nitrogen atom has 5 valence electrons: therefore, each atom is electrically neutral in molecular nitrogen. Nitrogen, as before, is electrically neutral. A neutral carbon has 4 valence electrons, and so carbon in this species, with an electron count of 5, has a unit negative charge. The spe ...
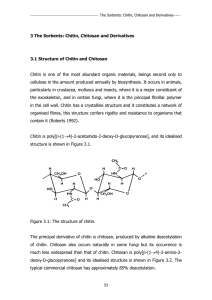
3 The Sorbents: Chitin, Chitosan and Derivatives
... metal ions to prepare chitosan-based adsorption gels that are insoluble in aqueous solutions without losing their high adsorption capacity. This concept is similar to that proposed by Nishide et al. (1976), who prepared crosslinked poly(4-vinylpyridine) resins with high selectivity to the metal ion ...
... metal ions to prepare chitosan-based adsorption gels that are insoluble in aqueous solutions without losing their high adsorption capacity. This concept is similar to that proposed by Nishide et al. (1976), who prepared crosslinked poly(4-vinylpyridine) resins with high selectivity to the metal ion ...
Amphiphilic Sugar Metal Carbenes
... are a very broad field of compounds involving also living organism; as an example hemoglobin and myoglobin are porphyrins bonded to an iron atom. Organometallic compounds are also involved in the production of fine chemicals and pharmaceuticals. One known organometallic compound is the ruthenium-bin ...
... are a very broad field of compounds involving also living organism; as an example hemoglobin and myoglobin are porphyrins bonded to an iron atom. Organometallic compounds are also involved in the production of fine chemicals and pharmaceuticals. One known organometallic compound is the ruthenium-bin ...
Chapter15 odd probs
... a filled outer shell, carbon forms covalent bonds to other atoms in molecules (e.g., methane, CH4), network covalent solids (e.g., diamond) and polyatomic ions (e.g., carbonate, CO32–). b) Since carbon has 4 valence shell electrons, it forms four covalent bonds to attain an octet. c) Two noble gas c ...
... a filled outer shell, carbon forms covalent bonds to other atoms in molecules (e.g., methane, CH4), network covalent solids (e.g., diamond) and polyatomic ions (e.g., carbonate, CO32–). b) Since carbon has 4 valence shell electrons, it forms four covalent bonds to attain an octet. c) Two noble gas c ...
1 Structure, Properties, and Preparation of Boronic Acid - Wiley-VCH
... from the boronate substituent [40]. Another evidence for the rather weak electronwithdrawing character of boronic esters comes from their modest stabilizing effect on boronyl-substituted carbanions, where their effect has been compared to that of a phenyl group (see Section 1.3.8.3). 1.2.2.2 Physica ...
... from the boronate substituent [40]. Another evidence for the rather weak electronwithdrawing character of boronic esters comes from their modest stabilizing effect on boronyl-substituted carbanions, where their effect has been compared to that of a phenyl group (see Section 1.3.8.3). 1.2.2.2 Physica ...
D. Bagchi, S. Chaudhuri, S. Sardar, S. Choudhury, N. Polley, P
... In this study analytical grade chemicals without further purication were used for synthesis. Curcumin, Zn(OAc)2$2H2O, Cu(OAc)2$H2O, CuCl2$2H2O were purchased from SigmaAldrich. As a suitable solvent for synthesis of metallo– curcumin complexes methanol was used (Merck). Millipore water was used as ...
... In this study analytical grade chemicals without further purication were used for synthesis. Curcumin, Zn(OAc)2$2H2O, Cu(OAc)2$H2O, CuCl2$2H2O were purchased from SigmaAldrich. As a suitable solvent for synthesis of metallo– curcumin complexes methanol was used (Merck). Millipore water was used as ...
oxazolines. their preparation, reactions, and
... the lower molecular weight amides can be distilled without instead of the more common procedure of using an azeocyclizing. tropic agent. This is claimed to reduce the reaction time to When amino alcohols having complete substitution on the less than 12 hr.4 Oxazoline esters or diesters are also form ...
... the lower molecular weight amides can be distilled without instead of the more common procedure of using an azeocyclizing. tropic agent. This is claimed to reduce the reaction time to When amino alcohols having complete substitution on the less than 12 hr.4 Oxazoline esters or diesters are also form ...
A study of complexes Mg(NH3)n and Ag(NH3)n , where n 1–8
... tetrahedral complexes containing ligands such as acetonitrile [12] and pyridines [13–15] are known in the solid state. In a wide variety of neat solvents (water, pyridine, acetonitrile, trimethylphosphate, N,N-dimethylformamide, 1,1,3,3-tetramethylurea, dimethylsulfoxide, n-alkylamines and ethylened ...
... tetrahedral complexes containing ligands such as acetonitrile [12] and pyridines [13–15] are known in the solid state. In a wide variety of neat solvents (water, pyridine, acetonitrile, trimethylphosphate, N,N-dimethylformamide, 1,1,3,3-tetramethylurea, dimethylsulfoxide, n-alkylamines and ethylened ...
PowerPoint ******
... (3) Methyl groups at axial positions of six-membered rings are almost ideally positioned to migrate because the bonds between the methyl groups and the rings are nearly coplanar with the empty orbitals of the cations. Equatorial substituents are unlikely to migrate. (4) Steric factors may also signi ...
... (3) Methyl groups at axial positions of six-membered rings are almost ideally positioned to migrate because the bonds between the methyl groups and the rings are nearly coplanar with the empty orbitals of the cations. Equatorial substituents are unlikely to migrate. (4) Steric factors may also signi ...
Answers to SelectedTextbook Questions
... arsenic varies dramatically depending on the species containing the arsenic atoms. For example, whereas elemental arsenic is toxic, the arsenic containing species in lobster are not. Both Vitamin B12 and Visudyne are porphyrin‐based. A natural product is a compound produced by a living organis ...
... arsenic varies dramatically depending on the species containing the arsenic atoms. For example, whereas elemental arsenic is toxic, the arsenic containing species in lobster are not. Both Vitamin B12 and Visudyne are porphyrin‐based. A natural product is a compound produced by a living organis ...
handbook of inorganic chemistry
... Specific heat 0.215 cal/g.°C (0.900 J/g.°C); heat capacity 5.81 cal/mol.°C (24.3 J/mol.°C); ∆Hfus (2.54 kcal/mol (10.6 kJ/mol); ∆Hvap 67.9 kcal/mol (284 kJ/mol); E° in aqueous soln. (acidic) at 25°C for the reaction Al3+ + 3e– —› Al(s) , –1.66V; S°298 6.77 cal/degree mol. K (28.3 J/degree mol.K) Pro ...
... Specific heat 0.215 cal/g.°C (0.900 J/g.°C); heat capacity 5.81 cal/mol.°C (24.3 J/mol.°C); ∆Hfus (2.54 kcal/mol (10.6 kJ/mol); ∆Hvap 67.9 kcal/mol (284 kJ/mol); E° in aqueous soln. (acidic) at 25°C for the reaction Al3+ + 3e– —› Al(s) , –1.66V; S°298 6.77 cal/degree mol. K (28.3 J/degree mol.K) Pro ...
2. PROPERTIES OF ZIRCONIUM 2.1 Physical Properties
... Sulphate ions have a strong affinity for zirconium and therefore other sulphate salts of zirconium are known, as well as various anionic sulphato-complexes in solutions. These salts are referred to as normal salts to distinguish them from the basic and acid salts. The key to this definition lies in ...
... Sulphate ions have a strong affinity for zirconium and therefore other sulphate salts of zirconium are known, as well as various anionic sulphato-complexes in solutions. These salts are referred to as normal salts to distinguish them from the basic and acid salts. The key to this definition lies in ...
Handbook of Inorganic Chemicals Pradyot Patnaik, Ph.D.
... Mohs hardness is based on a scale from 1 to 10 units in which diamond, the hardest substance, is given a value of 10 Mohs and talc given a value of 0.5. Vapor pressure is exerted by a solid or liquid in equilibrium with its own vapor. All liquids have vapor pressures. Vapor pressure depends on tempe ...
... Mohs hardness is based on a scale from 1 to 10 units in which diamond, the hardest substance, is given a value of 10 Mohs and talc given a value of 0.5. Vapor pressure is exerted by a solid or liquid in equilibrium with its own vapor. All liquids have vapor pressures. Vapor pressure depends on tempe ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.