* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PowerPoint ******
Hydroformylation wikipedia , lookup
Aromaticity wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Aromatization wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Homoaromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
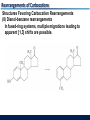
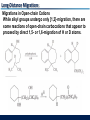
Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements 1,2-Shifts of migrating groups to empty orbitals in carbocations or toward partially empty orbitals in developing carbocations are the most common rearrangements of organic molecules. Especially, migration of hydrogen atom or alkyl or aryl groups in carbocations are called “WagnerMeerwein Rearrangements” Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements “Wagner-Meerwein Rearrangements” do not involve the bond breaking and formation because the following reaction show the retention of configuration Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements “Wagner-Meerwein Rearrangements” show that the substituents invariably end up on the face of the ring from which they started. Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements “Wagner-Meerwein Rearrangements” are intramolecular reactions which have TS resembling cyclic arrays of three atomic orbitals called as “corner-protonated cyclopropanes” Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements “Wagner-Meerwein Rearrangements” proceed most easily when the carbocations are converted to more stable forms. - Secondary cation to tertiary cation - Simple cation to resonance-stabilized cation Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements “Wagner-Meerwein Rearrangements” also proceed in the cases of same stability of carbocations. - Secondary cation to secondary cation - Tertiary cation to tertiary cation Migrations to Electron-Deficient Carbons Wagner-Meerwein Rearrangements “Wagner-Meerwein Rearrangements” must form the final products that are thermodynamically more stable than the starting materials. Some processes proceeding do appear to require uphill steps (formation of less stable carbocation). The Nature of The Migrating Groups Migratory Aptitudes (1) The relative yields of products from migrations of different groups are not related to the abilities of the groups to migrate (2) Geometric factors can be important in migration. A methyl group at the ring juncture of the two fused carbocyclic rings will migrate in preference to the migration of a primary or secondary alkyl group forming part of one of the rings. Migrations to Electron-Deficient Carbons Migratory Aptitudes (3) Methyl groups at axial positions of six-membered rings are almost ideally positioned to migrate because the bonds between the methyl groups and the rings are nearly coplanar with the empty orbitals of the cations. Equatorial substituents are unlikely to migrate. (4) Steric factors may also significantly affect the open-chain cations. Non-migrating substituents are forced into “eclipsed position in the TS for rearrangement. Migrations to Electron-Deficient Carbons Migratory Tendencies In some cases, migration tendencies can be determined by comparing the rates of migration of different groups in reactions with identical stationary groups. Degenerate Rearrangement – The products have the same structure as the starting material. Ethyl group migrate 13~55 times as fast as methyl group Migrations to Electron-Deficient Carbons Migratory Tendencies It is possible to correct observed migratory aptitudes for electronic and steric effects of stationary groups. Example : pinacol rearrangement methyl : ethyl : t-butyl = 1 : 17 : 4000 Migrations to Electron-Deficient Carbons Migratory Tendencies The general trend is that migration tendencies of alkyl groups in carbocation rearrangements increase with increasing substitution at the migrating carbon atoms. Migrations to Electron-Deficient Carbons Migratory Tendencies Hydrogens usually migrate more rapidly than alkyl group in Wagner-Meerwein rearrangements. The hydrogen atom is only a slightly better migrator than the methyl group and is probably a less effective migrator than most larger alkyl groups. Rearrangements of Carbocations Competition with Other Reactions The major products from many reactions in which carbocations are intermediates are usually formed without carbon skeleton rearrangements. In these reactions, nucleophiles react with carbocations more rapidly than rearrangements. Rearrangements of Carbocations Rearrangements Under Minimally Nucleophilic Conditions If nucleophiles react exceptionally slowly with carbocations, it is because the reaction mixtures contain strong Lewis acids that complex with the nucleophiles and minimize their reactivities. (Friedel-Crafts alkylation reaction) Rearrangements of Carbocations Rearrangements Under Minimally Nucleophilic Conditions Carbocations can also have long lifetimes in superacids, which are polar solvents with very low nucleophilicities. - FSO3H, CF3SO3H - BF3 in HF, AsF5 in FSO3H, SO2ClF in liquid SO2, SbF5 in FSO3H (Magic acid) Rearrangements of Carbocations Rearrangements of Alkanes Even alkanes having the lack of reactivity in ionic reactions appear to rearrange rapidly in superacids or under FriedelCrafts conditions. Pines and Wackher “Cationic chain mechanism for the Isomerization of alkanes in the presence of Lewis acids And promoters” Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements Several types of organic molecules in which carbocation rearrangements are particularly likely to occur, even in the presence of good nucleophiles (1) The neopentyl system 2,2-Dimethylpropyl halides or sulfonates cannot undergo E2 or SN2 reactions Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (2) Relief of ring strain Rearrangements are particularly likely to occur if they result in a 3- or 4-membered ring expanding to form a less strained structure. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (2) Relief of ring strain Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (2) Relief of ring strain In the very strained polycyclic system, relief of angle strain is achieved by contraction of a cyclobutane ring. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (3) Pinacol and semipinacolic rearrangements Tetramethylethylene glycol (pinacol) rearrange in acid to form tert-butyl methyl ketone (pinacolone) Formation of a new bond (p bond) provides the driving force Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (3) Pinacol and semipinacolic rearrangements b-Halo alcohols and 1,2-epoxides are common starting materials for semipinacolic rearrangements. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (3) Pinacol and semipinacolic rearrangements b-Halo alcohols and 1,2-epoxides are common starting materials for semipinacolic rearrangements. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (4) Rearrangements of a-hydroxyaldehydes and a-hydroxyketones (acyloin rearrangement) In acid-catalyzed rearrangements of a-hydroxyaldehydes or ketones, the migrating groups migrate to carbon atoms of protonated carbonyl groups rather than to carbocation centers. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (4) Rearrangements of a-hydroxyaldehydes and a-hydroxyketones (acyloin rearrangement) Difference between acyloin and pinacolic rearrangements - Competing elimination and substitution reactions of the alcohol functions are unlikely to occur in acyloin one - Pinacol rearrangements are usually highly exothermic, but acyloin rearrangements are often similar in energy, so that acyloin one are readily reversible - Acyloin rearrangements can take place under basic as well as acidic conditions Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (4) Rearrangements of a-hydroxyaldehydes and a-hydroxyketones (acyloin rearrangement) Acyloin rearrangements can take place under basic as well as acidic conditions. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (4) Rearrangements of a-hydroxyaldehydes and a-hydroxyketones (acyloin rearrangement) Many other aldehydes and ketones can undergo rearrangements in acid to form isomeric carbonyl compounds. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (5) The benzilic acid rearrangement If diphenyl-1,2-dione (benzil) is heated in strong basic solutions, it is quantitatively converted to salts of benzilic acid Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (5) The benzilic acid rearrangement Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (5) The benzilic acid rearrangement Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (6) Dienone-phenol rearrangements Ortho-cyclohexadienones undergo acid-catalyzed rearrangements to yield phenols or esters of phenols Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (6) Dienone-phenol rearrangements Take place in solution of sulfuric acid in acetic acid or acetic anhydride Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (6) Dienone-phenol rearrangements 1,3-Shifts of migrating cycloalkyl rings Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (7) Allyl and benzyl group migrations in dienone-phenol rearrangements Acid-catalyzed migrations of benzyl groups in orthocyclohexadienones can proceed by [1,5] shifts as well as [1,2] shifts Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (7) Allyl and benzyl group migrations in dienone-phenol rearrangements Migrations of allyl groups in ortho- and para-cyclohexadienones can proceed by [3,3] shifts as well as [1,2] shifts Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (8) Dienol-benzene rearrangements Cyclohexadienols are very acid-sensitive compounds that easily rearrange to form aromatic rings. Rearrangements of Carbocations Structures Favoring Carbocation Rearrangements (8) Dienol-benzene rearrangements In fused-ring systems, multiple migrations leading to apparent [1,3] shifts are possible. Long-Distance Migrations Migrations in Open-chain Cations While alkyl groups undergo only [1,2]-migration, there are some reactions of open-chain carbocations that appear to proceed by direct 1,5- or 1,6-migration of H or D atoms. Long-Distance Migrations Migrations in Open-chain Cations