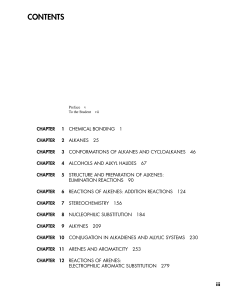
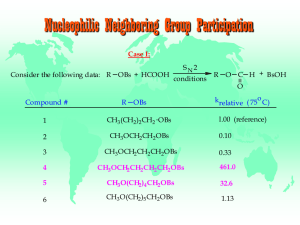
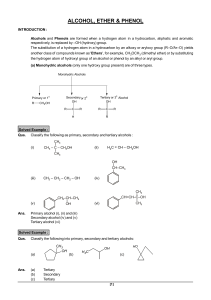
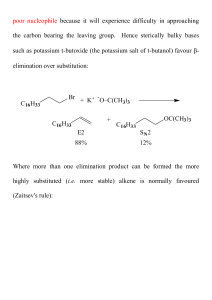
Study Guide and Solutions Manual
... organic reactions. A relatively few fundamental mechanisms suffice to describe almost every reaction you will encounter. Once learned and understood, these mechanisms provide a valuable means of categorizing the reactions of organic molecules. There will be numerous facts to learn in the course of t ...
... organic reactions. A relatively few fundamental mechanisms suffice to describe almost every reaction you will encounter. Once learned and understood, these mechanisms provide a valuable means of categorizing the reactions of organic molecules. There will be numerous facts to learn in the course of t ...
Chapter 16 Aldehydes and Ketones
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
Amphiphilic Sugar Metal Carbenes
... hydrogenation while K. B. Sharpless3 got the other half price for his work on catalysed chiral oxidation reactions. In 2005 Y. Chauvin,4 R. R. Schrock5 and R. H. Grubbs6 received the Nobel price for their work on the methatesis reaction methodology. This reaction involves a metal carbene and an olef ...
... hydrogenation while K. B. Sharpless3 got the other half price for his work on catalysed chiral oxidation reactions. In 2005 Y. Chauvin,4 R. R. Schrock5 and R. H. Grubbs6 received the Nobel price for their work on the methatesis reaction methodology. This reaction involves a metal carbene and an olef ...
Sample
... HBr to alkenes in 1869. Markovnikov stated : MARKOVNIKOV'S RULE : The addition of a proton acid to the double bond of an alkene result in a product with the acid proton bonded to the carbon atom that already holds the greater number of hydrogen atoms. This is the original statement of Markovnikov's ...
... HBr to alkenes in 1869. Markovnikov stated : MARKOVNIKOV'S RULE : The addition of a proton acid to the double bond of an alkene result in a product with the acid proton bonded to the carbon atom that already holds the greater number of hydrogen atoms. This is the original statement of Markovnikov's ...
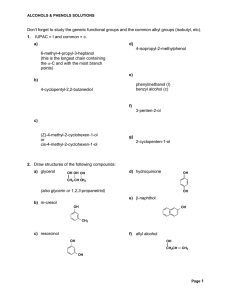
1 SCH4U1 HYDROCARBONS Certain organic compounds contain
... This works for simple alkanes such as butane (C4H10) and pentane (C5H12). However, it becomes hopeless when larger alkanes are considered. For example there are 5 isomers of hexane (C6H14), 9 isomers of heptane (C7H16) and 75 isomers of decane (C10H22). Another problem arises as far as nomenclature ...
... This works for simple alkanes such as butane (C4H10) and pentane (C5H12). However, it becomes hopeless when larger alkanes are considered. For example there are 5 isomers of hexane (C6H14), 9 isomers of heptane (C7H16) and 75 isomers of decane (C10H22). Another problem arises as far as nomenclature ...
What is Organic Chemistry?
... Organic chemistry is essential to the understanding of the intricate details of life The interactions and reactions of organic molecules are what define living systems. One needs to understand bonding, structure, properties, reactions, and synthesis to understand natural systems. CHAPTER 1 INTRODUCT ...
... Organic chemistry is essential to the understanding of the intricate details of life The interactions and reactions of organic molecules are what define living systems. One needs to understand bonding, structure, properties, reactions, and synthesis to understand natural systems. CHAPTER 1 INTRODUCT ...
Hypervalent Iodine Reagents in High Valent Transition Metal
... to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond formation. Key to the advancement of this chemistry has been the identification of oxidants t ...
... to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond formation. Key to the advancement of this chemistry has been the identification of oxidants t ...
Chalogen-Nitrogen Chemistry
... The chemistry of selenium– and tellurium–nitrogen compounds has progressed more slowly but, in the last ten years, there have been numerous developments in these areas also, as a result of the creative contributions of both inorganic and organic synthetic chemists. Significant differences are appare ...
... The chemistry of selenium– and tellurium–nitrogen compounds has progressed more slowly but, in the last ten years, there have been numerous developments in these areas also, as a result of the creative contributions of both inorganic and organic synthetic chemists. Significant differences are appare ...
Ylide Ligands - DIGITAL.CSIC, el repositorio institucional
... Not only the preparative methods specified, but also the bonding properties [12] of the ylides –mostly at the E=C bond– and some interesting organic applications [13,14] have been the subject of detailed revision works. In summary, the chemistry shown in Scheme 2 constitutes an useful set of tools ...
... Not only the preparative methods specified, but also the bonding properties [12] of the ylides –mostly at the E=C bond– and some interesting organic applications [13,14] have been the subject of detailed revision works. In summary, the chemistry shown in Scheme 2 constitutes an useful set of tools ...
$doc.title
... calculations 65 seem to indicate accumulation of negative charge on the 2- and 5-carbon atoms and a positive charge on the sulfur atom. Coordination can occur through the sulfur atom (8), through the 2- or 5-carbon or both (r] 1) or through the n-clouds of the C(2)=C(3) or C(4)=C(5) bonds (fl2) or b ...
... calculations 65 seem to indicate accumulation of negative charge on the 2- and 5-carbon atoms and a positive charge on the sulfur atom. Coordination can occur through the sulfur atom (8), through the 2- or 5-carbon or both (r] 1) or through the n-clouds of the C(2)=C(3) or C(4)=C(5) bonds (fl2) or b ...
PowerPoint ******
... “Wagner-Meerwein Rearrangements” must form the final products that are thermodynamically more stable than the starting materials. Some processes proceeding do appear to require uphill steps (formation of less stable carbocation). ...
... “Wagner-Meerwein Rearrangements” must form the final products that are thermodynamically more stable than the starting materials. Some processes proceeding do appear to require uphill steps (formation of less stable carbocation). ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.