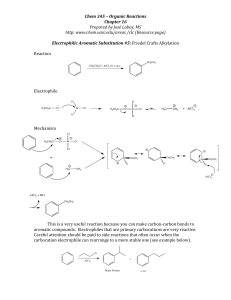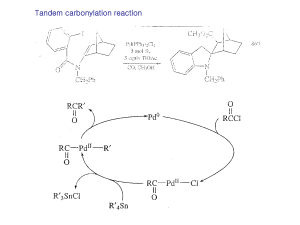
EAS Friedel-Crafts Alkylation
... The Friedel-‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-‐ alkyla ...
... The Friedel-‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-‐ alkyla ...
Lecture6-Organometallic Chemistry
... are coordinatively unsaturated (having an open coordination site or being weakly coordinated) Square-planar 16-electron complexes are coordinatively unsaturated ML4 complexes of Pd(II), Pt(II) and Rh(I) [RhCl(PPh3)3] – hydrogenation catalyst ...
... are coordinatively unsaturated (having an open coordination site or being weakly coordinated) Square-planar 16-electron complexes are coordinatively unsaturated ML4 complexes of Pd(II), Pt(II) and Rh(I) [RhCl(PPh3)3] – hydrogenation catalyst ...
슬라이드 1
... The main advantage of nickel is that it reacts more readily with arylchlorides and methanesulfonates than does the Pd system. These reactants may be more economical than iodides or triflates in large-scale synthesis. ...
... The main advantage of nickel is that it reacts more readily with arylchlorides and methanesulfonates than does the Pd system. These reactants may be more economical than iodides or triflates in large-scale synthesis. ...
01-43 chimica.qxd
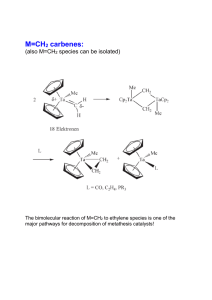
... Olefin metathesis is generally reversible and limited to an equilibrium. In many cases, however, the transformation may be brought to completion based on simple thermodynamic considerations. For instance, ringopening processes are enthalpically driven due to the relief of ring strain, whereas ring-c ...
... Olefin metathesis is generally reversible and limited to an equilibrium. In many cases, however, the transformation may be brought to completion based on simple thermodynamic considerations. For instance, ringopening processes are enthalpically driven due to the relief of ring strain, whereas ring-c ...
Ch 26 C-C bond formation
... Usefulness of Metathesis Reactions • Because olefin metathesis is an equilibrium process and with many alkene substrates yields a mixture of starting material and two or more alkene products, it is useless for preparative processes. • However, with terminal alkenes, one metathesis product is ethyle ...
... Usefulness of Metathesis Reactions • Because olefin metathesis is an equilibrium process and with many alkene substrates yields a mixture of starting material and two or more alkene products, it is useless for preparative processes. • However, with terminal alkenes, one metathesis product is ethyle ...
9 carbene complexes in olefin metathesis and ring
... results are obtained with Mo, W, and Re catalysts. The soluble catalysts, which have received extensive study in both academic and industrial laboratories, are of three major types. One family might be designated Ziegler catalysts because they are based on combinations of alkyl aluminum, magnesium, ...
... results are obtained with Mo, W, and Re catalysts. The soluble catalysts, which have received extensive study in both academic and industrial laboratories, are of three major types. One family might be designated Ziegler catalysts because they are based on combinations of alkyl aluminum, magnesium, ...
... Johnson Matthey have published an informative 82-page brochure, “The Catalyst Technical Handbook”, which covers the use of catalysts for chemical reactions important in industrial synthesis. The handbook recommends platinum group metal homogeneous, heterogeneous and FibreCatm anchored homogeneous ca ...
Organometallic Reactions and Catalysis
... • The catalyst hydrogenizes terminal and internal olefins. • Examine Table 14-3. ...
... • The catalyst hydrogenizes terminal and internal olefins. • Examine Table 14-3. ...
Losing and Gaining Electrons
... The strength of the metal-ligand bond is often judged by the bond length, M-L. This is affected by the force constant of the bond (“k”) and the steric hindrance of the ligand. The steric hindrance of the ligand is judged by the “cone angle” (i.e., the solid angle) that the ligand occupies around the ...
... The strength of the metal-ligand bond is often judged by the bond length, M-L. This is affected by the force constant of the bond (“k”) and the steric hindrance of the ligand. The steric hindrance of the ligand is judged by the “cone angle” (i.e., the solid angle) that the ligand occupies around the ...
The Shell Higher Olefins Process (SHOP)
... 100 – 110 bar) in a polar solvent (1,4-butanediol) to give a mixture of linear, even-numbered αolefins (C4 – C40) with a Flory-Schultz distribution (immiscible with the catalyst solution): ...
... 100 – 110 bar) in a polar solvent (1,4-butanediol) to give a mixture of linear, even-numbered αolefins (C4 – C40) with a Flory-Schultz distribution (immiscible with the catalyst solution): ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.