Honors Chemistry Organic Chemistry
... h. containing benzene or benzene-like structures i. from wood distillation / metabolized into formaldehyde j. benzene as a substituent k. reaction in the formation of esters l. phenol ...
... h. containing benzene or benzene-like structures i. from wood distillation / metabolized into formaldehyde j. benzene as a substituent k. reaction in the formation of esters l. phenol ...
lec-2- 211(ES +Add)
... on the addition of hydrogen halides to alkenes. •The rule states that : "when an unsymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the hydrogen adds to the carbon of the alkene that has the greater number of hydrogen substituents, and the halogen to the carbon of the alkene ...
... on the addition of hydrogen halides to alkenes. •The rule states that : "when an unsymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the hydrogen adds to the carbon of the alkene that has the greater number of hydrogen substituents, and the halogen to the carbon of the alkene ...
CHE 322
... 3. (8) Give the complete mechanism that shows why the reaction of butanal with a cyclic 2° amine followed by heating with acid produces an enamine that is nucleophilic at butanal’s former α-carbon. ...
... 3. (8) Give the complete mechanism that shows why the reaction of butanal with a cyclic 2° amine followed by heating with acid produces an enamine that is nucleophilic at butanal’s former α-carbon. ...
Word document format
... 1. Know how to prepare a functional group and what can be made from a functional group. For example, know how to prepare alkenes and what can be made from an alkene. The best way to memorize these reactions is to make flashcards. Include stereospecificity of reaction, where needed. This is most like ...
... 1. Know how to prepare a functional group and what can be made from a functional group. For example, know how to prepare alkenes and what can be made from an alkene. The best way to memorize these reactions is to make flashcards. Include stereospecificity of reaction, where needed. This is most like ...
Networking reactions for organic synthesis of the future
... Engineering (ISIS), University of Strasbourg, seeks to hire outstanding researchers at the Ph.D. and post-doctoral level. The main research thrust of this newly established laboratory is the development of a new approach to organic synthesis based on networking multiple reactions within one vessel. ...
... Engineering (ISIS), University of Strasbourg, seeks to hire outstanding researchers at the Ph.D. and post-doctoral level. The main research thrust of this newly established laboratory is the development of a new approach to organic synthesis based on networking multiple reactions within one vessel. ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
PowerPoint 프레젠테이션
... - alkenes are one of the most important building block - abundant in nature's chemicals: vitamin A, quinine, chlorophyll - manmade organic chemicals: pharmaceuticals, perfumes, flavoring ...
... - alkenes are one of the most important building block - abundant in nature's chemicals: vitamin A, quinine, chlorophyll - manmade organic chemicals: pharmaceuticals, perfumes, flavoring ...
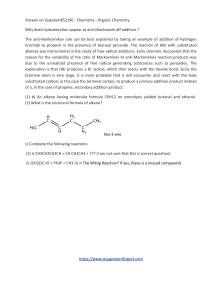
Answer on Question#52196 - Chemistry
... reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation is that HBr produces a Br radical, which then reacts with the double bond. Since the bromine atom i ...
... reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation is that HBr produces a Br radical, which then reacts with the double bond. Since the bromine atom i ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.