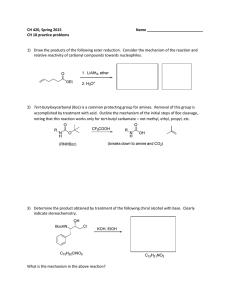
Get PDF - Wiley Online Library
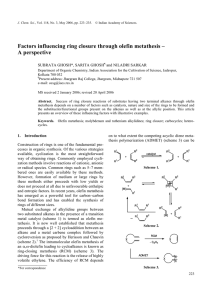
... pared to regular protected amines. Reacting 4 with 4bromo-1-butene (6a) and 5-bromo-1-pentene (6b) resulted in the formation of RCM precursors 7a and 7b in yields of 49 and 59 %, respectively. Alkylation of Boc-protected 5 with 6b proceeded slightly better and the corresponding product 8 was isolate ...
... pared to regular protected amines. Reacting 4 with 4bromo-1-butene (6a) and 5-bromo-1-pentene (6b) resulted in the formation of RCM precursors 7a and 7b in yields of 49 and 59 %, respectively. Alkylation of Boc-protected 5 with 6b proceeded slightly better and the corresponding product 8 was isolate ...
- M E S KVM College Valanchery.
... (a) CH3CH2ONa : No, since it is an oxygen-metal bond (b) CH3CH2Li : Yes there is a carbon-metal bond (c) CH3CH2BH2 : No, boron is a non-metal (d) (CH3CH2)2Zn : Yes there are 2 carbon-metal bonds (e) CH3CH2MgBr : Yes there is a carbon-metal bond (f) CH3C≡CNa : Yes there is a carbon-metal bond ...
... (a) CH3CH2ONa : No, since it is an oxygen-metal bond (b) CH3CH2Li : Yes there is a carbon-metal bond (c) CH3CH2BH2 : No, boron is a non-metal (d) (CH3CH2)2Zn : Yes there are 2 carbon-metal bonds (e) CH3CH2MgBr : Yes there is a carbon-metal bond (f) CH3C≡CNa : Yes there is a carbon-metal bond ...
1. Dehydration Synthesis 2. Fermentation
... Requires two alcohols. Uses a dehydrating agent (or enzyme) to remove an H from one hydroxyl and an OH from the other. This becomes water. ...
... Requires two alcohols. Uses a dehydrating agent (or enzyme) to remove an H from one hydroxyl and an OH from the other. This becomes water. ...
OMC-Karlin-Spring-`09-Lecture-Set
... stability.”, later accorded to the aromatic character of Cp– groups. The sandwich compound structure was described later; this led to new metallocenes chemistry (1973 Nobel prize, Wilkinson & Fischer). The Fe atom is assigned to the +2 oxidation state (Mössbauer spectroscopy). The bonding nature in ...
... stability.”, later accorded to the aromatic character of Cp– groups. The sandwich compound structure was described later; this led to new metallocenes chemistry (1973 Nobel prize, Wilkinson & Fischer). The Fe atom is assigned to the +2 oxidation state (Mössbauer spectroscopy). The bonding nature in ...
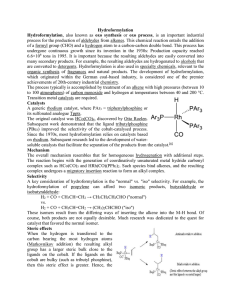
Hydroformylation Hydroformylation, also known as oxo synthesis or
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
Catalytic Hydrogenation of Alkenes: Relative Stability of
... Removal of a secondary hydrogen (C3 in the starting bromide) is sterically more difficult than abstracting a more exposed methyl hydrogen when a hindered base is used. The transition state leading to the more stable product is increased in energy by steric interference with the bulky base. An E2 rea ...
... Removal of a secondary hydrogen (C3 in the starting bromide) is sterically more difficult than abstracting a more exposed methyl hydrogen when a hindered base is used. The transition state leading to the more stable product is increased in energy by steric interference with the bulky base. An E2 rea ...
Fulltext PDF
... olefin metathesis catalysts. What is noteworthy about these catalysts is that they are stable in air, tolerate water, and can operate at elevated temperatures. On the other hand, the molybdenum-based catalysts prepared by Richard Schrock and coworkers are highly air and moisture sensitive but are mo ...
... olefin metathesis catalysts. What is noteworthy about these catalysts is that they are stable in air, tolerate water, and can operate at elevated temperatures. On the other hand, the molybdenum-based catalysts prepared by Richard Schrock and coworkers are highly air and moisture sensitive but are mo ...
Organic Chemistry (I) chapter 3 alkanes
... 6. Which of the following Newman projections represents "sighting down" the C4-C5 bond of 4,5-di-isopropyloctane in the conformation in which the two isopropyl groups are anti with respect to each other? ...
... 6. Which of the following Newman projections represents "sighting down" the C4-C5 bond of 4,5-di-isopropyloctane in the conformation in which the two isopropyl groups are anti with respect to each other? ...
Combustion, Addition and Elimination Objective Combustion Example
... is weaker than the single bond, this bond can break and then we have free bonding electrons where another element or functional group can be added. In general: ...
... is weaker than the single bond, this bond can break and then we have free bonding electrons where another element or functional group can be added. In general: ...
Nucleophilic Substitution Reaction
... In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can b ...
... In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can b ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.