* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nucleophilic Substitution Reaction
Kinetic isotope effect wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
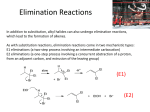
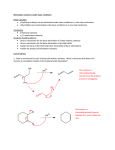
Nucleophilic Substitution Reaction Part-5 Elimination Reactions Elimination reactions, in which two groups are removed from a molecule, not being replaced by another group, are the reverse of addition reactions. Usually they involve the loss of two substituents from vicinal atoms resulting in the formation of a double or triple bond. Most commonly a proton is lost from one carbon whereas a nucleophile is lost from the adjacent carbon; these two carbon atoms are usually referred to as - and -carbons, respectively. This type of elimination reaction is known as -elimination or a 1,2-elimination reaction. The most familiar examples of b-elimination reactions include dehydrohalogenation of alkyl halides, dehydration of alcohols, pyrolysis of esters and the Hofmann degradation of quaternary ammonium hydroxides. There are a few reactions in which both the groups are lost from the same carbon atom. These are called -elimination reactions and the most common example of this type is the generation of dichlorocarbene from chloroform. In analogy with substitution reaction,b-elimination reactions are divided into E1 (Elimination, unimolecular) and E2 (Elimination, bimolecular) reactions. (i) E2 reaction : The Bimolecular mechanism Most of the elimination reactions are successful only when carried out in the presence of a strong base. The formation of ethylene on the treatment of ethyl bromide with sodium ethoxide is an example of this type. The rate of alkene formation is proportional to the concentrations of ethyl bromide as well as that of sodium ethoxide. Two types of mechanism are praposed which are in agreement with the kinetic data. In the first mechanism the base abstracts a proton from the -carbon and simultaneously the leaving group departs from the -carbon along with the pair of bonding electrons. In the second mechanism the base rapidly removes proton from the -carbon resulting in the formation of carbanion which loses the leaving group in a rate-determining step. Since the conditions of base-catalyzed elimination reactions do not allow the formation of an unstabilized carbanion, it is reasonable to presume if some part is formed it must be either rapidly reconverted to the substrate or converted to the product alkene. In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can be made very easily by means of labelling experiments. Consider the reaction of 2-phenylethyl bromide (I) and ethoxide to yield styrene (II) which follows second order kinetics. The reaction may follow either mechanism. The first step of the E1cB mechanism is reversible and hence when the reaction is carried out in C2H5OD instead of C2H5OH, the intermediate carbanion should pick up deuterium.