* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Elimination Reactions
Enantioselective synthesis wikipedia , lookup
Marcus theory wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Petasis reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
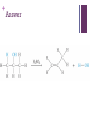
+ Elimination Reactions + In Elimination reactions, atoms are removed from an organic compound to form an alkene. Elimination reactions are though of as the reverse of Addition reactions. + Modeling Elimination Reactions Build the following reactant H2SO4 is a strong acid that is NOT a reactant, it just makes the reaction occur faster. + Using only the atoms you have in the models, rearrange the atoms to form two products. (you many need a different number or type of bonds) What did you do? Draw the product. + Answer + General equation of Elimination Reactions forming an Alkene Y and Z will combine An alkene will be formed + Elimination Reactions These reactions are useful because alkenes are used to manufacture many products such as: Carpets Clothing Upholstery Plastics + Elimination Reactions of Alcohols When alcohols are heated with a strong acid: An alkene and water are formed ACID + The acid is called a catalyst because it speeds up the reaction but is NOT one of the reactants. That is why it is written over the arrow. H2SO4(sulfuric acid) is a strong acid commonly used in elimination reactions + Examples Draw the structural formulas for the organic products that are predicted to form in the following elimination reactions. Assume the acid is sulfuric acid. 1-propanol 2-butanol (there are two options, show both) + Elimination Reactions of Haloalkanes When haloalkanes are heated with a strong base: An alkene, and ionic compound and water are formed + Examples Draw the structural formulas for the organic products that are predicted to form in the following elimination reactions. + KOH + There are two options for this reaction. Be sure to show both. + NaOH