Alkenes 4 - ChemWeb (UCC)
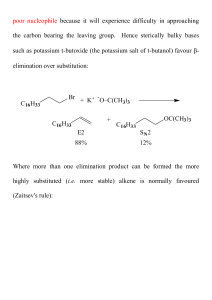
... mercurinium or bromonium cation bond-breaking of the C-Hg or C-Br bond is more advanced than bond-formation with the incoming nucleophile. Hence the SN2 transition state for this reaction has partial carbocationic (i.e. SN1) character and therefore nucleophilic attack is at the most substituted carb ...
... mercurinium or bromonium cation bond-breaking of the C-Hg or C-Br bond is more advanced than bond-formation with the incoming nucleophile. Hence the SN2 transition state for this reaction has partial carbocationic (i.e. SN1) character and therefore nucleophilic attack is at the most substituted carb ...
GPS semester review
... ____ 36. Activation energy is the minimum amount of energy needed for a chemical reaction to begin. ____ 37. Exothermic reactions do not require any activation energy. ____ 38. When a chemical equation contains the same number of atoms on both sides, the equation is balanced. ____ 39. Catalysts are ...
... ____ 36. Activation energy is the minimum amount of energy needed for a chemical reaction to begin. ____ 37. Exothermic reactions do not require any activation energy. ____ 38. When a chemical equation contains the same number of atoms on both sides, the equation is balanced. ____ 39. Catalysts are ...
Chapter 12 384 12.1 A system is isolated if it exchanges neither
... According to data in Chemistry and Life Box, a 55-kg person walking 6.0 km/hr consumes 1090 kJ/hr: 1 hr t = 2500 kJ = 2.3 hr. 1090 kJ Therefore, to consume the additional energy a person must walk: 6.0 km distance = 2.3 hr = 1.4 km. 1 hr 12.18 To work this problem, use data in ...
... According to data in Chemistry and Life Box, a 55-kg person walking 6.0 km/hr consumes 1090 kJ/hr: 1 hr t = 2500 kJ = 2.3 hr. 1090 kJ Therefore, to consume the additional energy a person must walk: 6.0 km distance = 2.3 hr = 1.4 km. 1 hr 12.18 To work this problem, use data in ...
OC 2/e Ch 11
... 11 Synthesis of Epoxides-3 Problem: given the stereospecificity of this scheme, show that cis-2-butene gives cis-2,3-dimethyloxirane H H3 C ...
... 11 Synthesis of Epoxides-3 Problem: given the stereospecificity of this scheme, show that cis-2-butene gives cis-2,3-dimethyloxirane H H3 C ...
Chapter 8 PowerPoint - Southeast Online
... • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecules NH3 • Since we count molecules by moles: 3 moles H2 1 mole N2 2 moles NH3 Tro's “Introductory Chemist ...
... • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecules NH3 • Since we count molecules by moles: 3 moles H2 1 mole N2 2 moles NH3 Tro's “Introductory Chemist ...
Late Transition Metal Amido Complexes: Electronic
... Lappart herby established the synthetic strategies and structural motifs of the amido ligand (NR2-) and furthermore explored the reactivity of amido metal bonds.[2a] During the past decades, this type of ligands have developed an increasing dominant role in ligand design due to their unique and ...
... Lappart herby established the synthetic strategies and structural motifs of the amido ligand (NR2-) and furthermore explored the reactivity of amido metal bonds.[2a] During the past decades, this type of ligands have developed an increasing dominant role in ligand design due to their unique and ...
Supplemental Problems
... such as wood, bones, and fossils. While alive, living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its body no longer takes in new carbon-14. Thus, by ...
... such as wood, bones, and fossils. While alive, living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its body no longer takes in new carbon-14. Thus, by ...
Experimental details
... pressure as well as the very intense ionizing radiation emitted. The relevant materials in nuclear technological applications are required to be stable and durable after having withstood these extreme conditions. The materials stability under high temperature and pressure is primarily controlled by ...
... pressure as well as the very intense ionizing radiation emitted. The relevant materials in nuclear technological applications are required to be stable and durable after having withstood these extreme conditions. The materials stability under high temperature and pressure is primarily controlled by ...
SUPPORTED LIGANDS FOR METAL CATALYZED REACTIONS Rocío Marcos Escartín ISBN:
... fulfil two important requirements: i) The bond-formation between the Lewis acid and the reagent/reactant needs to be reversible and ii) Lewis acid-reactant binding should be stronger than Lewis acid-product binding (in order to avoid product inhibition of the reaction).[2] The way in which Lewis aci ...
... fulfil two important requirements: i) The bond-formation between the Lewis acid and the reagent/reactant needs to be reversible and ii) Lewis acid-reactant binding should be stronger than Lewis acid-product binding (in order to avoid product inhibition of the reaction).[2] The way in which Lewis aci ...
Application of Novel Phosphine Ligands in Palladium
... new catalytic cycle. Often, these not chemical but physical reaction steps are rate-determining for the whole reaction. The third part of catalyzed reactions constitutes the transformations promoted by biomolecules, namely enzymes. Thus, nature can be considered as the world’s leading catalyst desig ...
... new catalytic cycle. Often, these not chemical but physical reaction steps are rate-determining for the whole reaction. The third part of catalyzed reactions constitutes the transformations promoted by biomolecules, namely enzymes. Thus, nature can be considered as the world’s leading catalyst desig ...