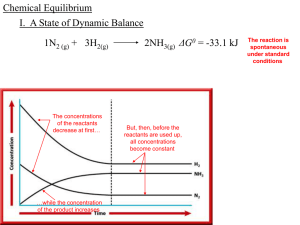
Chemical Equilibrium - local.brookings.k12.sd.us
... reaction proceeds, the ____ rate of the ________ forward _________ reaction continues to ________ decrease and the ____ rate reverse ________ reaction continues of the ________ increase until the two _____ rates are to ________ _____, equal and the system has reached a chemical __________ equilbrium ...
... reaction proceeds, the ____ rate of the ________ forward _________ reaction continues to ________ decrease and the ____ rate reverse ________ reaction continues of the ________ increase until the two _____ rates are to ________ _____, equal and the system has reached a chemical __________ equilbrium ...
Anexo I. Modelos teóricos de análisis de sistemas de valencia mixta.
... de onda del estado excitado y er es el operador de la transición dipolar. Si tenemos en cuenta un acoplamiento débil entre el dador y el aceptor, las funciones de onda del dador φd y del aceptor φa se pueden considerar aisladas; los coeficientes de combinación de las funciones de onda son αad = - αd ...
... de onda del estado excitado y er es el operador de la transición dipolar. Si tenemos en cuenta un acoplamiento débil entre el dador y el aceptor, las funciones de onda del dador φd y del aceptor φa se pueden considerar aisladas; los coeficientes de combinación de las funciones de onda son αad = - αd ...
Chapter 15 Chemical Equilibrium
... 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiometry of the reaction (that is, use the coefficients in the balanced chemical equation) to calculate the c ...
... 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiometry of the reaction (that is, use the coefficients in the balanced chemical equation) to calculate the c ...
Palladium(II)-Catalyzed Oxidative Cyclization Strategies Andreas K. Å. Persson
... palladium(II) readily forms π-complexes with a wide range of unsaturated hydrocarbons such as alkynes, alkenes and allenes. The coordination to palladium renders these fragments susceptible towards nucleophilic attack and/or migratory insertion. Many palladium(II)-catalyzed oxidative processes, suc ...
... palladium(II) readily forms π-complexes with a wide range of unsaturated hydrocarbons such as alkynes, alkenes and allenes. The coordination to palladium renders these fragments susceptible towards nucleophilic attack and/or migratory insertion. Many palladium(II)-catalyzed oxidative processes, suc ...
Elementary Steps, the Role of Chemisorbed Oxygen, and the Effects
... constant exceeding 500. Thus, even if CO desorbed before forming CO2, it would oxidize via reactions with O* at any reactor residence time required for detectable CH4 conversion, making direct partial oxidation impractical as a molecular route to H2!CO mixtures on Pd. CH4 turnover rates and effective ...
... constant exceeding 500. Thus, even if CO desorbed before forming CO2, it would oxidize via reactions with O* at any reactor residence time required for detectable CH4 conversion, making direct partial oxidation impractical as a molecular route to H2!CO mixtures on Pd. CH4 turnover rates and effective ...
Types of Chemical Reactions
... • A molecular/formula unit equation is one in which the reactants and products are written as if they were molecules/formula units, even though they may actually exist in solution as ions. Calcium hydroxide + sodium carbonate F.U. ...
... • A molecular/formula unit equation is one in which the reactants and products are written as if they were molecules/formula units, even though they may actually exist in solution as ions. Calcium hydroxide + sodium carbonate F.U. ...
aa-2005-38-71-negishi - University of Windsor
... The palladium-catalyzed cross-coupling of an organometal (R1M) with an organic electrophile (R2X) has emerged over the past thirty years as one of the most general and selective methods for carbon–carbon-bond formation (eq 1). Currently, it appears to be generally superior to related methods involvi ...
... The palladium-catalyzed cross-coupling of an organometal (R1M) with an organic electrophile (R2X) has emerged over the past thirty years as one of the most general and selective methods for carbon–carbon-bond formation (eq 1). Currently, it appears to be generally superior to related methods involvi ...
AP Chemistry-midterm review
... a. 1.36 J/g• C b. 4.18 J/g• C c. 1.11 J/g• C d. 0.73 J/g• C e. 1.54 J/g• C ____ 28. If a 10.0 g ball of iron at 160.0 C is dropped into 50.0 g of water at 20.0 C in an insulated container, what will be the final temperature of the water? The specific heat of iron is 0.444 J/g• C and that of water is ...
... a. 1.36 J/g• C b. 4.18 J/g• C c. 1.11 J/g• C d. 0.73 J/g• C e. 1.54 J/g• C ____ 28. If a 10.0 g ball of iron at 160.0 C is dropped into 50.0 g of water at 20.0 C in an insulated container, what will be the final temperature of the water? The specific heat of iron is 0.444 J/g• C and that of water is ...
File
... As we saw in the lesson on LeChatelier's Principle: Addition of a catalyst speeds up the forward reaction and the reverse reaction by the same amount. Therefore, it does not cause any shift of the equilibrium. Because there is no shift, the value of the Keq will also remain unchanged. ...
... As we saw in the lesson on LeChatelier's Principle: Addition of a catalyst speeds up the forward reaction and the reverse reaction by the same amount. Therefore, it does not cause any shift of the equilibrium. Because there is no shift, the value of the Keq will also remain unchanged. ...
Laboratories to be performed
... 1. You should always be prepared for class and ready to begin working as soon as the bell rings. We have a lot of material to cover, and I always look forward to seeing your smiling faces. If the door is closed upon your arrival to class, it means that you are tardy. If this occurs more than 5 times ...
... 1. You should always be prepared for class and ready to begin working as soon as the bell rings. We have a lot of material to cover, and I always look forward to seeing your smiling faces. If the door is closed upon your arrival to class, it means that you are tardy. If this occurs more than 5 times ...
CH221 CLASS 13
... Synopsis. Class 13 considers some important alkene addition reactions, from both the mechanistic and the synthetic viewpoint. Halogenation, halohydrin formation and hydration reactions are all discussed here. Hydration focuses on the oxymercuration and hydroboration /oxidation procedures. Orientatio ...
... Synopsis. Class 13 considers some important alkene addition reactions, from both the mechanistic and the synthetic viewpoint. Halogenation, halohydrin formation and hydration reactions are all discussed here. Hydration focuses on the oxymercuration and hydroboration /oxidation procedures. Orientatio ...