* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 15 Chemical Equilibrium
Chemical potential wikipedia , lookup
Crystallization wikipedia , lookup
Marcus theory wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
Pseudo Jahn–Teller effect wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Chemical reaction wikipedia , lookup
Colloidal crystal wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Planck's law wikipedia , lookup
Stoichiometry wikipedia , lookup
Rate equation wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Thermodynamics wikipedia , lookup
Transition state theory wikipedia , lookup
Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 15 Chemical Equilibrium Equilibrium © 2009, Prentice-Hall, Inc. Overview of Equilibrium – Chapter 15 • write Keq expression, in units of pressure or concentration • assess the Keq with regard to relative concentrations of reactants and products • manipulate chemical equations and Keq – reciprocal, multiplication, application of Hess’s Law • heterogeneous equilibria – what is included in Keq? • calculate Keq, including use of RICE tables Equilibrium © 2009, Prentice-Hall, Inc. Overview of Equilibrium – Chapter 15 • calculate Q and predict the direction of a reaction by relating Q to Keq • calculate equilibrium concentrations of reactants and/or products when given Keq • apply Le Châtelier’s Principle to predict responses of the equilibrium system to changes in reactant or product concentrations, changes in pressure or volume, changes in temperature • describe and explain the effects of catalysts on equilibrium Equilibrium © 2009, Prentice-Hall, Inc. The Concept of Equilibrium Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate. Equilibrium © 2009, Prentice-Hall, Inc. The Concept of Equilibrium • As a system approaches equilibrium, both the forward and reverse reactions are occurring. • At equilibrium, the forward and reverse reactions are proceeding at the same rate. Equilibrium © 2009, Prentice-Hall, Inc. A System at Equilibrium Once equilibrium is achieved, the amount of each reactant and product remains constant. Equilibrium © 2009, Prentice-Hall, Inc. Depicting Equilibrium Since, in a system at equilibrium, both the forward and reverse reactions are being carried out, we write its equation with a double arrow. N2O4 (g) 2 NO2 (g) Equilibrium © 2009, Prentice-Hall, Inc. Dynamic Equilibrium • A dynamic equilibrium exists when the rates of the forward and reverse reactions are equal. • There is no further change in [reactant] or [product]. • How fast you get to equilibrium depends on kinetics. Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant • Forward reaction: N2O4 (g) 2 NO2 (g) • Rate Law: Rate = kf [N2O4] Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant • Reverse reaction: 2 NO2 (g) N2O4 (g) • Rate Law: Rate = kr [NO2]2 Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant • Therefore, at equilibrium Ratef = Rater kf [N2O4] = kr [NO2]2 • Rewriting this, it becomes kf [NO2]2 = kr [N2O4] Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant The ratio of the rate constants is a constant at that temperature, and the expression becomes kf Keq = kr [NO2]2 = [N2O4] Equilibrium © 2009, Prentice-Hall, Inc. Summary 1. At equilibrium, the concentrations of reactants and products no longer change with time. 2. For equilibrium to occur, neither reactants nor products can escape from the system. 3. At equilibrium a particular ratio of concentration terms equals a constant. Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant • Consider the generalized reaction aA + bB cC + dD • The equilibrium expression for this reaction would be [C]c[D]d Kc = [A]a[B]b Equilibrium © 2009, Prentice-Hall, Inc. The Equilibrium Constant Since pressure is proportional to concentration for gases in a closed system, the equilibrium expression can also be written (PCc) (PDd) Kp = (PAa) (PBb) Equilibrium © 2009, Prentice-Hall, Inc. Keq vs Kc vs Kp • Keq = the general expression for equilibrium constant expressions. • Kc = Keq for which molar concentrations were used to evaluate the constant (i.e. subscript “c” = concentration). • Kc includes – Ka (weak acids) and Kb (weak bases) (Chapter 16) – Ksp (solubility product) (Chapter 17) – Kw (water) • Kp = Keq where “p” stands for pressure Equilibrium © 2009, Prentice-Hall, Inc. Relationship Between Kc and Kp • From the Ideal Gas Law we know that PV = nRT • Rearranging it, we get n P= RT V If we express volume in liters the quantity (n/V) is equivalent to molarity. Equilibrium © 2009, Prentice-Hall, Inc. Relationship Between Kc and Kp Plugging this into the expression for Kp for each substance, the relationship between Kc and Kp becomes Kp = Kc (RT)n where n = (moles of gaseous product) - (moles of gaseous reactant) Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.1 (p. 632) Write the equilibrium expression for Keq for these three reactions: a) 2 O3(g) D 3 O2(g) b) 2 NO(g) + Cl2(g) D 2 NOCl(g) c) Ag+(aq) + 2 NH3(g) D Ag(NH3)2+(aq) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.1 Write the equilibrium expression for Keq for these three reactions: a) H2(g) + I2(g) D 2 HI(g) b) Cd2+(aq) + 4 Br-(aq) D CdBr42-(aq) Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Can Be Reached from Either Direction As you can see, the ratio of [NO2]2 to [N2O4] remains constant at this temperature no matter what the initial concentrations of NO2 and N2O4 are. Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Can Be Reached from Either Direction This is the data from the last two trials from the table on the previous slide. Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Can Be Reached from Either Direction It doesn’t matter whether we start with N2 and H2 or whether we start with NH3: we will have the same proportions of all three substances at equilibrium. Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.2 (p. 634) In the synthesis of ammonia from nitrogen and hydrogen, N2(g) + 3 H2(g) D 2 NH3(g) Kc = 9.60 at 300oC, R = 0.0821 Latm/molK Calculate Kp for this reaction at this temperature. (4.34 x 10-3) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.2 For the equilibrium 2 SO3(g) D 2 SO2(g) + O2(g), Kc is 4.08 x 10-3 at 1000 K. Calculate the value for Kp. (0.335) Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Constants and Units • Keq – described using activities rather than [ ] or P no units • Activity in an ideal mixture = [ ] relative to 1 M ([reference]) or P relative to 1 atm (reference P) Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Constants and Units e.g. 0.010 M activity = 0.010 M = 0.010 1M same number, no units e.g. Keq = (PNO2/Pref)2 (PN2O4/Pref) • Results = units cancel Molarity and pressure can be used in the same Keq expression • For pure substances (ie. solids or liquids), the [reference] = itself, 1 Equilibrium © 2009, Prentice-Hall, Inc. What Does the Value of K Mean? • If K>>1, the reaction is product-favored; product predominates at equilibrium. Equilibrium © 2009, Prentice-Hall, Inc. What Does the Value of K Mean? • If K>>1, the reaction is product-favored; product predominates at equilibrium. • If K<<1, the reaction is reactant-favored; reactant predominates at equilibrium. Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.3 The following diagrams represent three different systems at equilibrium, all in the same size containers. a) Without doing any calculations, rank the three systems in order of increasing equilibrium constant, Kc. b) If the volume of the containers is 1.0 L and each sphere represents 0.10 mol, calculate Kc for each system. Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.3 The equilibrium constant for the reaction H2(g) + I2(g) D 2 HI(g) varies with temperature as follows: Kp = 792 at 298 K; Kp = 54 at 700 K. Is the formation of HI favored more at the higher or lower temperature? Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.4 (p. 637) The equilibrium constant for the reaction of N2 with O2 to form NO equals Kc = 1 x 10-30 at 25oC. N2(g) + O2(g) D 2 NO(g) Using this information, write the equilibrium constant expression and calculate the equilibrium constant for the following reaction: 2 NO(g) D N2(g) + O2(g) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.4 For the formation of NH3 from N2 and H2, N2(g) + H2(g) D 2 NH3(g), Kp = 4.34 x 10-3 at 300oC. What is the value of Kp for the reverse reaction? (2.30 x 102) Equilibrium © 2009, Prentice-Hall, Inc. Overview of Equilibrium – Chapter 15 Where we have been: • write Keq expression, in units of pressure or concentration • assess the Keq with regard to relative concentrations of reactants and products Equilibrium © 2009, Prentice-Hall, Inc. Overview of Equilibrium – Chapter 15 Where we are going: • manipulate chemical equations and Keq – reciprocal, multiplication, application of Hess’s Law • heterogeneous equilibria – what is included in Keq? • calculate Keq, including use of RICE tables • calculate Q and predict the direction of a reaction by relating Q to Keq • calculate equilibrium concentrations of reactants and/or products when given Keq Equilibrium © 2009, Prentice-Hall, Inc. Overview of Equilibrium – Chapter 15 • apply Le Châtelier’s Principle to predict responses of the equilibrium system to changes in reactant or product concentrations, changes in pressure or volume, changes in temperature • describe and explain the effects of catalysts on equilibrium Equilibrium © 2009, Prentice-Hall, Inc. Manipulating Equilibrium Constants 1. The equilibrium constant of a reaction in the reverse reaction is the reciprocal of the equilibrium constant of the forward reaction. 2. The equilibrium constant of a reaction that has been multiplied by a number is the equilibrium constant raised to a power that is equal to that number. 3. The equilibrium constant for a net reaction made up of two or more steps is the product of the equilibrium constants for the individual steps. Equilibrium © 2009, Prentice-Hall, Inc. Manipulating Equilibrium Constants 1. The equilibrium constant of a reaction in the reverse reaction is the reciprocal of the equilibrium constant of the forward reaction. In the opposite direction: N2O4 (g) D 2 NO2 (g) 2 NO2 (g) D N2O4 (g) Keq = [NO2]2 = 0.212 [N2O4] Keq = [N2O4] = 4.72 [NO2]2 Keq for these reactions are reciprocals of each other. Equilibrium © 2009, Prentice-Hall, Inc. Manipulating Equilibrium Constants 2. The equilibrium constant of a reaction that has been multiplied by a number is the equilibrium constant raised to a power that is equal to that number. N2O4(g) D 2 NO2(g) Kc = [NO2]2 = 0.212 [N2O4] 2 N2O4(g) D 4 NO2(g) Kc = [NO2]4 = (0.212)2 [N2O4]2 Equilibrium © 2009, Prentice-Hall, Inc. Manipulating Equilibrium Constants 3. The equilibrium constant for a net reaction made up of two or more steps is the product of the equilibrium constants for the individual steps. Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.5 (p. 638) Given the following information, HF(aq) D H+(aq) + F-(aq) H2C2O4(aq) D 2 H+(aq) + C2O42-(aq) Kc = 6.8 x 10-4 Kc = 3.8 x 10-6 determine the value of Kc for the following reaction: 2 HF(aq) + C2O42-(aq) D 2 F-(aq) + H2C2O4(aq) (0.12) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.5 Given the following information at 700 K, H2(g) + I2(g) D 2HI(g) Kp = 54.0 N2(g) + 3 H2(g) D 2 NH3(g) Kp = 1.04 x 10-4 determine the value of Kp (at 700 K) 2 NH3(g) + 3 I2(g) D 6 HI(g) + N2(g) (1.51 x 109) Equilibrium © 2009, Prentice-Hall, Inc. Heterogeneous Equilibrium Equilibrium © 2009, Prentice-Hall, Inc. Heterogeneous Equilibria • Equilibria in which all reactants and products are present in the same phase are called homogeneous equilibria. • Equilibria in which one or more reactants or products are present in a different phase are called heterogeneous equilibria. Equilibrium © 2009, Prentice-Hall, Inc. The Concentrations of Solids and Liquids Are Essentially Constant Both can be obtained by multiplying the density of the substance by its molar mass — and both of these are constants at constant temperature. Equilibrium © 2009, Prentice-Hall, Inc. The Concentrations of Solids and Liquids Are Essentially Constant Therefore, the concentrations of solids and liquids do not appear in the equilibrium expression. PbCl2(s) D Pb2+(aq) + 2 Cl-(aq) Kc = [Pb2+] [Cl-]2 Equilibrium © 2009, Prentice-Hall, Inc. CaCO3(s) D CaO(s) + CO2(g) As long as some CaCO3 or CaO remain in the system, the amount of CO2 above the solid will remain the same. Equilibrium © 2009, Prentice-Hall, Inc. Heterogeneous Equilibria Systems where the solvent is involved as a reactant or product and the solutes are present at low concentrations. e.g. dissociation of a weak acid H2O(l) + CO32–(aq) D OH–(aq) + HCO3–(aq) Kc = [OH–][HCO3–] / [CO32–] Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.6 (p. 640) Write the equilibrium-constant Kc for each of the following reactions: a) CO2(g) + H2(g) D CO(g) + H2O(l) Kc = b) SnO2(s) + 2 CO(g) D Sn(s) + 2 CO2(g) Kc = Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.6 Write the equilibrium-constant expressions for each of the following reactions: a) Cr(s) + 3 Ag+(aq) D Cr3+(aq) + 3 Ag(s) Kc = b) 3 Fe(s) + 4 H2O(g) D Fe3O4(s) + 4 H2(g) Kp = Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.7 (p. 641) Each of the following mixtures was placed in a closed container and allowed to stand. Which of these mixtures is capable of attaining the equilibrium CaCO3(s) D CaO(s) + CO2(g) a) pure CaCO3 b) CaO and a pressure of CO2 > Kp c) some CaCO3 and a pressure of CO2 > Kp d) CaCO3 and CaO Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.7 When added to Fe3O4(s) in a closed container, which one of the following substances – H2(g), H2O(g), O2(g) - will allow equilibrium to be established in the reaction 3 Fe(s) + 4 H2O(g) D Fe3O4(s) + 4 H2(g) Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Calculations Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.8 (p. 642) A mixture of hydrogen and nitrogen in a reaction vessel is allowed to attain equilibrium at 472oC. The equilibrium mixture of gases was analyzed and found to contain 7.38 atm H2, 2.46 atm N2, and 0.166 atm NH3. From these data calculate the equilibrium constant, Kp, for N2(g) + 3 H2(g) D 2 NH3(g) (2.79 x 10-5) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.8 An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25oC: [HC2H3O2] = 1.65 x 10-2 M; [H+] = 5.44 x 10-4 M; and [C2H3O2-] = 5.44 x 10-4 M. Calculate the equilibrium constant, Kc, for the ionization of acetic acid at 25oC. The reaction is HC2H3O2(aq) D H+(aq) + C2H3O2-(aq) (1.79 x 10-5) Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium Calculations 1. Tabulate all the known initial and equilibrium concentrations of the species that appear in the equilibrium-constant expression. 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiometry of the reaction (that is, use the coefficients in the balanced chemical equation) to calculate the changes in concentration of all the other species in the equilibrium. 4. From the initial concentrations and the changes in concentration, calculate the equilibrium concentrations. These are then used to evaluate the equilibrium constant. Equilibrium © 2009, Prentice-Hall, Inc. An Equilibrium Problem (Sample Exercise 15.9, p. 643) A closed system initially containing 1.000 x 10-3 M H2 and 2.000 x 10-3 M I2 at 448 C is allowed to reach equilibrium. Analysis of the equilibrium mixture shows that the concentration of HI is 1.87 x 10-3 M. Calculate Kc at 448 C for the reaction taking place, which is H2(g) + I2(g) D 2 HI(g) Equilibrium © 2009, Prentice-Hall, Inc. What Do We Know? Reaction: Initially H2(g) + I2(g) D 2 HI(g) [H2], M [I2], M [HI], M 1.000 x 10-3 2.000 x 10-3 0 Change At equilibrium 1.87 x 10-3 Equilibrium © 2009, Prentice-Hall, Inc. [HI] Increases by 1.87 x 10-3 M Reaction: Initially H2(g) + I2(g) D 2 HI(g) [H2], M [I2], M [HI], M 1.000 x 10-3 2.000 x 10-3 0 Change +1.87 x 10-3 At equilibrium 1.87 x 10-3 Equilibrium © 2009, Prentice-Hall, Inc. Stoichiometry tells us [H2] and [I2] decrease by half as much. Reaction: H2(g) + I2(g) D 2 HI(g) [H2], M [I2], M [HI], M Initially 1.000 x 10-3 2.000 x 10-3 0 Change -9.35 x 10-4 -9.35 x 10-4 +1.87 x 10-3 At equilibrium 1.87 x 10-3 Equilibrium © 2009, Prentice-Hall, Inc. We can now calculate the equilibrium concentrations of all three compounds… Reaction: H2(g) + I2(g) D 2 HI(g) [H2], M [I2], M [HI], M Initially 1.000 x 10-3 2.000 x 10-3 0 Change -9.35 x 10-4 -9.35 x 10-4 +1.87 x 10-3 6.5 x 10-5 1.065 x 10-3 1.87 x 10-3 At equilibrium Equilibrium © 2009, Prentice-Hall, Inc. …and, therefore, the equilibrium constant. [HI]2 Kc = [H2] [I2] = (1.87 x 10-3)2 (6.5 x 10-5)(1.065 x 10-3) = 51 Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.9 Sulfur trioxide decomposes at high temperature in a sealed container: 2 SO3(g) D 2 SO2(g) + O2(g) Initially the vessel is charged at 1000 K with SO3(g) at a partial pressure of 0.500 atm. At equilibrium the SO3 partial pressure is 0.200 atm. Calculate the value of Kp at 1000 K. (0.338) Equilibrium © 2009, Prentice-Hall, Inc. The Reaction Quotient (Q) • Q gives the same ratio the equilibrium expression gives, but for a system that is not at equilibrium. • To calculate Q, one substitutes the initial concentrations of reactants and products into the equilibrium expression. C D Q a b A B c d Equilibrium © 2009, Prentice-Hall, Inc. The Reaction Quotient • Note: Q = Keq only at equilibrium. • If Q < Keq then the forward reaction must occur to reach equilibrium. • If Q > Keq then the reverse reaction must occur to reach equilibrium. Products are consumed, reactants are formed. Q decreases until it equals Keq. Equilibrium © 2009, Prentice-Hall, Inc. If Q = K, the system is at equilibrium. Equilibrium © 2009, Prentice-Hall, Inc. If Q > K, there is too much product, and the equilibrium shifts to the left. Equilibrium © 2009, Prentice-Hall, Inc. If Q < K, there is too much reactant, and the equilibrium shifts to the right. Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.10 (p. 645) At 448oC the equilibrium constant, Kc, for the reaction H2(g) + I2(g) 2 HI(g) is 51. Predict how the reaction will proceed to reach equilibrium at 448oC if we start with 2.0 x 10-2 mol of HI, 1.0 x 10-2 mole of H2, and 3.0 x 10-2 mol of I2 in a 2.00-L container. (Q = 1.3, so reaction must proceed from left to right) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.10 At 1000 K the value of Kp for the reaction 2 SO3(g) D 2 SO2(g) + O2(g) is 0.338. Calculate the value for Qp, and predict the direction in which the reaction will proceed toward equilibrium if the initial partial pressures of reactants are PSO3 = 0.16 atm; PSO2 = 0.41 atm; PO2 = 2.5 atm. (Qp = 16; Qp > Kp, so reaction will proceed from right to left) Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.11 (p. 646) For the Haber process, N2(g) + 3 H2(g) D 2 NH3(g), Kp = 1.45 x 10-5 at 500oC. In an equilibrium mixture of the three gases at 500oC, the partial pressure of H2 is 0.928 atm and that of N2 is 0.432 atm. What is the partial pressure of NH3 in this equilibrium mixture? (2.24 x 10-3 atm) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.11 At 500 K the reaction PCl5(g) D PCl3(g) + Cl2(g) has Kp = 0.497. In an equilibrium mixture at 500 K, the partial pressure of PCl5 is 0.860 atm and that of PCl3 is 0.350 atm. What is the partial pressure of Cl2 in the equilibrium mixture? (1.22 atm) Equilibrium © 2009, Prentice-Hall, Inc. Warmup – Equilibrium p.661, 15.31 A mixture of 0.100 mol of NO, 0.050 mol of H2, and 0.100 mol of H2O is placed in a 1.0 L vessel at 300 K. The following equilibrium is established: 2 NO(g) + 2 H2(g) D N2(g) + 2 H2O(g) At equilibrium, there are 0.062 mol of NO. a) Calculate the equilibrium concentrations of H2, N2, and H2O. b) Calculate Keq . Equilibrium © 2009, Prentice-Hall, Inc. Warmup – Equilibrium p.661, 15.31 A mixture of 0.100 mol of NO, 0.050 mol of H2, and 0.100 mol of H2O is placed in a 1.0 L vessel at 300 K. The following equilibrium is established: 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) At equilibrium, there are 0.062 mol of NO. a) Calculate the equilibrium concentrations of H2, N2, and H2O. (0.012 mol, 0.019 mol, 0.138 mol) b) Calculate Keq . (650) Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.12 (p. 646) A 1.000-L flask is filled with 1.000 mol of H2 and 2.000 mol of I2 at 448oC. The value of the equilibrium constant, Kc, for the reaction H2(g) + I2(g) D 2 HI(g) at 448oC is 50.5. What are the partial pressures of H2, I2, and HI in the flask at equilibrium? ([H2] = 0.065 M, [I2] = 1.065 M, [HI] = 1.87 M) Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.12 For the equilibrium, PCl5(g) D PCl3(g) + Cl2(g), the equilibrium constant, Kp, has the value 0.497 at 500 K. A gas cylinder at 500 K is charged with PCl5(g) at an initial pressure of 1.66 atm. What are the equilibrium pressures of PCl5, PCl3, and Cl2 at this temperature? (PPCl5 = 0.967 atm, PPCl3 = PCl2 = 0.693 atm) Equilibrium © 2009, Prentice-Hall, Inc. Le Châtelier’s Principle Equilibrium © 2009, Prentice-Hall, Inc. Le Châtelier’s Principle “If a system at equilibrium is disturbed by a change in temperature, pressure, or the concentration of one of the components, the system will shift its equilibrium position so as to counteract the effect of the disturbance.” Equilibrium © 2009, Prentice-Hall, Inc. The Haber Process As P increases, the amount of ammonia present at equilibrium increases. As T increases, the amount of ammonia at equilibrium decreases. Equilibrium Can this be predicted? © 2009, Prentice-Hall, Inc. The Haber Process The transformation of nitrogen and hydrogen into ammonia (NH3) is of tremendous significance in agriculture, where ammonia-based fertilizers are of utmost importance. Equilibrium © 2009, Prentice-Hall, Inc. The Haber Process If H2 is added to the system, N2 will be consumed and the two reagents will form more NH3. Equilibrium © 2009, Prentice-Hall, Inc. The Haber Process This apparatus helps push the equilibrium to the right by removing the ammonia (NH3) from the system as a liquid. Equilibrium © 2009, Prentice-Hall, Inc. Effects of Volume and Pressure Changes Le Châtelier’s principle predicts that if pressure is increased, the system will shift to counteract the increase. • That is, the system shifts to remove gases and decrease pressure. • An increase in pressure favors the direction that has fewer moles of gas. Equilibrium © 2009, Prentice-Hall, Inc. Effects of Volume and Pressure Changes • In a reaction with the same number of moles of gas in the products and reactants, changing the pressure has no effect on the equilibrium. • No change will occur if we increase the total gas pressure by the addition of a gas that is not involved in the reaction. Equilibrium © 2009, Prentice-Hall, Inc. The Effect of Changes in Temperature Co(H2O)62+(aq) + 4Cl–(aq) D CoCl42–(aq) + 6H2O(l) H > 0 Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.13 (p. 653) Consider the following equilibrium: N2O4(g) D 2 NO2(g) Ho = 58.0 kJ In what direction will the equilibrium shift when each of the following changes is made to a system at equilibrium: a) add N2O4 b) remove NO2 c) increase the total pressure by adding N2(g) d) increase the volume e) decrease the temperature? Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.13 For the reaction PCl5(g) D PCl3(g) + Cl2(g) Ho = 87.9 kJ in what direction will the equilibrium shift when a) Cl2(g) is removed; b) the temperature is decreased; c) the volume of the reaction system is increased; d) PCl3(g) is added? Equilibrium © 2009, Prentice-Hall, Inc. Sample Exercise 15.14 (p. 653) a)Using the standard heat of formation data in Appendix C, determine the standard enthalpy change for the reaction N2(g) + 3 H2(g) D 2 NH3(g) 0 0 2 mol x -46.19 kJ/mol = -92.38 kJ/mol b)Determine how the equilibrium constant for this reaction should change with temperature. Equilibrium © 2009, Prentice-Hall, Inc. Equilibrium © 2009, Prentice-Hall, Inc. Practice Exercise 15.14 The enthalpy change for the reaction 2 POCl3(g) D 2 PCl3(g) + O2(g) -542.2 kJ/mol -288.07 kJ/mol = (2 mol x -288.07 kJ/mol) – (2 mol x -542.2 kJ/mol = + 508.3 kJ Use this result to determine how the equilibrium constant for the reaction should change with temperature. Equilibrium © 2009, Prentice-Hall, Inc. Catalysts Equilibrium © 2009, Prentice-Hall, Inc. Catalysts Catalysts increase the rate of both the forward and reverse reactions. Equilibrium © 2009, Prentice-Hall, Inc. Catalysts When one uses a catalyst, equilibrium is achieved faster, but the equilibrium composition remains unaltered. Equilibrium © 2009, Prentice-Hall, Inc. Sample Integrative Exercise 15 At temperatures near 800oC, steam passed over hot coke (a form of carbon obtained from coal) reacts to form CO and H2: C(s) + H2O(g) D CO(g) + H2(g) The mixture of gases that results is an important industrial fuel called water gas. a) At 800oC the equilibrium constant for this reaction is Kp = 14.1. What are the equilibrium partial pressures of H2O, CO, and H2 in the equilibrium mixture at this temperature if we start with solid carbon and 0.100 mol of H2O in a 1.00-L vessel? b) What is the minimum amount of carbon required to achieve equilibrium under these conditions? Equilibrium © 2009, Prentice-Hall, Inc. Sample Integrative Exercise 15 c) What is the total pressure in the vessel at equilibrium? d) At 25oC the value of Keq for this reaction is 1.7 x 10-21. Is the reaction exothermic or endothermic? e) To produce the maximum amount of CO and H2 at equilibrium, should the pressure of the system be increased or decreased? Equilibrium © 2009, Prentice-Hall, Inc.


















































































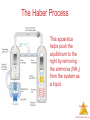























![[A, 8-9]](http://s1.studyres.com/store/data/006655537_1-7e8069f13791f08c2f696cc5adb95462-150x150.png)
