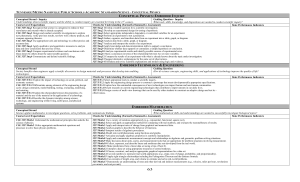
Elementary Principles of Chemical Processes, 3rd Update Edition
... 200 papers on chemical process engineering and engineering education and presented hundreds of seminars, workshops, and short courses in both categories to industrial and research institutions and universities throughout the United States and abroad. Since 1991 he has codirected the National Effecti ...
... 200 papers on chemical process engineering and engineering education and presented hundreds of seminars, workshops, and short courses in both categories to industrial and research institutions and universities throughout the United States and abroad. Since 1991 he has codirected the National Effecti ...
Version PREVIEW – Exam 3 – JOHNSON – (53140) 1 This print
... For which of the following processes does the entropy of the universe decrease? 1. None of these is correct. correct ...
... For which of the following processes does the entropy of the universe decrease? 1. None of these is correct. correct ...
pdf - arXiv
... Theory (QFT) as a consequence of the reality of the zero point energy [7]. Lifshitz extended the Casimir calculus of perfect metal plates at zero temperature to the more general case of dielectric plates at any finite temperature [15], which is one of the most famous and more used formulas of Casimi ...
... Theory (QFT) as a consequence of the reality of the zero point energy [7]. Lifshitz extended the Casimir calculus of perfect metal plates at zero temperature to the more general case of dielectric plates at any finite temperature [15], which is one of the most famous and more used formulas of Casimi ...
Unit 8 Chemical Equilibrium Focusing on Acid
... changes, which are balanced because they are occurring at equal rates, within a closed system. What we observe directly is the net effect—neither an increase nor a decrease in any measurable property. Chemistry involves the study of change in chemical substances. To predict and control chemical chan ...
... changes, which are balanced because they are occurring at equal rates, within a closed system. What we observe directly is the net effect—neither an increase nor a decrease in any measurable property. Chemistry involves the study of change in chemical substances. To predict and control chemical chan ...
David - Collegiate Quiz Bowl Packet
... Questions by David Farris and editors—round 1 1. They are bounded by Seifert (pron. SEE-fert) surfaces, and different projections of one are related by Reidemeister (REE-de-my-ster) moves. Their invariants include the fundamental group of their complements and the HOMFLY, Alexander and Jones polynom ...
... Questions by David Farris and editors—round 1 1. They are bounded by Seifert (pron. SEE-fert) surfaces, and different projections of one are related by Reidemeister (REE-de-my-ster) moves. Their invariants include the fundamental group of their complements and the HOMFLY, Alexander and Jones polynom ...
CHAPTER 16 ACID-BASE EQUILIBRIA AND SOLUBILITY
... Buffer (a) will be a more effective buffer because the concentrations of acid and base components are ten times higher than those in (b). Thus, buffer (a) can neutralize 10 times more added acid or base compared to buffer (b). ...
... Buffer (a) will be a more effective buffer because the concentrations of acid and base components are ten times higher than those in (b). Thus, buffer (a) can neutralize 10 times more added acid or base compared to buffer (b). ...
Go FIGure
... athletic injuries (▶ Figure 13.5). The packs consist of a pouch of water and the solid salt sealed off from the water—MgSO41s2 for hot packs and NH4NO31s2 for cold packs. When the pack is squeezed, the seal separating the solid from the water is broken and a solution forms, either increasing or decr ...
... athletic injuries (▶ Figure 13.5). The packs consist of a pouch of water and the solid salt sealed off from the water—MgSO41s2 for hot packs and NH4NO31s2 for cold packs. When the pack is squeezed, the seal separating the solid from the water is broken and a solution forms, either increasing or decr ...
Energetics
... • H is more easily measured than H as most reactions happen in open vessels. i.e. at constant pressure. • The absolute values of H and H cannot ...
... • H is more easily measured than H as most reactions happen in open vessels. i.e. at constant pressure. • The absolute values of H and H cannot ...
Using cryo-electron microscopy to determine thermodynamic and
... can be used to directly view the different microstructures of most self-assembled surfactant systems to resolutions of about 2 nm. There have been several recent reviews showing the ability of cryo-TEM to image different amphiphilic systems [16 –18]. However, the additional step of quantifying the i ...
... can be used to directly view the different microstructures of most self-assembled surfactant systems to resolutions of about 2 nm. There have been several recent reviews showing the ability of cryo-TEM to image different amphiphilic systems [16 –18]. However, the additional step of quantifying the i ...
Solutions - ChemConnections
... Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of H ...
... Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of H ...
Chemistry - A Quantitative Science
... They also need to know how much energy is required or how much heat is generated by a reaction. They must also understand how the reaction occurs so that they can optimize the reaction conditions. These are the types of problems addressed in this text. We begin our study of the quantitative aspects ...
... They also need to know how much energy is required or how much heat is generated by a reaction. They must also understand how the reaction occurs so that they can optimize the reaction conditions. These are the types of problems addressed in this text. We begin our study of the quantitative aspects ...
Determination of Equilibrium Constants for Reactions between Nitric
... KI solution with ultraviolet light or activated carbon.12−14 Also, a comparative study of three absorbents by Yu et al.15 showed that ammoniacal cobalt(II) complexes had advantages over the two compared absorbents when simulated flue gas was treated with about 5% oxygen. Moreover, the main byproducts ...
... KI solution with ultraviolet light or activated carbon.12−14 Also, a comparative study of three absorbents by Yu et al.15 showed that ammoniacal cobalt(II) complexes had advantages over the two compared absorbents when simulated flue gas was treated with about 5% oxygen. Moreover, the main byproducts ...
File
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
CHAPTER 6 ENERGY RELATIONSHIPS IN CHEMICAL REACTIONS
... The system is the specific part of the universe that is of interest to us. The surroundings are the rest of the universe outside the system. An open system can exchange mass and energy, usually in the form of heat with its surroundings. A closed system allows the transfer of energy (heat) but not ma ...
... The system is the specific part of the universe that is of interest to us. The surroundings are the rest of the universe outside the system. An open system can exchange mass and energy, usually in the form of heat with its surroundings. A closed system allows the transfer of energy (heat) but not ma ...
X: Ag, Ca, In, Li, Na, Sn, Sr and Zn
... additions of Li, Na, Ca, Zn, Ag, In, Sr, and Sn can improve the mechanical properties of Mgbased alloys, by forming secondary precipitates in the Mg matrix. In developing new magnesium alloys, it is important to understand their constitution (microstructure) and thermodynamic behaviour. Obtaining su ...
... additions of Li, Na, Ca, Zn, Ag, In, Sr, and Sn can improve the mechanical properties of Mgbased alloys, by forming secondary precipitates in the Mg matrix. In developing new magnesium alloys, it is important to understand their constitution (microstructure) and thermodynamic behaviour. Obtaining su ...
chapter 5 gases
... The system is the specific part of the universe that is of interest to us. The surroundings are the rest of the universe outside the system. An open system can exchange mass and energy, usually in the form of heat with its surroundings. A closed system allows the transfer of energy (heat) but not ma ...
... The system is the specific part of the universe that is of interest to us. The surroundings are the rest of the universe outside the system. An open system can exchange mass and energy, usually in the form of heat with its surroundings. A closed system allows the transfer of energy (heat) but not ma ...
TR-00-13 - Svensk Kärnbränslehantering AB
... mainly as CuCO3(aq) but also as Cu(CO3)22−. The pH-dependence of the carbonate ion is such that the stabilisation is stronger at the higher pH of the bulk than at a lower pH of a corrosion pit. The acid-base couple H2CO3(aq)-HCO3− is a major pH-regulating system in many natural waters as well as in ...
... mainly as CuCO3(aq) but also as Cu(CO3)22−. The pH-dependence of the carbonate ion is such that the stabilisation is stronger at the higher pH of the bulk than at a lower pH of a corrosion pit. The acid-base couple H2CO3(aq)-HCO3− is a major pH-regulating system in many natural waters as well as in ...
Determination of Equilibrium Constants for the
... at the B3LYP/6-311++G(3df,3pd) level. These complexes are similar to the well-known adducts between HO2 and other atmospheric species such as H2O. Hermans et al.7,17 also performed approximated G2Mc// B3LYP/cc-pVTZ calculations on the reactions of HO2 with these carbonyls, but they went one step fur ...
... at the B3LYP/6-311++G(3df,3pd) level. These complexes are similar to the well-known adducts between HO2 and other atmospheric species such as H2O. Hermans et al.7,17 also performed approximated G2Mc// B3LYP/cc-pVTZ calculations on the reactions of HO2 with these carbonyls, but they went one step fur ...