Chapter 19 Homework Problems Answers
... The slower that the energy extraction is performed, the greater is the total amount of energy that can be obtained. This is the same as saying that the most energy is available from a process that occurs reversibly. ...
... The slower that the energy extraction is performed, the greater is the total amount of energy that can be obtained. This is the same as saying that the most energy is available from a process that occurs reversibly. ...
IIT-JEE (Advanced) - Brilliant Public School Sitamarhi
... Limiting Reagent : It is very important concept in chemical calculation. It refers to reactant ...
... Limiting Reagent : It is very important concept in chemical calculation. It refers to reactant ...
Section – B - About iTutoring
... (2). What is called chemical equilibrium? The equilibrium established in chemical reactions is called chemical equilibrium. (3). How can be said that equilibrium is dynamic in nature? The equilibrium is dynamic and not steady as the forward and the reverse reactions occur with the same velocity at t ...
... (2). What is called chemical equilibrium? The equilibrium established in chemical reactions is called chemical equilibrium. (3). How can be said that equilibrium is dynamic in nature? The equilibrium is dynamic and not steady as the forward and the reverse reactions occur with the same velocity at t ...
SQA Advanced Higher Chemistry Unit 2 Principles of Chemical
... Which of the following statements applies to this equation? 1. Calcium carbonate reacts with hydrochloric acid to produce calcium chloride solution, water and carbon dioxide. 2. One formula unit of calcium carbonate reacts with two formula units of hydrochloric acid to produce one formula unit each ...
... Which of the following statements applies to this equation? 1. Calcium carbonate reacts with hydrochloric acid to produce calcium chloride solution, water and carbon dioxide. 2. One formula unit of calcium carbonate reacts with two formula units of hydrochloric acid to produce one formula unit each ...
Physics Test with ans.
... 70. An insulated container, filled with water, contains a thermometer and a paddle wheel. The paddle wheel can be rotated by an external source. This apparatus can be used to determine: A. specific heat of water B. relation between kinetic energy and absolute temperature C. thermal conductivity of w ...
... 70. An insulated container, filled with water, contains a thermometer and a paddle wheel. The paddle wheel can be rotated by an external source. This apparatus can be used to determine: A. specific heat of water B. relation between kinetic energy and absolute temperature C. thermal conductivity of w ...
Phase behavior of clathrate hydrates: a model for single and
... reference temperature and pressure (T0 ; P0 ), usually taken to be the freezing point of water and zero pressure. The other terms in Eq. (5) account for temperature and pressure e)ects on the chemical potential and for the activity of water. In general, the reference chemical potential of water is a ...
... reference temperature and pressure (T0 ; P0 ), usually taken to be the freezing point of water and zero pressure. The other terms in Eq. (5) account for temperature and pressure e)ects on the chemical potential and for the activity of water. In general, the reference chemical potential of water is a ...
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
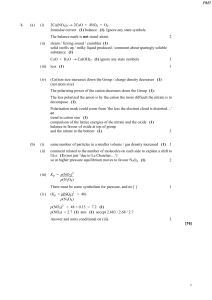
... Kp = pSO32/ pSO22 × pO2 (= 3.00 × 104) (1) 3.00 × 104 = pSO32 / 0.1 × 0.1 × 0.5 (1) if no expression for Kp is given this correct substitution can score 2 marks pSO32 = 150 pSO3 = 12.25 (1) Ratio of SO3 = ...
... Kp = pSO32/ pSO22 × pO2 (= 3.00 × 104) (1) 3.00 × 104 = pSO32 / 0.1 × 0.1 × 0.5 (1) if no expression for Kp is given this correct substitution can score 2 marks pSO32 = 150 pSO3 = 12.25 (1) Ratio of SO3 = ...
EIT Review S2012 Part 2 Dr. J. Mack CSUS Department of Chemistry
... • Notice that the basic form of the equilibrium constant expression is the same as for Kc. In some cases, the numerical values of Kc and Kp are the same. They are different when the numbers of moles of gaseous reactants and products are different. EIT (2) ...
... • Notice that the basic form of the equilibrium constant expression is the same as for Kc. In some cases, the numerical values of Kc and Kp are the same. They are different when the numbers of moles of gaseous reactants and products are different. EIT (2) ...
Thermodynamics and Phase Diagrams
... loosely, t is the number of possible equivalent microstates in a macrostate, that is the number of quantum states for the system which are accessible under the applicable conditions of energy, volume, etc. For example, for a system which can be described by a set of single-particle energy levels, t ...
... loosely, t is the number of possible equivalent microstates in a macrostate, that is the number of quantum states for the system which are accessible under the applicable conditions of energy, volume, etc. For example, for a system which can be described by a set of single-particle energy levels, t ...
From Kinetics to Equilibrium
... reaction rate or rate of reaction. How do chemists express reaction rates? Consider how the rates of other processes are expressed. For example, the Olympic sprinter in Figure 12.1 can run 100 m in about 10 s, resulting in an average running rate of 100 m/10 s or about 10 m/s. The running rate of a ...
... reaction rate or rate of reaction. How do chemists express reaction rates? Consider how the rates of other processes are expressed. For example, the Olympic sprinter in Figure 12.1 can run 100 m in about 10 s, resulting in an average running rate of 100 m/10 s or about 10 m/s. The running rate of a ...
View/Open
... The magnitudes of changes observed under the two conditions are different. ∆E) is the heat change accompanying a chemical reaction at The change in internal energy (∆ constant volume because no external work is performed. However at constant pressure not only does the change in internal energy take ...
... The magnitudes of changes observed under the two conditions are different. ∆E) is the heat change accompanying a chemical reaction at The change in internal energy (∆ constant volume because no external work is performed. However at constant pressure not only does the change in internal energy take ...
x - mrs. leinweber`s wiki
... 1. initial equilibrium state 2. shifting non-equilibrium state 3. new equilibrium state ...
... 1. initial equilibrium state 2. shifting non-equilibrium state 3. new equilibrium state ...