Thermodynamics of Ion Association in the Saturated Solution of
... In turn, most of the theories that have been used to predict the extend of solubility of an ionic compound in a given solvent or in a mixed solvent and related ion association are based on changes in the electrostatic properties of the solvent, solute and ion solvation as well as on the ionic streng ...
... In turn, most of the theories that have been used to predict the extend of solubility of an ionic compound in a given solvent or in a mixed solvent and related ion association are based on changes in the electrostatic properties of the solvent, solute and ion solvation as well as on the ionic streng ...
Entropy and Free Energy
... the entire molecule moves through space [9 Section 3.1]; rotational energy, in which the molecule spins about an axis running through its center of mass; and vibrational energy, in which atoms of a molecule move relative to one another. ...
... the entire molecule moves through space [9 Section 3.1]; rotational energy, in which the molecule spins about an axis running through its center of mass; and vibrational energy, in which atoms of a molecule move relative to one another. ...
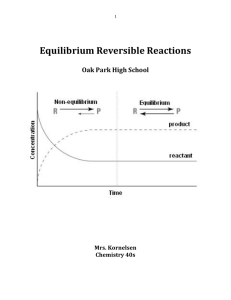
Equilibrium Reversible Reactions
... For example, in a class of 20 students, 10 students could represent sodium ions and 10 students could represent chloride ions. Have 4 sodium ions and 4 chloride ions link arms on the left side of the room to represent sodium chloride particles. Have the remaining 12 students stand on the right side ...
... For example, in a class of 20 students, 10 students could represent sodium ions and 10 students could represent chloride ions. Have 4 sodium ions and 4 chloride ions link arms on the left side of the room to represent sodium chloride particles. Have the remaining 12 students stand on the right side ...
PDF file - Comp Chem - University of Minnesota Twin Cities
... distance from the centre of mass for three nanoparticles. Nevertheless, by using the appropriate variable, one can still make useful analyses and predictions, but there are dramatic surprises in store if one is only accustomed to bulk thermodynamics.9,10 For example, not only does the condition of c ...
... distance from the centre of mass for three nanoparticles. Nevertheless, by using the appropriate variable, one can still make useful analyses and predictions, but there are dramatic surprises in store if one is only accustomed to bulk thermodynamics.9,10 For example, not only does the condition of c ...
Chemical thermodynamics - Mahesh Tutorials Science
... The first law talks about the conservation of energy in a process but does not speak of the feasibility of a process. It does not tell whether a process will happen on its own i.e. whether the process is spontaneous or not. A spontaneous process is one which happens on its own. Example, heat always ...
... The first law talks about the conservation of energy in a process but does not speak of the feasibility of a process. It does not tell whether a process will happen on its own i.e. whether the process is spontaneous or not. A spontaneous process is one which happens on its own. Example, heat always ...
Document
... (a) Each box contains 10 spheres. The amount of product in each varies as follows: (i) 6, (ii) 1, (iii) 8. Thus, the equilibrium constant varies in the order (ii) < (i) < (iii). (b) In (i) we have 0.60 mol/L product and 0.40 mol/L reactant, giving Kc = 0.60/0.40 = 1.5. (You will get the same result ...
... (a) Each box contains 10 spheres. The amount of product in each varies as follows: (i) 6, (ii) 1, (iii) 8. Thus, the equilibrium constant varies in the order (ii) < (i) < (iii). (b) In (i) we have 0.60 mol/L product and 0.40 mol/L reactant, giving Kc = 0.60/0.40 = 1.5. (You will get the same result ...
Thermochemistry
... Over 90% of the energy we use comes originally from the sun. Every day, the sun provides the earth with almost 10,000 times the amount of energy necessary to meet all of the world’s energy needs for that day. Our challenge is to find ways to convert and store incoming solar energy so that it can be ...
... Over 90% of the energy we use comes originally from the sun. Every day, the sun provides the earth with almost 10,000 times the amount of energy necessary to meet all of the world’s energy needs for that day. Our challenge is to find ways to convert and store incoming solar energy so that it can be ...
Chapter 1: Matter and Measurement
... Heats of Reaction and Calorimetry Work The First Law of Thermodynamics Heats of Reaction: U and H The Indirect Determination of H: Hess’s Law Standard Enthalpies of Formation Fuels as Sources of Energy Focus On Fats, Carbohydrates and Energy Storage ...
... Heats of Reaction and Calorimetry Work The First Law of Thermodynamics Heats of Reaction: U and H The Indirect Determination of H: Hess’s Law Standard Enthalpies of Formation Fuels as Sources of Energy Focus On Fats, Carbohydrates and Energy Storage ...
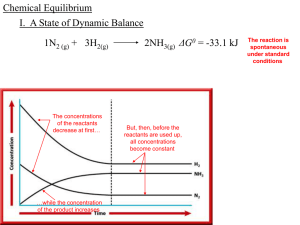
Equilibrium
... the equilibrium constant has the same value regardless of the initial amounts of each reaction component. It does, however, depend on the temperature of the reaction. Equilibrium is defined as a condition in which the rates of the forward and reverse reactions are equal. A change in temperature will ...
... the equilibrium constant has the same value regardless of the initial amounts of each reaction component. It does, however, depend on the temperature of the reaction. Equilibrium is defined as a condition in which the rates of the forward and reverse reactions are equal. A change in temperature will ...