3 CO 2 (g) + 4 H 2 O(l)
... Therefore the overall enthalpy change is the same, independent of the route we took to get there. Therefore, as a consequence of enthalpy being a state property, the difference in enthalpy between two systems under a given set of conditions is constant. As far as chemists are concerned there are thr ...
... Therefore the overall enthalpy change is the same, independent of the route we took to get there. Therefore, as a consequence of enthalpy being a state property, the difference in enthalpy between two systems under a given set of conditions is constant. As far as chemists are concerned there are thr ...
24. The following reaction is at equilibrium
... (B) If Q = K there is no change. (C) If Q > K, the reaction goes to the left. (D) The system will never come to equilibrium. (E) If Q < K for a particular reaction, the final equilibrium mixture will have more reactants than the original mixture. 23. Syngas, a mixture of CO and H2 gases, is very ind ...
... (B) If Q = K there is no change. (C) If Q > K, the reaction goes to the left. (D) The system will never come to equilibrium. (E) If Q < K for a particular reaction, the final equilibrium mixture will have more reactants than the original mixture. 23. Syngas, a mixture of CO and H2 gases, is very ind ...
module-iv --- combustion thermodynamic applied termodynamics
... exothermic. If energy is consumed during a reaction, H will have a positive value; the reaction is said to be endothermic. The enthalpy change for a chemical change is independent of the method or path by which the change is carried out as long as the initial and final substances are brought to the ...
... exothermic. If energy is consumed during a reaction, H will have a positive value; the reaction is said to be endothermic. The enthalpy change for a chemical change is independent of the method or path by which the change is carried out as long as the initial and final substances are brought to the ...
The First Law of Thermodynamics Does Not Predict Spontaneous
... One Ne atom. At a given instant, an Ne atom in the left flask has its energy in one of some number (W ) of microstates. Opening the stopcock increases the volume, which increases the number of possible locations and the number of translational energy level. Thus, the system has 21, or 2, times as ...
... One Ne atom. At a given instant, an Ne atom in the left flask has its energy in one of some number (W ) of microstates. Opening the stopcock increases the volume, which increases the number of possible locations and the number of translational energy level. Thus, the system has 21, or 2, times as ...
Pdf - Text of NPTEL IIT Video Lectures
... Chemical potential can be defined either as rate of change of internal energy with respect to the number of moles of that component, when entropy, volume and composition of the other components remains same; or it can be defined as, the rate of change of enthalpy with respect to the number of moles ...
... Chemical potential can be defined either as rate of change of internal energy with respect to the number of moles of that component, when entropy, volume and composition of the other components remains same; or it can be defined as, the rate of change of enthalpy with respect to the number of moles ...
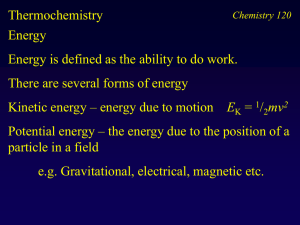
Thermochemistry (Ch 8)
... compound is converted from a solid to a liquid without a change in temperature. “Latent” means hidden; the heat absorbed/released during a phase change does not cause the temperature to change. Note: Hfus for water is _____________________________ LATENT HEAT OF VAPORIZATION, Hvap - the enthalpy c ...
... compound is converted from a solid to a liquid without a change in temperature. “Latent” means hidden; the heat absorbed/released during a phase change does not cause the temperature to change. Note: Hfus for water is _____________________________ LATENT HEAT OF VAPORIZATION, Hvap - the enthalpy c ...
Solidification in heat packs: I. Nucleation rate
... The expression for the number of incipient nuclei in Eq. 5 requires the calculation of the free energy of forming one incipient nucleus, ⌬⍀ nU . We can represent the energy of forming a cluster of n molecules, ⌬⍀ n , as a sum of three contributions, from volume effects ⌬G® Žmelting., surface effects ...
... The expression for the number of incipient nuclei in Eq. 5 requires the calculation of the free energy of forming one incipient nucleus, ⌬⍀ nU . We can represent the energy of forming a cluster of n molecules, ⌬⍀ n , as a sum of three contributions, from volume effects ⌬G® Žmelting., surface effects ...
Solution-Solubility-Equilibrium
... The rule like dissolves like where like refers to similarities in polarities of substances, has useful application for predicting solubilities. (Polarity is discussed in the Chemical Bonding Unit) In general, polar and ionic solutes tend to be more soluble in polar solvents and non polar solutes ten ...
... The rule like dissolves like where like refers to similarities in polarities of substances, has useful application for predicting solubilities. (Polarity is discussed in the Chemical Bonding Unit) In general, polar and ionic solutes tend to be more soluble in polar solvents and non polar solutes ten ...
Physical Science 2014 - SC3208 IC Scope and Sequence
... Measure distance and time to determine speed. Introduction to Forces Describe the concept of force. Distinguish between balanced and unbalanced forces and their effect on motion. Explain how to determine the net force on an object. Friction Describe friction and explain what causes it to occur. Expl ...
... Measure distance and time to determine speed. Introduction to Forces Describe the concept of force. Distinguish between balanced and unbalanced forces and their effect on motion. Explain how to determine the net force on an object. Friction Describe friction and explain what causes it to occur. Expl ...
Thermodynamics of Electric and Magnetic Systems
... The systems which contain electric or magnetic media have besides the electric (respectively magnetic) properties also the common properties of a thermodynamic systems (that is thermal, volumic, chemical); moreover, there are correlations between the electric (magnetic) properties and the thermal or ...
... The systems which contain electric or magnetic media have besides the electric (respectively magnetic) properties also the common properties of a thermodynamic systems (that is thermal, volumic, chemical); moreover, there are correlations between the electric (magnetic) properties and the thermal or ...
15.0 EquilibriumIHS2014
... increasing the container volume. Then the equilibrium shifts to the left (the side with more moles of gas) • At B, the temperature is increased. Then the equilibrium shifts to left. • At C, C2H6(g) is added to the system. Then the equilibrium shifts to the left. • At D, no shift in equilibrium posit ...
... increasing the container volume. Then the equilibrium shifts to the left (the side with more moles of gas) • At B, the temperature is increased. Then the equilibrium shifts to left. • At C, C2H6(g) is added to the system. Then the equilibrium shifts to the left. • At D, no shift in equilibrium posit ...
CHAPtER 2 Energy calculations
... The amount of energy produced by a chemical reaction is directly proportional to the amount of substance initially present. If, as in the above example, twice as much reactant is used, then twice as much energy can be produced. • If a reaction is reversed, ∆H is equal to, but opposite in sign, to t ...
... The amount of energy produced by a chemical reaction is directly proportional to the amount of substance initially present. If, as in the above example, twice as much reactant is used, then twice as much energy can be produced. • If a reaction is reversed, ∆H is equal to, but opposite in sign, to t ...
Measuring Rates
... The analysis of kinetic data demands a basic understanding of the mathematical relationships between concentration and time for chemical reactions with different rate orders. It also requires that we understand how experimental data can be represented in different ways to elicit underlying trends. D ...
... The analysis of kinetic data demands a basic understanding of the mathematical relationships between concentration and time for chemical reactions with different rate orders. It also requires that we understand how experimental data can be represented in different ways to elicit underlying trends. D ...
Multiple Representations of Knowledge - AP Central
... exerts the force and the system object on which the force is exerted. For example, for the force exerted by the earth on the object, we write FE on O, where E indicates the earth and O stands for the system object. We do not use such terms as the weight of an object or the tension in a rope because ...
... exerts the force and the system object on which the force is exerted. For example, for the force exerted by the earth on the object, we write FE on O, where E indicates the earth and O stands for the system object. We do not use such terms as the weight of an object or the tension in a rope because ...
Physical Chemistry Lab Report Rubric –Veldman Fall 2012
... two sucrose combustion trials. However, this average value does not quite compare to the literature value, and the percent error equates to 18.50%. Figure 2, however, display the data as expected; initial temperature is stable, there is a steep and steady increase once the bomb is ignited, and then ...
... two sucrose combustion trials. However, this average value does not quite compare to the literature value, and the percent error equates to 18.50%. Figure 2, however, display the data as expected; initial temperature is stable, there is a steep and steady increase once the bomb is ignited, and then ...
Chapter 15a
... There are three types of problems encountered with weak acids or bases: dissociation, buffers or hydrolysis. We'll look at each type in detail. Dissociation of a Weak Acid In this type of problem, you will be asked to find the hydronium ion concentration and/or the pH of a weak acid whose initial co ...
... There are three types of problems encountered with weak acids or bases: dissociation, buffers or hydrolysis. We'll look at each type in detail. Dissociation of a Weak Acid In this type of problem, you will be asked to find the hydronium ion concentration and/or the pH of a weak acid whose initial co ...
Growing Negative Pressure in Dissolved Solutes: Raman - HAL-Insu
... which can be also called strain, is a vectorial quantity)1. One interest to study the tensile (or stretched) liquids is that they are metastable with respect to their vapor: there are superheated liquids. The extent of superheating for one liquid (extreme tensile strength) and its behavior in this p ...
... which can be also called strain, is a vectorial quantity)1. One interest to study the tensile (or stretched) liquids is that they are metastable with respect to their vapor: there are superheated liquids. The extent of superheating for one liquid (extreme tensile strength) and its behavior in this p ...