The effect of confinement on chemical reactions
... ethanol to produce ethyl acetate, CH3COOH + C2H5OH $ C2H5OOCCH3 + H2O, is a common industrial process in the synthesis of organic solvents. There is experimental evidence [16] that when this reaction is carried out in porous materials, the selectivity towards ethyl acetate depends on the adsorbent u ...
... ethanol to produce ethyl acetate, CH3COOH + C2H5OH $ C2H5OOCCH3 + H2O, is a common industrial process in the synthesis of organic solvents. There is experimental evidence [16] that when this reaction is carried out in porous materials, the selectivity towards ethyl acetate depends on the adsorbent u ...
Binnie thermochemistry
... in the bomb calorimeter is constant, what is measured is really the change in internal energy, E, not H. • For most reactions, the difference is very small. Thermochemistry ...
... in the bomb calorimeter is constant, what is measured is really the change in internal energy, E, not H. • For most reactions, the difference is very small. Thermochemistry ...
Thermochemistry
... 1.Introduction to Energy changes Energy is the capacity to do work. There are many/various forms of energy like heat, electric, mechanical, and/ or chemical energy.There are two types of energy: (i)Kinetic Energy(KE) ;the energy in motion. (ii)Potential Energy(PE); the stored/internal energy. Energ ...
... 1.Introduction to Energy changes Energy is the capacity to do work. There are many/various forms of energy like heat, electric, mechanical, and/ or chemical energy.There are two types of energy: (i)Kinetic Energy(KE) ;the energy in motion. (ii)Potential Energy(PE); the stored/internal energy. Energ ...
Test bank questions
... Which of the following is a true statement about chemical equilibria in general? A. At equilibrium the total concentration of products equals the total concentration of reactants, that is, [products] = [reactants]. B. Equilibrium is the result of the cessation of all chemical change. C. There is onl ...
... Which of the following is a true statement about chemical equilibria in general? A. At equilibrium the total concentration of products equals the total concentration of reactants, that is, [products] = [reactants]. B. Equilibrium is the result of the cessation of all chemical change. C. There is onl ...
Thermodynamic Investigation of the AINC and AICN Isomers by
... For AlCN, the theoretical values from Ma et al.1 were used: r Al-C52.014 and r C-N51.171, because Gerasimov et al.8 found that the rotational constant for the ground state of this molecule, B5(0.167460.0046) cm21, is equal to that calculated,1 B50.1672 cm21; this means that the intermolecular distan ...
... For AlCN, the theoretical values from Ma et al.1 were used: r Al-C52.014 and r C-N51.171, because Gerasimov et al.8 found that the rotational constant for the ground state of this molecule, B5(0.167460.0046) cm21, is equal to that calculated,1 B50.1672 cm21; this means that the intermolecular distan ...
Energetics - chemistryatdulwich
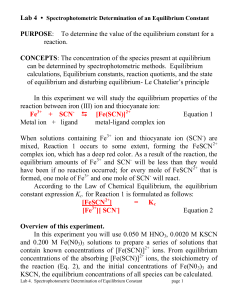
... Work, w, is difficult to measure whilst q is a lot easier as you will see later; so in order to measure E accurately it is advisable to either keep w to a minimum or get rid of it altogether by ensuring that the system cannot do any work i.e. conduct the reaction in a closed system like a bomb calo ...
... Work, w, is difficult to measure whilst q is a lot easier as you will see later; so in order to measure E accurately it is advisable to either keep w to a minimum or get rid of it altogether by ensuring that the system cannot do any work i.e. conduct the reaction in a closed system like a bomb calo ...
(a) From , 2013 General Chemistry I
... by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until its pressure has fallen to 1.20 atm. In step 2, it is heated and allowed to expand against a constant pressure of 1.20 atm until its volume is 20 ...
... by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until its pressure has fallen to 1.20 atm. In step 2, it is heated and allowed to expand against a constant pressure of 1.20 atm until its volume is 20 ...
File - Mr Weng`s IB Chemistry
... • Total energy is conserved in chemical reactions. • Chemical reactions that involve transfer of heat between the system and the surroundings are described as endothermic or exothermic. • The enthalpy change (∆H) for chemical reactions is indicated in kJ mol-1. • ∆H values are usually expressed unde ...
... • Total energy is conserved in chemical reactions. • Chemical reactions that involve transfer of heat between the system and the surroundings are described as endothermic or exothermic. • The enthalpy change (∆H) for chemical reactions is indicated in kJ mol-1. • ∆H values are usually expressed unde ...
Precipitation of salts in freezing seawater and ozone depletion
... 3 and CO3 ), within 0.1−0.2 pH-units around an average value of 8.2 at the surface. Because of the high amount of salts dissolved in it (its average salinity S (Millero et al., 2008) being 35), seawater does not entirely freeze solid when exposed to subzero temperature. The formation of ice is accom ...
... 3 and CO3 ), within 0.1−0.2 pH-units around an average value of 8.2 at the surface. Because of the high amount of salts dissolved in it (its average salinity S (Millero et al., 2008) being 35), seawater does not entirely freeze solid when exposed to subzero temperature. The formation of ice is accom ...
Physics
... state Kirchoff’s Laws; show conservation of charge in a circuit with the help of Kirchoff’s 1st law; show conservation of energy in a circuit with the help of Kirchoff’s 2nd law; define potential divider with two examples; briefly explain the construction and working of a rheostat with the help of a ...
... state Kirchoff’s Laws; show conservation of charge in a circuit with the help of Kirchoff’s 1st law; show conservation of energy in a circuit with the help of Kirchoff’s 2nd law; define potential divider with two examples; briefly explain the construction and working of a rheostat with the help of a ...