Lecture notes for Challenges in the Physics of Life and Energy, part
... industrialized nations (2) there is a zoo of units (3) energy and power are mixed up and (4) energy is very cheap. We illustrate our bad intuition by two examples. The energy to climb the Kalimanjaro is just which is for a typical person roughly 80 kg × 10 m s−2 × 5700 m = 4 6 MJ. The energy f ...
... industrialized nations (2) there is a zoo of units (3) energy and power are mixed up and (4) energy is very cheap. We illustrate our bad intuition by two examples. The energy to climb the Kalimanjaro is just which is for a typical person roughly 80 kg × 10 m s−2 × 5700 m = 4 6 MJ. The energy f ...
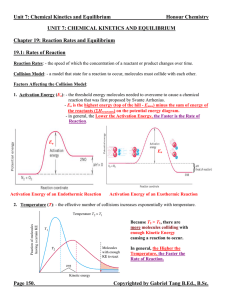
Unit 7 Reaction Rates and Equilibrium Notes
... Equilibrium Expression: - an expression relating the concentrations or pressures of the reactants and products when they are at the state of equilibrium. - it takes the form of the individual products raised to the power of their respective coefficients divided by the individual reactants raised to ...
... Equilibrium Expression: - an expression relating the concentrations or pressures of the reactants and products when they are at the state of equilibrium. - it takes the form of the individual products raised to the power of their respective coefficients divided by the individual reactants raised to ...
Seeking the Chemical Roots of Darwinism: Bridging between
... Needless to say this replicative type of stability is not applicable at the individual level, but rather at the population level. The individual entities within that population are continually being turned over, so that it is the replicator population as a whole that is perceived as stable and forms ...
... Needless to say this replicative type of stability is not applicable at the individual level, but rather at the population level. The individual entities within that population are continually being turned over, so that it is the replicator population as a whole that is perceived as stable and forms ...
Physical-chemical properties of complex natural fluids
... vapour and melts, as well as subvalence state of metals during transport processes. Based on chemical analyses of gases and condensates from high-temperature fumaroles of the Kudryavy volcano (i Iturup, Kuril Arc, Russia), a thermodynamic simulation of transport and deposition of ore- and rock-formi ...
... vapour and melts, as well as subvalence state of metals during transport processes. Based on chemical analyses of gases and condensates from high-temperature fumaroles of the Kudryavy volcano (i Iturup, Kuril Arc, Russia), a thermodynamic simulation of transport and deposition of ore- and rock-formi ...
Hydrogen dissociation under equilibrium and non
... Computer simulations are a useful tool for a realistic study of systems under conditions that are not easy to achieve in a laboratory, such as high temperature, pressure, concentration and other extreme conditions. Quantum mechanics is one option, but as these simulations becomes very time consuming ...
... Computer simulations are a useful tool for a realistic study of systems under conditions that are not easy to achieve in a laboratory, such as high temperature, pressure, concentration and other extreme conditions. Quantum mechanics is one option, but as these simulations becomes very time consuming ...
Thermophysical Properties of High-Temperature Reacting Mixtures
... (1) Calculation of the equilibrium composition and the thermodynamic properties, including mass density, molar weight, sonic velocity, heat capacity ratio, enthalpy, entropy, and specific heat, taking into account the influence of the formation of solid carbon. (2) Calculation of the transport coeff ...
... (1) Calculation of the equilibrium composition and the thermodynamic properties, including mass density, molar weight, sonic velocity, heat capacity ratio, enthalpy, entropy, and specific heat, taking into account the influence of the formation of solid carbon. (2) Calculation of the transport coeff ...
Thermodynamics of Combustion
... reasonable assessment of what would be expected under good combustion conditions. It cannot really be used to assess emissions levels but it can be extended to this simply by the introduction of additional equations: the basic approach is still valid. The manner in which combustion takes place is go ...
... reasonable assessment of what would be expected under good combustion conditions. It cannot really be used to assess emissions levels but it can be extended to this simply by the introduction of additional equations: the basic approach is still valid. The manner in which combustion takes place is go ...
PREPARMACY PHYSICAL CHEMISTRY THERMOCHEMISTRY
... ammonium hydroxide is dissociated into ions. In general, the heat of dissociation of a weak acid or weak base may be defined as the change in enthalpy of the system when one mole of it is dissociated into ions. ...
... ammonium hydroxide is dissociated into ions. In general, the heat of dissociation of a weak acid or weak base may be defined as the change in enthalpy of the system when one mole of it is dissociated into ions. ...
Ch.1-Matter and Change
... In the liquid state, matter has a definite volume, but an indefinite shape. In the gaseous state, matter has neither definite volume nor definite shape. Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
... In the liquid state, matter has a definite volume, but an indefinite shape. In the gaseous state, matter has neither definite volume nor definite shape. Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
Chapter 5
... heat exchanged under constant pressure. Note that, like the internal energy of a system, enthalpy cannot be measured directly and it is not possible to know the amount of enthalpy present in a chemical sample. However, enthalpy change, and therefore relative enthalpy, can be measured. Enthalpy is a ...
... heat exchanged under constant pressure. Note that, like the internal energy of a system, enthalpy cannot be measured directly and it is not possible to know the amount of enthalpy present in a chemical sample. However, enthalpy change, and therefore relative enthalpy, can be measured. Enthalpy is a ...
Chemical Equilibrium
... • Equilibrium [ ] of products are placed in the numerator. • Equilibrium [ ] of reactants are placed in the denominator. • Each [ ] term is raised to an exponent equal to its coefficient in the balanced equation. • If there is more than 1 product or reactant, the terms are multiplied. • Solids and l ...
... • Equilibrium [ ] of products are placed in the numerator. • Equilibrium [ ] of reactants are placed in the denominator. • Each [ ] term is raised to an exponent equal to its coefficient in the balanced equation. • If there is more than 1 product or reactant, the terms are multiplied. • Solids and l ...
2.26 MB - KFUPM Resources v3
... phase diagram since all three phases are separated by three lines, namely the sublimation line (between solid and vapor regions), the vaporization line (between liquid and vapor regions), and the melting line (between solid and ...
... phase diagram since all three phases are separated by three lines, namely the sublimation line (between solid and vapor regions), the vaporization line (between liquid and vapor regions), and the melting line (between solid and ...
Thermodynamics and Statistical Mechanics
... controls the flow of heat from one body to another when they are brought into thermal contact. We shall also attempt to understand the relationship between heat and mechanical work. For instance, to what extent does the heat content of a body increase when mechanical work is done on it? More importa ...
... controls the flow of heat from one body to another when they are brought into thermal contact. We shall also attempt to understand the relationship between heat and mechanical work. For instance, to what extent does the heat content of a body increase when mechanical work is done on it? More importa ...
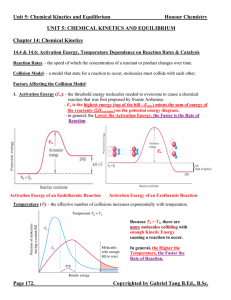
Unit 5: Chemical Kinetics and Equilibrium
... 1. When K >> 1, the equilibrium system favours the products. There are more products than reactants at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than r ...
... 1. When K >> 1, the equilibrium system favours the products. There are more products than reactants at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than r ...
Studies of Lithium Hydride Systems. I. Solid
... These equations must satisfy the Gibbs- Duhem equation within the assumption that the coefficients are independent of the temperature. The values of the excess chemical potentials of both components were then smoothed as a function of Xl simultaneously by a leastsquares method with the use of eq. 6 ...
... These equations must satisfy the Gibbs- Duhem equation within the assumption that the coefficients are independent of the temperature. The values of the excess chemical potentials of both components were then smoothed as a function of Xl simultaneously by a leastsquares method with the use of eq. 6 ...
Chemical Engineering Principles of CVD Processes
... The reactor is surrounded by furnace -The substrate and reactor walls are at the same temperature -The deposition is not only on the substrate but also on the inside of the reactor walls Contamination: a risk that particles will become detached (spall) from the walls & fall down during the depositio ...
... The reactor is surrounded by furnace -The substrate and reactor walls are at the same temperature -The deposition is not only on the substrate but also on the inside of the reactor walls Contamination: a risk that particles will become detached (spall) from the walls & fall down during the depositio ...
Spontaneous Change: Entropy and Gibbs Energy
... so. Entropy is the yardstick for measuring the dispersal of energy. In this chapter, we will continue to interpret observations about macroscopic systems by using a microscopic point of view. We will develop a conceptual model for understanding entropy and learn how to evaluate entropy changes for a ...
... so. Entropy is the yardstick for measuring the dispersal of energy. In this chapter, we will continue to interpret observations about macroscopic systems by using a microscopic point of view. We will develop a conceptual model for understanding entropy and learn how to evaluate entropy changes for a ...